Fragmentation of polyploid megakaryocytes into platelets has great relevance for blood homeostasis. Apoptotic cell death is a highly regulated genetic program, which has been observed in mature megakaryocytes fragmenting into platelets. The antiapoptotic protein BclxL has been reported as up-regulated during megakaryocytic differentiation in vitro, but absent during late megakaryopoiesis. Our study focused on examining BclxL levels in megakaryocytes in vivo and in assessing the effect of its overexpression in transgenic mice (via the platelet factor 4 [PF4] promoter) on megakaryocyte development and platelet fragmentation. Interestingly, in the wild-type and less in PF4-driven transgenic mice, BclxL was not detected in a fraction of the large mature megakaryocytes, suggesting a regulation on the protein level. BclxL overexpression was associated with a moderate increase in megakaryocyte number, with no significant change in ploidy level or platelet counts. When the mice were challenged by induction of immune thrombocytopenia, the rate of platelet recovery was significantly slower in the transgenic mice as compared with controls. Moreover, proplatelet formation in vitro by transgenic megakaryocytes was limited. Transgenic megakaryocytes displayed poorly developed platelet demarcation membranes and cell margin extensions. Our study indicates that regulated expression of BclxL in megakaryocytes is important for the development of cells with a high potential to fragment into platelets.
Introduction
Megakaryopoiesis is a complex process, which is completed by platelet formation in bone marrow (BM) sinusoids accompanied by apoptotic death of senescent cells.1 Mpl ligand, also referred to as thrombopoietin (TPO) or megakaryocyte growth and development factor (MGDF),2,3 elevates the number and ploidy of megakaryocytes and increases platelet levels in vivo. Although the terminal differentiation of mammalian megakaryocytes into platelets has great relevance for blood homeostasis, the mechanism of platelet formation is still a matter of controversy.4 5
Apoptotic cell death is a highly regulated genetic program,6 and although it has been observed in mature megakaryocytes, it remains to be determined how the induction of apoptosis relates to normal megakaryocyte maturation and to the release of functional platelets. Recent studies attempted to investigate the relationship existing between megakaryocyte maturation, apoptosis, and proplatelet formation in culture of CD34+-derived megakaryocytes.7,8 Although unable to investigate in depth the subordination of events, this study showed that apoptosis occurred predominantly in mature megakaryocytes rather than in immature megakaryoblasts. Moreover, the kinetics of platelet release into the culture supernatants corresponded to the onset of apoptosis in these cells. This temporal correlation suggested that maximal platelet production and megakaryocyte apoptosis are closely related events. In a recent study, it was shown that the antiapoptotic protein BclxL is abundant in differentiating megakaryocytes in culture, but absent from mature prefragmenting cells.9
To explore the role of deregulated expression of BclxL in megakaryocyte development and platelet fragmentation in vivo, we generated transgenic mice overexpressing the antiapoptotic human BclxL gene in megakaryocytes.10 The transgenic phenotype observed included a decrease in the number of megakaryocytes undergoing apoptosis and a change in the morphology of the demarcation membrane structure in megakaryocytes, associated with a low frequency of cells able to produce proplatelets. The platelets produced, however, displayed no change in reactivity in response to different agonists. These results support the hypothesis that cellular changes associated with BclxL overexpression in megakaryocytes are important for thrombopoiesis.
Materials and methods
Plasmid construction
The plasmid PF4-hBclxL-SV40 was constructed by using gene fragments from several different plasmids: pPF4GH, which contains the rat 1.1-kb PF4 promoter in which the original PF4 5′ untranslated region (UTR) was included, linked to human growth hormone gene (HGH)11; PF4SV40 construct, which contains the rat platelet factor 4 (PF4) promoter and 400 bp of SV40 small t intron and polyA tail12; and pSFFV-hBclxL-NEO plasmid, which contains 800 bp of human full-length BclxL cDNA (a generous gift from Dr D. D. Chaplin, Howard Hughes Medical Institute, Washington University School of Medicine, St Louis, MO). The construction involved conventional methods, as we used before.13 The resulting plasmid, referred to as PF4-hBclxL-SV40, was confirmed by DNA sequencing, and subsequently digested with NdeI to excise the PF4-hBclxL-SV40 fragment used for microinjections.
Generation of transgenic mice
The above construct was used for the generation of transgenic mice (on FVB strain), which were then examined for transgene integration and expression, as described before.13
BM isolation and cell culture
Bone marrow (BM) was harvested from femurs of control or transgenic mice as described before.11 The isolated cells were plated at a concentration of 5 × 106 cells/mL in Iscove modified Dulbecco medium (IMDM; Gibco BRL, Gaithersburg, MD), supplemented with 20% horse serum (Gibco BRL), 2%l-glutamine (Gibco BRL), 50 U/mL penicillin (Gibco BRL), 50 U/mL streptomycin (Gibco, BRL), and 25 ng/mL of the Mpl ligand, pegylated recombinant murine MGDF (PEG-rmMGDF; generous gift from Amgen, Thousand Oaks, CA; referred to as MGDF) and cultured at 37°C in humidified chamber with 5% CO2.
Proplatelet formation assay
For proplatelet formation assay, BM was harvested and cultured as described above. For the first 3 days of culture, 25 ng/mL MGDF was added. Day 3 BM cultures were used to obtain an enriched population of megakaryocytes, using indirect immunomagnetic negative separation with Dynabeads M-450 in accordance with the manufacturer's protocol with some modifications (Dynal, Oslo, Norway). Briefly, BM cultured cells were resuspended at a concentration of 5 × 106 cells/mL in 0.1% bovine serum albumin (BSA; Sigma, St Louis, MO), 0.6% sodium citrate and 2% fetal bovine serum (FBS; Gibco, BRL) in phosphate-buffered saline (PBS). Purified rat antimouse IgG: Mac-1α (Pharmingen, San Diego, CA, no. 01711A), TER-119 (Pharmingen, no. 09081), and Gr-1 (Pharmingen, no. 01211A) were added at a final concentration of 5 μg/mL and incubated for 30 minutes at 4°C on a rotor. Cells, covered with primary antibodies were washed in 0.1% BSA, 0.6% sodium citrate, and 2% FBS in PBS. Washed Dynabeads M-450 covered with sheep antirat IgG were added to a final concentration of 2 × 107 beads/mL in 0.1% BSA, 2% FBS in PBS and incubated for 40 minutes at 4°C with rotation. Negatively selected megakaryocytes were pipetted off with the supernatant on a Dynal magnetic particle concentrator (MPC), washed twice in IMDM, and plated at a concentration of 1 × 105cells/well in 24-well plates in IMDM, supplemented with 10% FBS, 2%l-glutamine in the presence or absence of 25 ng/mL MGDF. Proplatelets were detected and scored by phase contrast microscopy (inverted microscope Olympus IX 70, Olympus America, Bellingham, MA) on days 5 and 6 of culture. The total number of megakaryocytes making proplatelets was determined and normalized to the total number of megakaryocytes in the well.
Acetylcholinesterase assay and immunohistochemistry
The BM megakaryocytes were identified by in situ staining for acetylcholinesterase as described elsewhere.14,15 For immunohistochemistry, both primary BM cells and negatively selected BM megakaryocytes were used after 3-day culture in the presence of MGDF (see above). BM cells were cytospun on microscope slides at a concentration of 1 × 106 or 1 × 105cells/slide respectively, fixed, and stained as described before.16 Cy3-conjugated goat antirabbit IgG (Jackson Immunoresearch Laboratories, West Grove, PA) was used in conjugation with 10 μg/mL primary rabbit anti-BclxL antibody (Santa Cruz Biotechnology, Santa Cruz, CA, no. sc 7195), which specifically recognizes BclxL in rat BM cell extract. Rat anti-CD41 conjugated to fluorescein isothiocyanate (FITC; Pharmingen) or Cy3 (Jackson Laboratories) was used for staining megakaryocytes. DNA was stained with 20 μM Hoechst (Sigma).
Examination of transgene expression in BclxL transgenic mice by PCR-based cDNA amplification
To further confirm transgene expression, RNA isolated from total BM was subjected to reverse transcription (RT) and polymerase chain reaction (PCR)–based cDNA amplification. For this purpose BM was harvested from femurs of control or transgenic mice and cultured for 3 days as described. Total RNA was isolated with TRIzol reagent (Gibco BRL). First-strand cDNA synthesis was carried out following a protocol of SMART cDNA Library Construction Kit (Clontech, Palo Alto, CA), using specific sense: 5′-GCAGTGGTAACAACGCAGAGTACGCGGG-3′ (with 3′ annealing to CAP sequences in mRNAs), and antisense: 5′-GCAGTGGTAACAACGCAGAGTACTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTVN primers. For cDNA amplification, the Expand Long Template PCR system (Boehringer Mannheim, Indianapolis, IN), the above sense primer and 20 cycles of PCR reactions were used. Amplified DNA was electrophoresed on 1% agarose gel, transferred to nylon membrane, and tested with 32P-labeled probes as indicated.
Platelet counts and volume, blood cell counts, and TPO immunoassay
Induction of ITP and platelet recovery studies
Age- and sex-matched transgenic and wild-type mice (8-week-old mice each weighing about 25 g) were injected in the tail vein with 25 μg of the antibody pOp3 (dissolved in PBS/BSA), a rat IgG2a against the N-terminal 45-kDa region of mouse glycoprotein Ibα (GPIbα). This antibody is platelet/megakaryocyte specific and was used before to induce immune thrombocytopenia (ITP).19Following the injection, platelet levels were determined as a function of time. Injection of mice with PBS/BSA did not cause a significant change in platelet level.
Ploidy analysis
Detection of apoptotic cells
Apoptosis was determined in an enriched population of BM megakaryocytes, obtained by magnetic beads separation as described above, on day 6 of culture. The ApopTag Plus Peroxidase in situ Apoptosis Detection Kit (Intergene, Purchase, NY) was used to assay apoptosis, following the manufacturer's instructions for suspension cell cultures.
Preparation of BM for transmission electron microscopy
Bone marrow was flushed from femurs with fixative (4.3% glutaraldehyde in 0.1 M Veronal buffer, pH 7.4), washed once in fixative, and incubated for 60 minutes at 4°C. After fixation, cells were washed once in 0.1 M Veronal buffer and postfixed in 1% osmium tetroxide in 0.1 M Veronal buffer for 10 minutes at 4°C.21 The samples were dehydrated through a series of graded ethanol, infiltrated with resin composed of Araldite 502, DDSA (dodecenyl succinic anhydride), and BDMA (benzyldimethylamine; Ted Pella, Redding, CA) and finally embedded in the above-mentioned resin. Thin sections were cut and stained with uranyl acetate and lead citrate and viewed with a transmission electron microscope (Phillips 300 TEM).
PEG-rmMGDF injections into mice
Single injections of PEG-rmMGDF (50 μg/kg) into 3-month-old mice via the lateral tail vein were carried out as described previously.22 Control mice received a carrier injection of 1% normal mouse serum in PBS.
Platelet aggregation studies
For aggregation studies, platelets were prepared as described elsewhere.23 Briefly, blood was drawn from the abdominal aorta of 4-month-old mice into a 3.15% sodium citrate solution, keeping the ratio of 1 volume of anticoagulant to 9 volumes of blood. To obtain platelet-rich plasma (PRP), samples were centrifuged for 35 seconds at low speed (2700 rpm) in a Sorvall centrifuge (brakes off) at room temperature. To obtain platelet-poor plasma (PPP), the rest of the sample was centrifuged for 15 minutes at 1500g in a tabletop microfuge at room temperature. Platelets were stored at 37°C throughout the experiments and the cell count was adjusted in the final suspension to a concentration of 5 × 105 cells/μL in PRP. Aggregation was measured at 37°C by a turbidimetric method in a dual-channel Platelet Aggregation Profiler (Biodata, Horsham, PA). A 200-μL aliquot of platelet suspension was stirred at 1100 rpm and activated by the addition of 5 to 15 μL agonist.
Results
Generation of transgenic mice overexpressing the BclxLgene under the control of the PF4 promoter
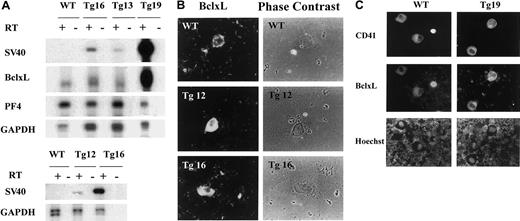
To explore the role of processes associated with programmed cell death in megakaryocyte development and platelet fragmentation, we created a transgenic mouse model in which the antiapoptotic humanBclxL gene was overexpressed in megakaryocytes via the rat PF4 tissue-specific promoter.11 15 We confirmed the expression of the transgene in megakaryocytes of 4 transgenic lines produced (Tg12, Tg13, Tg16, Tg19) by PCR-based cDNA amplification of BM cells (Figure 1A, see “Materials and methods”). This analysis revealed that all 4 transgenic founder lines expressed the transgene. This was concluded based on detection of SV40 (unique to the transgene) or BclxL, as compared with endogenous PF4 (a lineage-specific mRNA), or to glyceraldehyde-phosphate dehydrogenase (GAPDH). Based on this method of determination, Tg19 and Tg16 had significant levels of transgenic mRNA as compared with Tg13 and Tg12.
BclxL transgene expression in megakaryocytes.
(A) PCR-based cDNA amplification, showing transgene expression in BM of transgenic mice, as described in “Materials and methods.” Reverse-transcribed, amplified DNA was electrophoresed, transferred onto a nylon membrane, and analyzed with 32P-labeled probes as indicated. The reaction was carried out in the absence or presence of reverse transcriptase (RT) for transgenic lines (Tg) and wild-type (WT) mice. SV40 probe detects the transgene only, BclxL probe detects the endogenous and transgenic BclxL at similar positions, and both PF4 and GAPDH are used to evaluate and normalize for the efficiency of the RT reaction. The lower panel with SV40 probe shows twice the time of the exposure of the film as the top panel to permit visualization of the band in Tg12. Data are representative of 3 experiments. (B) BM cells derived from transgenic (Tg) or control (WT) mice were cytospun (as a dense layer of cells to allow focus on the rare megakaryocytes) and Cy3-labeled with rabbit antibodies to human BclxL (which also reacts with mouse BclxL). Transgenic megakaryocytes, identified based on size and morphology, displayed higher levels of BclxL, as compared with control (examinations were done blindly). The staining appeared cytosolic, as also described by others.42 The data are representative of 2 transgenic lines (2 mice per line in duplicates; original magnification, × 400). (C) Freshly derived BM cells were cytospun onto a slide and subjected to immunohistochemistry with anti-BclxL (middle panel). Megakaryocytes were identified based on staining with an antibody to CD41 (top panel) and DNA was stained with Hoechst (lower panel). No staining was observed with secondary antibody alone and no BclxL-specific fluorescence was observed on staining with CD41 alone and vice versa (not shown). The micrographs are of a representative field, further demonstrating enhanced, lineage-specific expression of BclxL in a transgenic line (original magnification, × 400).
BclxL transgene expression in megakaryocytes.
(A) PCR-based cDNA amplification, showing transgene expression in BM of transgenic mice, as described in “Materials and methods.” Reverse-transcribed, amplified DNA was electrophoresed, transferred onto a nylon membrane, and analyzed with 32P-labeled probes as indicated. The reaction was carried out in the absence or presence of reverse transcriptase (RT) for transgenic lines (Tg) and wild-type (WT) mice. SV40 probe detects the transgene only, BclxL probe detects the endogenous and transgenic BclxL at similar positions, and both PF4 and GAPDH are used to evaluate and normalize for the efficiency of the RT reaction. The lower panel with SV40 probe shows twice the time of the exposure of the film as the top panel to permit visualization of the band in Tg12. Data are representative of 3 experiments. (B) BM cells derived from transgenic (Tg) or control (WT) mice were cytospun (as a dense layer of cells to allow focus on the rare megakaryocytes) and Cy3-labeled with rabbit antibodies to human BclxL (which also reacts with mouse BclxL). Transgenic megakaryocytes, identified based on size and morphology, displayed higher levels of BclxL, as compared with control (examinations were done blindly). The staining appeared cytosolic, as also described by others.42 The data are representative of 2 transgenic lines (2 mice per line in duplicates; original magnification, × 400). (C) Freshly derived BM cells were cytospun onto a slide and subjected to immunohistochemistry with anti-BclxL (middle panel). Megakaryocytes were identified based on staining with an antibody to CD41 (top panel) and DNA was stained with Hoechst (lower panel). No staining was observed with secondary antibody alone and no BclxL-specific fluorescence was observed on staining with CD41 alone and vice versa (not shown). The micrographs are of a representative field, further demonstrating enhanced, lineage-specific expression of BclxL in a transgenic line (original magnification, × 400).
Expression on the protein level was verified by immunohistochemistry, using an antibody specific to BclxL. It should be pointed out that in studying megakaryocyte biology, one often has to rely on careful in situ examinations because of the rarity of this cell type in the marrow (< 0.05%). We noted that not all of the large mature megakaryocytes in the wild-type or transgenic mice stained positive for BclxL; for example, among 40 transgenic megakaryocytes surveyed in Tg19, only 70% displayed a strong staining with anti-BclxL, and only 50% of the wild-type megakaryocytes displayed a weak, but detectable, staining for endogenous BclxL. The remainders of the cells in each sample were BclxL negative. Similar results were obtained with Tg16 analyzed (data not shown), further suggesting that wild-type or PF4-driven BclxL levels are regulated on the protein level (representative fields are shown in Figure 1B-C and Figure 2B). In the transgenic or wild-type samples, we occasionally noted small-sized megakaryocytes with intense staining for BclxL (Figure 1C). This is also supported by a recent report, according to which BclxL is expressed in small size, developing megakaryocytes in culture, but not in mature cells.9 The fact the immunohistochemistry did not reveal a significant difference in the level of BclxL protein in different transgenic lines (eg, Tg12 and Tg16 shown in Figure 1B) could be due to saturating levels of BclxL mRNA in the transgenic megakaryocytes.
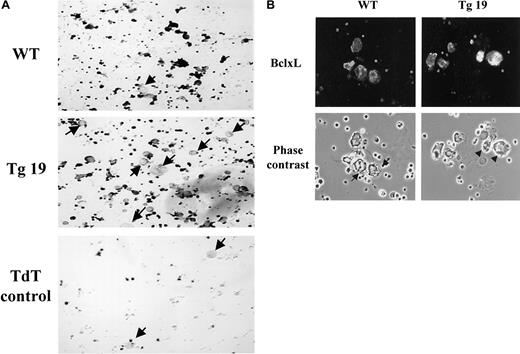
A decrease in the number of megakaryocytes undergoing apoptosis is detected in BclxL transgenic mice.
BM cultures of transgenic line 19 (Tg19) or control (WT) mice were enriched for megakaryocytes by indirect immunomagnetic negative separation. Following this procedure, there are still numerous, contaminating BM cells. (A) Apoptotic cells were detected by dark staining with the ApopTag Plus Peroxidase in situ Apoptosis Detection kit. Arrows point to some of the nonapoptotic megakaryocytes (original magnification, × 200). The experiment shown is representative of 3 performed. Terminal dioxyl transferase (TdT) enzyme control was always included in the study, because contaminant magnetic beads often give a positive signal. (B) Isolated megakaryocytes were cytospun and stained with Cy3-labeled rabbit antibodies to human BclxL (which also reacts with mouse BclxL; original magnification, × 400). In the center of the WT panel are proplatelet-forming cells with poor BclxL expression. The arrows point to cellular extensions (as also demonstrated in detail in Figure 7), which do not appear in megakaryocytes with high abundance of BclxL (eg, Tg19; indicated by arrowheads).
A decrease in the number of megakaryocytes undergoing apoptosis is detected in BclxL transgenic mice.
BM cultures of transgenic line 19 (Tg19) or control (WT) mice were enriched for megakaryocytes by indirect immunomagnetic negative separation. Following this procedure, there are still numerous, contaminating BM cells. (A) Apoptotic cells were detected by dark staining with the ApopTag Plus Peroxidase in situ Apoptosis Detection kit. Arrows point to some of the nonapoptotic megakaryocytes (original magnification, × 200). The experiment shown is representative of 3 performed. Terminal dioxyl transferase (TdT) enzyme control was always included in the study, because contaminant magnetic beads often give a positive signal. (B) Isolated megakaryocytes were cytospun and stained with Cy3-labeled rabbit antibodies to human BclxL (which also reacts with mouse BclxL; original magnification, × 400). In the center of the WT panel are proplatelet-forming cells with poor BclxL expression. The arrows point to cellular extensions (as also demonstrated in detail in Figure 7), which do not appear in megakaryocytes with high abundance of BclxL (eg, Tg19; indicated by arrowheads).
We also followed apoptosis in megakaryocytes in one of the transgenic lines using the TUNEL assay. This assay cannot be performed simultaneously with immunohistochemistry for BclxL because of quenching and overlap of colors. BM cultures were enriched for megakaryocytes as described in “Materials and methods.” The transgenic sample displayed a significant number of apoptotic cells, in accordance with the above conclusion that BclxL is absent in a fraction of the cells. This assay presented with a relatively high variability. For instance, in 3 experiments performed, a range of 1.5- to 1.9-fold reduction in the number of apoptotic cells was observed in the transgenic samples as compared with control (Figure 2A). Hence, we could only conclude that there is a trend of reduced numbers of apoptotic megakaryocytes in these transgenic mice. Figure 2B focuses on a culture of BM cells enriched for megakaryocytes in which BclxL is detected, showing that its level in the transgenic sample is higher than the typical level detected in a control cell. Note also in Figure 2B cells in the control culture (arrows), in which proplatelet formation is in process, and endogenous BclxL levels are low. Processes typical of proplatelet formation were routinely further confirmed by visualizing the cells at high magnification (eg, Figure 7A). Our survey of several fields (approximately 500 megakaryocytes) indicated that not all megakaryocytes with low level of BclxL are engaged in proplatelet formation, but those with high abundance of BclxL do not display proplatelet formation (eg, Tg19 in Figure 2B, arrowheads).
BclxL transgenic mice show an increase in the number of megakaryocytes and no change in ploidy level or in serum concentration of TPO
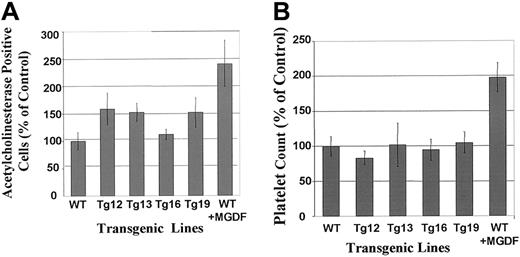
Megakaryocyte number could be under a tight homeostasis in vivo so that a change in the rate of apoptosis may cause a small or no increase in the steady-state level of megakaryocytes in the marrow. To examine this possibility, BM samples cytospun on slides were subjected to in situ staining for acetylcholinesterase, a unique marker for rodent and cat megakaryocytes14 (Figure3A). We determined a constant tendency of augmented megakaryocyte number in BM of transgenic animals, as compared with control, as follows: a 1.62-fold increase in Tg12 (P > .05), a 1.54-fold increase in Tg13 (P < .05), a 1.20-fold increase in Tg16 (P > .05), and a 1.60-fold increase in Tg19 (P < .05). The differences observed for Tg13 and Tg19, as compared with control, are statistically significant, as derived by t tests. MGDF increased megakaryocyte number in wild-type mice by 2.4-fold (P = .05; Figure 3A). Tg13 and Tg19 were also subjected to histologic evaluation of the spleen, as we described before.16 A blinded evaluation of 3 different splenic sections from each group revealed that the total number of megakaryocytes in each of these lines was increased by approximately 2-fold compared with control mice (not shown). The overall cellularity of the spleen in control and transgenic animals was similar. Spleen sections of MGDF-treated mice (analyzed as a control for this assay) displayed a 2.5-fold increase in the number of megakaryocytes, as also observed before.16 Taken together, this assay and the above in situ staining evaluation indicated a moderate, but significant, increase in megakaryocyte number in the transgenic lines 13 and 19. These changes were not accompanied by any significant shift in the ploidy level, as derived by t test evaluation of the data (Table 1). Because the number of megakaryocytes may affect the serum levels of TPO, we examined this parameter in Tg16 (which displayed no significant change in megakaryocyte number) and in Tg19 (which displayed an increase in megakaryocyte numbers) and found it to be similar to control mice (in the range of 3.7-4.1 × 103 pg/mL). This indicated that the phenotype of the transgenic mice was not based on secondary effects on the levels of TPO in the circulation.
Megakaryocytes are more numerous in BM of BclxL transgenic mice, but no increase in platelet level is detected.
(A) BM was harvested from femurs of WT or transgenic mice and cytospun; megakaryocytes were identified by in situ staining for acetylcholinesterase as described in “Materials and methods.” The data are presented as percentage of WT megakaryocytes, presented as averages ± SD for 4 different experiments (4 mice from each group), each performed in duplicates. The actual average number for WT is 52.3 ± 15.4 megakaryocytes/5 × 105 BM cells. Values were also obtained in MGDF-injected mice. (B) Platelet counts from the peripheral blood of WT and transgenic mice. The data are shown as percentage of WT values with averages ± SD for 8 mice for Tg12; for 16 mice each for Tg13, Tg16, and Tg19; and for 24 mice for WT. The actual average number for WT is 1.2 × 106 platelets in 1 μL blood. Values were also obtained in MGDF-injected mice.
Megakaryocytes are more numerous in BM of BclxL transgenic mice, but no increase in platelet level is detected.
(A) BM was harvested from femurs of WT or transgenic mice and cytospun; megakaryocytes were identified by in situ staining for acetylcholinesterase as described in “Materials and methods.” The data are presented as percentage of WT megakaryocytes, presented as averages ± SD for 4 different experiments (4 mice from each group), each performed in duplicates. The actual average number for WT is 52.3 ± 15.4 megakaryocytes/5 × 105 BM cells. Values were also obtained in MGDF-injected mice. (B) Platelet counts from the peripheral blood of WT and transgenic mice. The data are shown as percentage of WT values with averages ± SD for 8 mice for Tg12; for 16 mice each for Tg13, Tg16, and Tg19; and for 24 mice for WT. The actual average number for WT is 1.2 × 106 platelets in 1 μL blood. Values were also obtained in MGDF-injected mice.
Megakaryocyte ploidy in BclxL transgenic mice is not altered
| Mouse line . | Sample (n) . | Ploidy distribution (%) . | ||||||
|---|---|---|---|---|---|---|---|---|
| 2 N . | 4 N . | 8 N . | 16 N . | 32 N . | 64 N . | 128 N . | ||
| WT | 28 | 15.29 ± 3.62 | 9.44 ± 2.29 | 11.14 ± 2.89 | 47.30 ± 5.81 | 14.68 ± 4.09 | 1.37 ± 0.74 | 0.79 ± 0.60 |
| Tg12 | 16 | 14.99 ± 2.95 | 8.99 ± 3.05 | 14.39 ± 4.06 | 48.49 ± 2.92 | 11.32 ± 3.94 | 1.19 ± 0.53 | 0.65 ± 0.34 |
| Tg13 | 16 | 17.11 ± 4.34 | 9.03 ± 3.12 | 12.46 ± 4.89 | 47.93 ± 5.10 | 11.63 ± 3.54 | 1.23 ± 0.54 | 0.65 ± 0.39 |
| Tg16 | 24 | 17.02 ± 3.02 | 10.18 ± 2.89 | 10.42 ± 4.06 | 42.73 ± 7.76 | 17.03 ± 7.25 | 1.73 ± 1.00 | 0.84 ± 0.59 |
| Tg19 | 16 | 16.36 ± 4.28 | 9.51 ± 2.17 | 10.23 ± 2.67 | 48.05 ± 6.01 | 14.02 ± 3.62 | 1.29 ± 0.70 | 0.52 ± 0.38 |
| Mouse line . | Sample (n) . | Ploidy distribution (%) . | ||||||
|---|---|---|---|---|---|---|---|---|
| 2 N . | 4 N . | 8 N . | 16 N . | 32 N . | 64 N . | 128 N . | ||
| WT | 28 | 15.29 ± 3.62 | 9.44 ± 2.29 | 11.14 ± 2.89 | 47.30 ± 5.81 | 14.68 ± 4.09 | 1.37 ± 0.74 | 0.79 ± 0.60 |
| Tg12 | 16 | 14.99 ± 2.95 | 8.99 ± 3.05 | 14.39 ± 4.06 | 48.49 ± 2.92 | 11.32 ± 3.94 | 1.19 ± 0.53 | 0.65 ± 0.34 |
| Tg13 | 16 | 17.11 ± 4.34 | 9.03 ± 3.12 | 12.46 ± 4.89 | 47.93 ± 5.10 | 11.63 ± 3.54 | 1.23 ± 0.54 | 0.65 ± 0.39 |
| Tg16 | 24 | 17.02 ± 3.02 | 10.18 ± 2.89 | 10.42 ± 4.06 | 42.73 ± 7.76 | 17.03 ± 7.25 | 1.73 ± 1.00 | 0.84 ± 0.59 |
| Tg19 | 16 | 16.36 ± 4.28 | 9.51 ± 2.17 | 10.23 ± 2.67 | 48.05 ± 6.01 | 14.02 ± 3.62 | 1.29 ± 0.70 | 0.52 ± 0.38 |
Platelet number, volume and reactivity, and blood cell counts are moderately changed or unchanged in BclxL transgenic mice
Whereas MGDF injection into control mice increased platelet counts, platelet levels in all the PF4-hBclxL transgenic lines were not significantly different from those in control mice (Figure 3B). Please note that platelet levels were monitored in mice at age 6 to 12 weeks and the data were compiled, as shown in Figure 3. This was pursued to analyze platelet counts in most of the mice that were used for other analyses. This range of age, although small, may have contributed to the variability observed (SD) in platelet counts, in addition to the well-known variability that exists between individual mice of the same strain. However, when data were analyzed by comparing smaller groups of age-matched mice, there was still no significant difference in platelet counts in the transgenic and nontransgenic mice. By both types of group analysis, MGDF injection significantly increased platelet counts. Bleeding time was comparable in control and transgenic mice (not shown). The extent of platelet aggregation in response to adenosine diphosphate or arachidonic acid was similar in the control and transgenic mice (not shown). Also, platelet volumes in transgenic mice were not significantly different from those in control mice (Table2). Finally, the transgenic phenotype was not associated with any change in white or red blood cell counts (Table2). The statistical differences between groups were derived byt tests.
The distribution of erythroid and myeloid cells, and mean platelet volume is not altered in BclxL transgenic mice
| Mouse line . | Sample (n) . | Red blood cells (106/μL) . | White blood cells (103/μL) . | Mean platelet volume (fL) . |
|---|---|---|---|---|
| WT | 28 | 8.62 ± 1.51 | 5.31 ± 2.11 | 4.42 ± 0.23 |
| Tg12 | 16 | 6.60 ± 0.56 | 4.39 ± 1.70 | 4.74 ± 0.27 |
| Tg13 | 16 | 8.93 ± 1.35 | 5.53 ± 1.36 | 4.71 ± 0.36 |
| Tg16 | 24 | 7.58 ± 1.32 | 5.52 ± 0.64 | 4.47 ± 0.25 |
| Tg19 | 16 | 8.96 ± 0.43 | 5.58 ± 0.55 | 4.60 ± 0.32 |
| Mouse line . | Sample (n) . | Red blood cells (106/μL) . | White blood cells (103/μL) . | Mean platelet volume (fL) . |
|---|---|---|---|---|
| WT | 28 | 8.62 ± 1.51 | 5.31 ± 2.11 | 4.42 ± 0.23 |
| Tg12 | 16 | 6.60 ± 0.56 | 4.39 ± 1.70 | 4.74 ± 0.27 |
| Tg13 | 16 | 8.93 ± 1.35 | 5.53 ± 1.36 | 4.71 ± 0.36 |
| Tg16 | 24 | 7.58 ± 1.32 | 5.52 ± 0.64 | 4.47 ± 0.25 |
| Tg19 | 16 | 8.96 ± 0.43 | 5.58 ± 0.55 | 4.60 ± 0.32 |
Platelet levels in mice subjected to experimental ITP
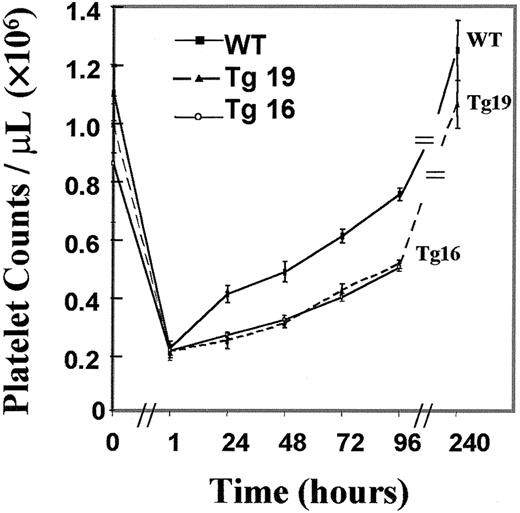
Platelet levels may be influenced by megakaryocyte ploidy and number as well as by the integrity of the membrane system within the mature megakaryocyte. Because the increased number of megakaryocytes in BclxL transgenic mice (Tg19) was not associated with any increase in the numbers of platelets, we speculated that under normal conditions, a tight regulation of platelet homeostasis prevents a significant change in platelet levels on BclxL overexpression. If this were the case, platelet elevation under stress might be different in the control and transgenic mice. To test this hypothesis, age- and sex-matched transgenic and wild-type mice were subjected to experimental ITP and platelet recovery was monitored. Tg19 and Tg16 were chosen for these experiments because both strains expressed the transgene (Figures 1 and2B), but only Tg19 showed a significant increase in megakaryocyte number, hence allowing us to examine if platelet recovery also depends on changes in the baseline level of megakaryocytes. As shown in Figure4, platelet recovery was significantly and similarly lower in both transgenic lines compared with control (P < .01), suggesting that megakaryocytes in the transgenic mice were impaired in their ability to fragment into platelets. In this type of analysis we observed a lesser variation in platelet counts as compared with the data in Figure 3B, most likely because emphasis was given to elect perfectly matching mice (age and sex) for this injection protocol.
Platelet recovery is slower in transgenic mice subjected to experimentally induced ITP.
The data are averages ± SD for 6 mice for Tg19, 3 mice for Tg16, and 8 wild-type (WT) mice. The differences between the transgenic lines and control were statistically significant (P < .01), as derived by t tests.
Platelet recovery is slower in transgenic mice subjected to experimentally induced ITP.
The data are averages ± SD for 6 mice for Tg19, 3 mice for Tg16, and 8 wild-type (WT) mice. The differences between the transgenic lines and control were statistically significant (P < .01), as derived by t tests.
Ultrastructural analysis of megakaryocytes of BclxL transgenic mice shows a marked change in the morphology of the demarcation membrane structures
There are several facets to the study of the mechanism by which BclxL overexpression may induce impairment in platelet fragmentation. These include, for instance, identification of the signaling pathways mediating the effect (which would typically require biochemical assays on abundant cells), structural changes within the cells (which could be performed in situ), and so forth. In the current study we elected to focus on examining potential changes in the intracellular demarcation membrane system, which have been described as important for platelet fragmentation (see “Discussion”). Electron microscopic analysis indicated that the majority of control megakaryocytes (> 90%) exhibited well-demarcated cytoplasmic territories with extensive dilation of the demarcation membrane system (Figure5A-B). As described in “Discussion,” this system has been described before as the origin of platelets. In contrast, the majority of transgenic megakaryocytes (> 75%) showed poorly developed demarcation territories that consisted of a very congested demarcation membrane system (Figure 5C-F, especially panels E and F). Although a small population of the megakaryocytes from the transgenic line (< 25%) did contain some areas of platelet territory delineation, the majority of megakaryocytes from these mice had a significant change in these membranes. Also, megakaryocytes of transgenic mice exhibited an extensive outer ring of cytoplasm with no typical platelet extensions (Figure 5D,F, arrows). In control megakaryocytes the peripheral sheet unfolds from the cell core and displays an extensive platelet-shedding pattern (Figure 5B, arrow). It is important to note that our analyses were conducted in 3 different mice for each group (control and transgenic), showing the same results. At least 50 megakaryocytes from these 3 different mice (control and transgenic) were analyzed. It should be also pointed out that the BclxL antibodies available thus far are not yielding a signal when used under the fixation methods necessary for electron microscopic analysis.
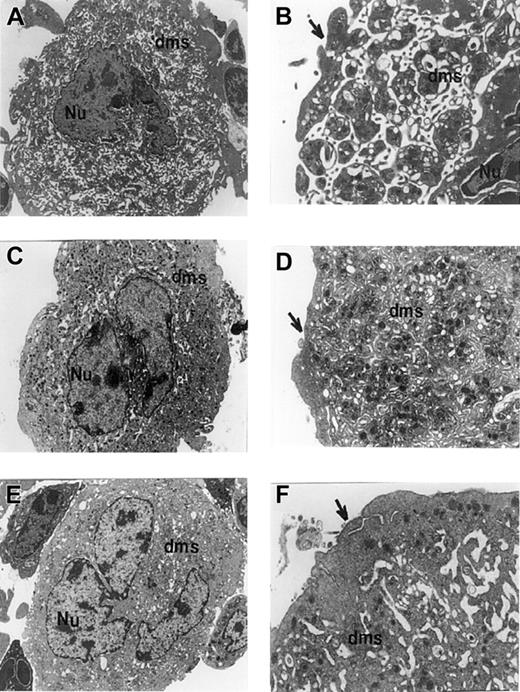
Transmission electron micrographs of megakaryocytes derived from control or BclxL transgenic mice.
Micrographs show megakaryocytes from WT control (A,B), Tg19 (C,D), and Tg16 (E,F) mice. At least 50 megakaryocytes from 3 different mice (control and transgenic) were analyzed. The nucleus (Nu) and demarcation membrane system (dms) are labeled. Note the poor dilation of demarcation membranes in panels C through F. Arrows point to peripheral sheet of cytoplasm which appearance is consistent with platelet shedding in control cells (A,B). Note the extensive outer ring of cytoplasm in panels C and D, and especially in panels E and F. Granule numbers are comparable in control and transgenic megakaryocytes. Original magnifications, × 4500 in panels A, C, and E; × 12 500 in panels B, D, and F.
Transmission electron micrographs of megakaryocytes derived from control or BclxL transgenic mice.
Micrographs show megakaryocytes from WT control (A,B), Tg19 (C,D), and Tg16 (E,F) mice. At least 50 megakaryocytes from 3 different mice (control and transgenic) were analyzed. The nucleus (Nu) and demarcation membrane system (dms) are labeled. Note the poor dilation of demarcation membranes in panels C through F. Arrows point to peripheral sheet of cytoplasm which appearance is consistent with platelet shedding in control cells (A,B). Note the extensive outer ring of cytoplasm in panels C and D, and especially in panels E and F. Granule numbers are comparable in control and transgenic megakaryocytes. Original magnifications, × 4500 in panels A, C, and E; × 12 500 in panels B, D, and F.
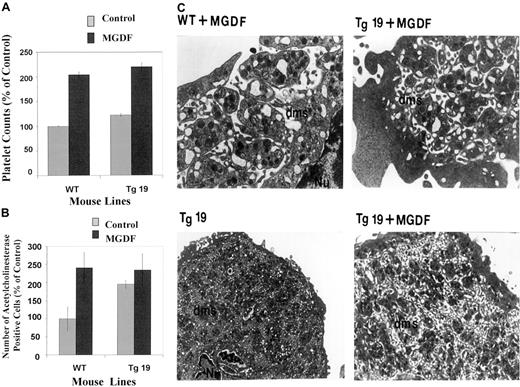
We next sought to investigate if a treatment of BclxL transgenic mice with MGDF would affect megakaryocyte expansion and platelet levels to the same extent as it does in control mice. MGDF caused a 2-fold increase in circulating platelets in control mice as well as in Tg19 (Figure 6A). The number of megakaryocytes did not change significantly in the transgenic line (P > .05; which had already an elevated baseline), whereas it increased almost by 2.5-fold in control animals on treatment with MGDF (Figure 6B). Electron microscopic analysis showed that MGDF-treated transgenic mice displayed more megakaryocytes (about 75% of 20 analyzed) containing membranous structures that were dilated as compared with nontreated transgenic mice (Figure 6C).
Effects of Mpl ligand on platelet and megakaryocyte levels and structure in the BclxL transgenic and control mice.
(A) Level of circulating platelets on day 5 after a single injection of PEG-rmMGDF (50 μg/kg) in the peripheral blood of WT and Tg19 mice. Numbers are averages ± SD for 3 different mice. (B) Number of acetylcholinesterase-positive cells in mouse BM after injection of PEG-rmMGDF (50 μg/kg). The numbers are averages ± SD for 2 mice, each with 3 determinations. Details are as in the legend for Figure 3. (C) Transmission electron micrograph of megakaryocytes from WT and Tg19 mice after PEG-rmMGDF injection. Original magnification, × 16 000 in the top panel and × 6800 in the lower panel.
Effects of Mpl ligand on platelet and megakaryocyte levels and structure in the BclxL transgenic and control mice.
(A) Level of circulating platelets on day 5 after a single injection of PEG-rmMGDF (50 μg/kg) in the peripheral blood of WT and Tg19 mice. Numbers are averages ± SD for 3 different mice. (B) Number of acetylcholinesterase-positive cells in mouse BM after injection of PEG-rmMGDF (50 μg/kg). The numbers are averages ± SD for 2 mice, each with 3 determinations. Details are as in the legend for Figure 3. (C) Transmission electron micrograph of megakaryocytes from WT and Tg19 mice after PEG-rmMGDF injection. Original magnification, × 16 000 in the top panel and × 6800 in the lower panel.
BM of transgenic mice has a lower number of megakaryocytes that produce proplatelets in vitro
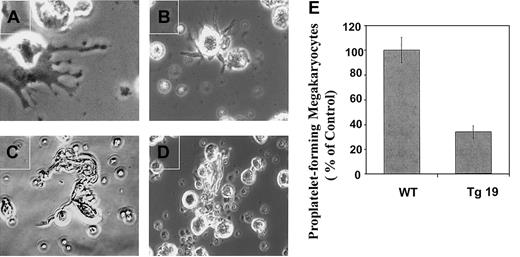
To further study the consequences of BclxL overexpression on thrombopoiesis, we used an in vitro model of proplatelet formation as used by others.24 As others, we noticed that control megakaryocytes form several types of extensions: extended cytoplasmic processes constricted at platelet-sized intervals (Figure7A), pseudopodialike processes (Figure7B), and mainly long, thin extensions (Figure 7C). As shown in Figure7E, cultures from Tg19 displayed a 3-fold decrease in the number of proplatelet-forming megakaryocytes (Figure 7D) as compared with control (P < .05).
The frequency of megakaryocytes capable of forming proplatelets is decreased in BM cultures derived from BclxL transgenic mice.
BM cultures of Tg19 or WT mice were enriched for megakaryocytes by indirect immunomagnetic negative separation. Isolated megakaryocytes were cultured for 3 days as described in “Materials and methods” and subjected to examination by phase contrast microscopy (A-D). Note 3 different types of cytoplasmic extensions for WT megakaryocytes: beaded proplatelets (A), pseudopodialike (B), and long, thin extensions (C), whereas mainly one type was observed for Tg19—long, thin extensions (D). Original magnification, × 600 for panel A and × 400 for panels B, C, and D. (E).The frequency of megakaryocytes making proplatelets was monitored. The data are averages ± SD for 3 experiments, with 2 wells analyzed in each.
The frequency of megakaryocytes capable of forming proplatelets is decreased in BM cultures derived from BclxL transgenic mice.
BM cultures of Tg19 or WT mice were enriched for megakaryocytes by indirect immunomagnetic negative separation. Isolated megakaryocytes were cultured for 3 days as described in “Materials and methods” and subjected to examination by phase contrast microscopy (A-D). Note 3 different types of cytoplasmic extensions for WT megakaryocytes: beaded proplatelets (A), pseudopodialike (B), and long, thin extensions (C), whereas mainly one type was observed for Tg19—long, thin extensions (D). Original magnification, × 600 for panel A and × 400 for panels B, C, and D. (E).The frequency of megakaryocytes making proplatelets was monitored. The data are averages ± SD for 3 experiments, with 2 wells analyzed in each.
Discussion
The process of megakaryocyte fragmentation into platelets in vitro has been clearly associated with apoptosis.7,25 Apoptosis has been described in mature megakaryocytes in BM26 and CD34+-derived cultured cells.8 An interest in apoptosis as a possible mechanism of platelet shedding emerged from the observation that the process of platelet fragmentation is reminiscent of the course of programmed cell death in its multiple features: ruffling, blebbing, and condensation of the plasma and nuclear membranes; reorganization and disruption of the cytoskeletal architecture; DNA fragmentation; and, finally, cell shrinkage and formation of apoptotic bodies.27 One of the major regulators of cytoskeletal organization and apoptosis is BclxL,10 the level of which is reduced in mature cultured megakaryocytes.9 To establish the role of apoptosis in platelet fragmentation in vivo, one may need to explore effects of deregulated expression in megakaryocytes of different proapoptotic as well as antiapoptotic proteins. In the current study, we have developed the first tool needed to approach this question in vivo. We aimed at addressing whether deregulated expression of BclxL has an effect on megakaryocyte development or fragmentation into functional platelets. To this end, transgenic mice were generated in which BclxL was overexpressed specifically in megakaryocytes via the PF4 promoter.
The transgenic phenotype of PF4-BclxL mice included a moderate increase in the number of megakaryocytes, as was also observed in MGDF-treated control mice (single-dose injection). In contrast to the latter case, however, the platelet number did not increase in mice overexpressingBclxL. This led us to suspect that more megakaryocytes in the transgenic mice have an impaired ability to release platelets. Interestingly, a different study described a 50% reduction in platelet counts in transgenic mice constitutively expressing theBcl-2 gene in the whole hematopoietic compartment.25 Because in this model Bcl-2overexpression was in all hematopoietic lineages, it was not determined how lineage redistribution may affect the number of mature megakaryocytes in vivo and platelet levels. The role of Bcl-2 family members in platelet fragmentation was also implicated in a recent study,28 showing that platelet levels are reduced by half in mice that lack the gene for Bim, a negative regulator of Bcl-2–like prosurvival factors. In this study, too, the lack ofBim in all lineages could have affected cytokine production and hence platelet levels. Our current investigation is novel in that it links directly changes in BclxL expression in megakaryocytes in vivo to megakaryocyte ultrastructure and platelet levels.
At the present time 2 models of thrombopoiesis exist. The platelet territory model has been suggested based on freeze-fracture and thin-section electron microscopic analysis of the internal membranes of megakaryocytes. According to this model, internal membranes demarcate fields or territories of prepackaged platelets, and fragmentation of the cytoplasm releases these platelets.29,30 An alternative flow model incorporates the observation that mammalian platelets assemble through intermediate long cytoplasmic extensions, called proplatelets, consisting of multiple platelet-sized beads along their length.24,31-33 Several studies have captured mammalian megakaryocytes extruding proplateletlike processes into the BM sinusoids.34,35 Additionally, cultured megakaryocytes were shown to develop various degrees of the demarcation membrane system in addition to proplatelet extensions.8,36 These 2 models are not viewed as mutually exclusive,4,37 because the position of long cytoplasmic extensions and proplatelet formation may be determined by the structure of intracellular membranes that protrude and connect to the cell surface. A linkage between poorly developed demarcation membranes and a lack of platelet fragmentation was observed in several genetically modified mouse models in which an increase or no change in megakaryocyte numbers was detectable.16,38 In our current study, examination of the sections of mouse BM cells by transmission electron microscopy revealed that the majority of control megakaryocytes exhibited well-demarcated cytoplasmic territories with extensive dilation of the demarcation membrane system and with large cisterns and vacuoles present. All of the above are believed to lead to demarcation of platelet fields in mature megakaryocytes.36
In contrast to control megakaryocytes, the majority of transgenic cells showed poorly developed demarcation territories that consisted of a very congested demarcation membrane system, which may prevent delineation of future platelet fields. In addition, megakaryocytes of transgenic mice exhibited an extensive outer ring of cytoplasm with poorly developed platelet extensions. We speculate that these significant ultrastructural changes did not result in a parallel decrease in platelet counts because they were only prevalent in about 75% of the cells, and because the total number of megakaryocytes in some of the transgenic mice was increased (in accordance, and as discussed below, only about 70% of the transgenic megakaryocytes displayed high levels of BclxL). For example, Tg19 demonstrates a 1.6-fold higher number of megakaryocytes, of which about 25% display normal demarcation membranes. Fragmentation from these normal cells, in addition to a potential fragmentation from the cells with impaired demarcation membranes, may account for maintaining an adequate platelet level in the transgenic mice. In agreement with the above-described ultrastructural characteristics, the BclxL transgenic mice had a poor rate of platelet recovery after experimentally induced ITP, as compared with control mice. Also in accordance, megakaryocytes isolated from BM of transgenic mice had a low frequency of cells able to produce proplatelets in vitro. We could not collect a sufficient number of platelets from this system for proper evaluation of level and activation potential.
Injection of MGDF reversed to a large extent the effects of BclxL on the ultrastructure of megakaryocytes and on platelet levels, suggesting that the profile of development of the demarcation membrane system in megakaryocytes is relevant to the determination of platelet levels. It is conceivable that high levels of this cytokine initiate signaling in mature megakaryocytes that promote destabilization of Bcl-2 proteins (endogenous and transgene) or its inactivation via posttranslational modifications.39,40 It is also possible that at this stage of cell maturation, a boost of MGDF increases Bax levels or induces its dephosphorylation and translocation to the mitochondrial membranes, resulting in a low ratio of BclxL/Bax, which would promote apoptosis.41 Future studies will address these possibilities.
Our current model clearly indicates that BclxL levels are regulated in mature megakaryocytes in vivo and that deregulated, high-level expression of BclxL impairs the ability of the cells to fragment into platelets. In addition, this study also provides a facet related to the mechanism responsible for this phenomenon by linking it to significant changes in the development of demarcation membrane structures.
We thank Dr Ramesh Shivdasani for reading the manuscript and for valuable insights on the assay of proplatelet formation, Dr Beatrice Hechler for assistance with platelet aggregation studies, and Dr Hou-Xiang Xie and Robin MacDonald at our BUSM Central Transgenic facility.
Prepublished online as Blood First Edition Paper, May 13, 2002; DOI 10.1182/blood-2001-12-0263.
Supported by National Institutes of Health grant NIH-HL58547 to K.R.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Katya Ravid, Boston University School of Medicine, Biochemistry, K225, 715 Albany St, Boston, MA 02118; e-mail:ravid@biochem.bumc.bu.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal