B cells of chronic lymphocytic leukemia (B-CLL) are resistant to transduction with most currently available vector systems. Using an optimized adenovirus-free packaging system, recombinant adeno-associated virus (rAAV) vectors coding for the enhanced green fluorescent protein (AAV/EGFP) and CD40 ligand (AAV/CD40L) were packaged and highly purified resulting in genomic titers up to 3 × 1011/mL. Cells obtained from 24 patients with B-CLL were infected with AAV/EGFP or AAV/CD40L at a multiplicity of infection (MOI) of 100 resulting in transgene expression in up to 97% of cells as detected by flow cytometry 48 hours after infection. Viral transduction could be specifically blocked by heparin. Transduction with AAV/CD40L resulted in up-regulation of the costimulatory molecule CD80 not only on infected CLL cells but also on noninfected bystander leukemia B cells, whereas this effect induced specific proliferation of HLA-matched allogeneic T cells. Vaccination strategies for patients with B-CLL using leukemia cells infected ex vivo by rAAV vectors now seems possible in the near future.
Introduction
B cells of chronic lymphocytic leukemia (B-CLL) are inefficient antigen-presenting cells because they lack costimulatory molecules for efficient T-cell activation.1 Moreover, CD40 ligand (CD40L), a critical molecule for T-cell activation, is down-regulated on T cells in patients with CLL.2 This defect can be partially corrected by gene transfer of CD40L into B-CLL cells. This strategy, using recombinant adenoviral vectors, induced an autologous immune recognition of B-CLL cells in vitro and in patients.3,4 However, transduction by recombinant adenovirus requires a high concentration of viral particles per cell (multiplicity of infection [MOI] up to 2000) because B-CLL cells lack the fiber receptor essential for adenoviral attachment.5Furthermore, the use of adenovirus has raised the question whether adenoviral fiber structures mediate an unspecific immune stimulation.6 In contrast, adeno-associated virus (AAV), a nonenveloped human parvovirus, has gained attention for human gene therapy, because it does not seem to be pathogenic. AAV vectors have been proven to be efficient gene transfer vehicles, in particular for solid tumors.7-10 However, sufficient gene transfer into primary B-CLL cells with AAV vectors has not been achieved so far. Previous efforts to transduce primary B-CLL cells with recombinant AAV (rAAV) were inefficient with transgene expression levels below 3%.11 A recent improvement in the AAV packaging process was the introduction of a plasmid that encodes the required adenoviral gene products for the helper virus.12,13 In addition, concentration of viral particles by density-gradient steps using iodixanol, a nonionic dimeric x-ray contrast solution, instead of cesium chloride yielded higher titers.14 By using these improvements, we could prepare high-titer rAAV stocks free of adenovirus to reassess the susceptibility of primary B-CLL cells for rAAV vectors.
Patients, materials, and methods
Patients, cells, and cell culture
After informed consent, peripheral blood was obtained from patients satisfying diagnostic criteria for B-CLL.15Mononuclear cells were isolated on a Ficoll/Hypaque (Seromed, Berlin, Germany) density gradient by centrifugation and depleted from monocytes by adherence to plastic tissue culture flasks. More than 98% of isolated cells coexpressed CD5 and CD19, as assessed by flow cytometry; therefore nonmalignant B cells did not constitute a meaningful fraction of the total cells isolated. Patients were either untreated or had not received cytoreductive treatment for a period of at least 1 month before investigation. All patients were clinically stable and free from infectious complications when blood samples were collected. Staging was performed according to the Binet classification. Clinical characteristics of the patients studied are summarized in Table1.
Clinical features of patients with B-CLL and transduction efficiency into primary CLL cells by AAV/EGFP and AAV/CD40L
| Patient . | Stage (Binet) . | Sex/age . | EGFP (%) . | EGFP (MFIR) . | CD40L (%) . | CD40L (MFIR) . |
|---|---|---|---|---|---|---|
| 1 | C | M/53 | 13.5 | 6.0 | ND | ND |
| 2 | A | F/56 | 15.5 | 3.6 | ND | ND |
| 3 | C | M/83 | 13.0 | 4.3 | ND | ND |
| 4 | A | M/61 | 42.4 | 5.3 | 77.8 | 209.0 |
| 5 | A | M/73 | 44.9 | 6.6 | 97.7 | 312.2 |
| 6 | A | M/64 | 41.0 | 8.4 | 15.0 | 2.5 |
| 7 | A | F/59 | 36.5 | 6.0 | 15.0 | 3.7 |
| 8 | A | F/65 | 24.5 | 3.4 | ND | ND |
| 9 | C | M/62 | 14.0 | 2.0 | ND | ND |
| 10 | C | M/64 | ND | ND | 13.4 | 3.0 |
| 11 | C | M/70 | 13.4 | 2.7 | 10.0 | 1.3 |
| 12 | A | M/53 | 16.0 | 2.6 | 48.0 | 19.3 |
| 13 | B | M/75 | ND | ND | 34.4 | 3.0 |
| 14 | C | M/57 | 28.0 | 4.7 | 31.8 | 3.5 |
| 15 | C | M/73 | ND | ND | 23.3 | 2.7 |
| 16 | B | M/53 | ND | ND | 45.5 | 11.4 |
| 17 | A | M/72 | 34.7 | 3.6 | ND | ND |
| 18 | B | F/63 | 41.4 | 5.2 | ND | ND |
| 19 | A | F/60 | 18.6 | 2.1 | ND | ND |
| 20 | A | M/46 | 34.0 | 3.7 | ND | ND |
| 21 | C | M/62 | 50.3 | 5.4 | 78.9 | 80.2 |
| 22 | B | M/70 | 63.0 | 15.2 | 81.5 | 164.9 |
| 23 | C | M/60 | ND | ND | 30.6 | 6.6 |
| 24 | C | M/41 | ND | ND | 56.6 | 14.0 |
| Patient . | Stage (Binet) . | Sex/age . | EGFP (%) . | EGFP (MFIR) . | CD40L (%) . | CD40L (MFIR) . |
|---|---|---|---|---|---|---|
| 1 | C | M/53 | 13.5 | 6.0 | ND | ND |
| 2 | A | F/56 | 15.5 | 3.6 | ND | ND |
| 3 | C | M/83 | 13.0 | 4.3 | ND | ND |
| 4 | A | M/61 | 42.4 | 5.3 | 77.8 | 209.0 |
| 5 | A | M/73 | 44.9 | 6.6 | 97.7 | 312.2 |
| 6 | A | M/64 | 41.0 | 8.4 | 15.0 | 2.5 |
| 7 | A | F/59 | 36.5 | 6.0 | 15.0 | 3.7 |
| 8 | A | F/65 | 24.5 | 3.4 | ND | ND |
| 9 | C | M/62 | 14.0 | 2.0 | ND | ND |
| 10 | C | M/64 | ND | ND | 13.4 | 3.0 |
| 11 | C | M/70 | 13.4 | 2.7 | 10.0 | 1.3 |
| 12 | A | M/53 | 16.0 | 2.6 | 48.0 | 19.3 |
| 13 | B | M/75 | ND | ND | 34.4 | 3.0 |
| 14 | C | M/57 | 28.0 | 4.7 | 31.8 | 3.5 |
| 15 | C | M/73 | ND | ND | 23.3 | 2.7 |
| 16 | B | M/53 | ND | ND | 45.5 | 11.4 |
| 17 | A | M/72 | 34.7 | 3.6 | ND | ND |
| 18 | B | F/63 | 41.4 | 5.2 | ND | ND |
| 19 | A | F/60 | 18.6 | 2.1 | ND | ND |
| 20 | A | M/46 | 34.0 | 3.7 | ND | ND |
| 21 | C | M/62 | 50.3 | 5.4 | 78.9 | 80.2 |
| 22 | B | M/70 | 63.0 | 15.2 | 81.5 | 164.9 |
| 23 | C | M/60 | ND | ND | 30.6 | 6.6 |
| 24 | C | M/41 | ND | ND | 56.6 | 14.0 |
ND indicates not determined.
HeLa cells were obtained from the American Type Culture Collection (Rockville, MD); the 293 cells were a gift from M. Lohse, Max-Planck-Institute of Biochemistry (Martinsried, Germany). Cells were cultured at 37°C in 5% CO2 in air, in culture medium consisting of Dulbecco modified Eagle medium (DMEM; Biochrom, Berlin, Germany) supplemented with 10% fetal calf serum (FCS; Biochrom), 2 mMl-glutamine (Biochrom), 100 U/mL penicillin (Biochrom), and 100μg/mL streptomycin (Biochrom).
HeLa/SF cells transfected with human CD40L cDNA were produced as previously described.16 Cells were γ-irradiated at 200 Gy, plated at 3 × 104 cells/well in 96-well plates in media, and incubated overnight at 37°C in a 5% CO2humidified atmosphere. Before addition of B-CLL cells, the feeder layers were washed twice with phosphate-buffered saline (PBS), and tumor cells were cultured at 2 × 106 cells/mL in Iscove medium (Gibco BRL, Berlin, Germany) supplemented with 20% heat-inactivated FCS, 2 mM/L l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. For functional assays, CLL cells were harvested, purified by Ficoll density gradient centrifugation, washed, and analyzed by flow cytometry.
Antibodies and reagents
Immunophenotyping was performed with the following monoclonal antibodies (mAbs) conjugated with fluorescein isothiocyanate (FITC), phycoerythrin (PE), or phycoerythrin cyanine 5 (PE-Cy5): CD5, CD19, CD80, anti-κ, anti-λ (Beckman Coulter, Krefeld, Germany). Fluorescein-conjugated mAbs specific for murine CD40L were purchased from BD Pharmingen (Heidelberg, Germany) and expression was controlled by an isotype hamster IgG3 mAb (BD Pharmingen). Heparin (10 000 U/mL; Braun, Melsungen, Germany) was used for blocking experiments with infectious AAV.
Plasmids
The adenoviral pXX6 plasmid was a kind gift of R. Samulski and described previously.17 The gene for enhanced green fluorescent protein (EGFP) was obtained by excising the Asp718-NotI fragment of pEGFP-N1 (Clontech, Heidelberg, Germany) and inserted into the Asp718-NotI site of psub/CEP4(Sal invers). psub/CEP4(Sal invers) is a derivative of psub201(+), which was digested with XbaI, blunt ended, and ligated to the blunt-ended 3923-bpSalI-NruI-fragment of pCEP4(Sal invers).18 pCEP4(Sal invers) differs from pCEP4 (Invitrogen, Groningen, Netherlands) by inversion of theSalI8-SalI3-1316-fragment. The construct pAAV/mCD40L contains the murine CD40L encoding gene driven by the cytomegalovirus (CMV) promoter. To generate pAAV/mCD40L, the 0.8-kb open reading frame (ORF) was released by BamHI digestion from pCEP4/mCD40L and ligated into an Asp718/NotI-digested psubCEP4(Sal invers).
rAAV vector production and purification
The 293 cells were seeded at 80% confluency and cotransfected by calcium phosphate with a total of 37.5 μg vector plasmid (pAAV/EGFP or pAAV/mCD40L), packaging plasmid pRC, and adenoviral plasmid pXX6 at a 1:1:1 molar ratio.19 Then, 48 hours after transfection cells were harvested and pelleted by low-speed centrifugation. Cells were resuspended in 150 mM NaCl, 50 mM Tris-HCl (pH 8.5), freeze-thawed several times, and treated with Benzonase (50 U/mL) for 30 minutes at 37°C.20 Cell debris was spun down at 3700g for 20 minutes at 4°C. Supernatant was purified by ammonium sulfate precipitation. The pellet was resuspended in PBS-MK buffer (1 × PBS, 1 mM MgCl2, and 2.5 mM KCl) and loaded onto an iodixanol gradient and purified as previously described.20 The iodixanol fraction was further purified by heparin affinity column chromatography and the virus dialyzed against PBS-MK before being used.21
Viral vector titering assays
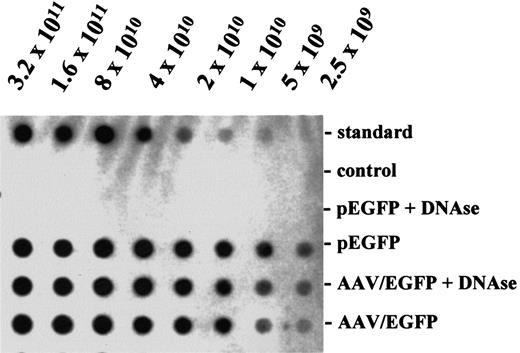
The vector particle titer for AAV/EGFP and AAV/CD40L was determined by treating vector dilutions with DNAse I (end concentration 0.5 μg/μL; Boehringer Mannheim, Mannheim, Germany) for 60 minutes at 25°C to remove putatively free viral genomes that could be subsequently hybridized with the probe. The viral preparations were then blotted in a serial 2-fold dilution and finally hybridized with a random-primed transgene specific probe by standard methods. Particle titers were determined by comparing the intensity of the hybridization signals with that obtained for the vector plasmid standard of known concentration blotted on the same membrane. The transducing titer was determined as follows: 7 × 104 HeLa cells per well were seeded in a 12-well plate (4 cm2); 24 hours later the cells were irradiated with 100 Gy and serial dilutions of the recombinant virus were added to the cells. After 48 hours, the number of transgene-expressing cells was quantified by flow cytometry.
AAV transduction
Primary CLL cells (5 × 105 cells/well in 96-well plates) were incubated in a total of 50 μL Iscove modified Dulbecco medium (IMDM) supplemented with 20% FCS and infectious AAV was added resulting in an MOI between 1 and 500. Cells were incubated for 2 hours at 37°C in 5% CO2 in air. Thereafter, infected cells were transferred on a γ-irradiated feeder layer expressing CD40L (HeLa/SF) as outlined above and 150 μL Iscove medium was added.
Flow cytometry
Forty-eight hours after AAV transduction, CLL cells were harvested, purified by Ficoll density gradient centrifugation, and washed. Specific, directly conjugated antibodies were applied to cells for 30 minutes in PBS, 4% FCS, 0.1% sodium azide, 20 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), and 5 mM EDTA (ethylenediaminetetraacetic acid), pH 7.3, on ice and washed. Nonspecific binding was controlled by incubation with isotypic controls (murine isotype IgG1 mAb and hamster isotype IgG3 mAb, BD Pharmingen). Fluorescence was measured with a Coulter Epics XL-MCL (Beckman Coulter). A minimum of 5000 cells was analyzed for each sample. The percentage of positive cells is defined as the fraction beyond the region of 99% of the control-stained cells. Data were analyzed with the use of WinMDI2.8 FACS software. We calculated the mean fluorescence intensity ratio (MFIR) to compare the relative staining intensities of 2 or more stained cell populations. The MFIR is the mean fluorescence intensity (MFI) of cells stained with a fluorochrome-conjugated antigen-specific mAb divided by the MFI of cells stained with a fluorochrome-conjugated isotype control mAb.3
Transactivation assay
CD40L-transduced or mock-infected B-CLL cells were prelabeled with a green fluorescent dye (CellTracker Green CMFDA, Molecular Probes, Eugene, OR) at a concentration of 1 μM for 15 minutes at 37°C. After extensive washing, labeled stimulator cells were cocultured with noninfected, nonstained CLL cells from the same patient at 37°C for another 48 hours. Expression of CD80 on noninfected, nonlabeled naive CLL cells was assessed by PE-conjugated anti-CD80 mAb (Beckman Coulter).
Mixed lymphocyte reaction
T cells derived from HLA.A2+ healthy donors were isolated to more than 95% purity by depleting CD19+, CD14+, CD56+, and CD16+ cells from blood mononuclear cells using the Pan T Cell Isolation Kit (Miltenyi Biotec, Bergisch Gladbach, Germany) and magnetic separation columns (Miltenyi Biotec). CLL cells isolated from HLA.A2+ patients were transduced with AAV (AAV/EGFP, AAV/CD40L) and after separation from HeLa/SF feeder cells coincubated for 72 hours with untransduced CLL cells (ratio 1:10) derived from the same patient. Irradiated (200 Gy) CLL cells were used as stimulators, cocultured at 1 × 104 cells/well in a final volume of 200 μL with allogeneic T cells at 1 × 105 cells/well in 96-well round-bottom plates, and incubated for 4 days in IMDM medium (Biochrom) supplemented with interleukin 2 (IL-2; 20 IU/mL; Panbiotech, Aidenbach, Germany) at 37°C in a 5% CO2 humidified atmosphere. During the last 6 hours of the 96-hour culture period, cells were pulsed with 0.5 μCi (0.0185 MBq) [3H]-thymidine (Amersham, Braunschweig, Germany). Cells were harvested onto glass fiber filters and dried, and the [3H]-thymidine incorporation was measured by scintillation spectrophotometry in a Wallac Microbeta Plus 1450 scintillation counter (Turku, Finland).
Statistics
Statistical associations between dependent subgroups were analyzed by the t test for paired samples; statistical associations between independent subgroups were carried out using the median 2-sample test. In the case of multiple comparisons Pvalues were adjusted by the Bonferroni method. A statistical significance was accepted for P < .05. The calculations were determined by the statistical software package SAS, version 8.2.
Results
Efficient packaging of high-titer, purified rAAV vectors coding for EGFP and CD40L with an improved packaging system
Using a new helper virus-free packaging protocol, efficient production of rAAV virions coding for EGFP and CD40L was achieved. The peak fraction of rAAV preparations contained a concentration of 3.8 × 1011 (SEM, 0.6 × 1011) viral particles per milliliter for AAV/EGFP and 2.4 × 1011(SEM, 0.8 × 1011) for AAV/CD40L, respectively. To exclude the possibility that contaminating DNA accounted for hybridization signals, viral stocks were preincubated with DNAse. No significant decrease in titer was seen, suggesting that the majority of viral particles detected was intact (Figure1). In several viral preparations infectious titers of 3.7 × 109/mL (SEM, 0.3 × 109) for AAV/EGFP and 4.7 × 109/mL (SEM, 0.3 × 109) for AAV/CD40L were achieved. This corresponds to an infectious-to-particle titer ratio of about 1:100 for AAV/EGFP and 1:50 for AAV/CD40L.
Genomic titers of rAAV.
AAV/EGFP preparations and the vector plasmid pEGFP were blotted in a serial 2-fold dilution and hybridized with a random-primed transgene specific probe by standard methods. Particle titers were determined by comparing the intensity of the hybridization signals with that obtained for the vector plasmid standard of known concentration blotted on the same membrane. A DNA fragment encoding resistance for neomycin served as negative control in the hybridization reaction. DNAse treatment (0.5 μg/μL DNAse I, Boehringer Mannheim; 60 minutes at 25°C) was performed to remove free, unpackaged viral genomes.
Genomic titers of rAAV.
AAV/EGFP preparations and the vector plasmid pEGFP were blotted in a serial 2-fold dilution and hybridized with a random-primed transgene specific probe by standard methods. Particle titers were determined by comparing the intensity of the hybridization signals with that obtained for the vector plasmid standard of known concentration blotted on the same membrane. A DNA fragment encoding resistance for neomycin served as negative control in the hybridization reaction. DNAse treatment (0.5 μg/μL DNAse I, Boehringer Mannheim; 60 minutes at 25°C) was performed to remove free, unpackaged viral genomes.
High expression of EGFP and CD40L on primary B-CLL after AAV transduction
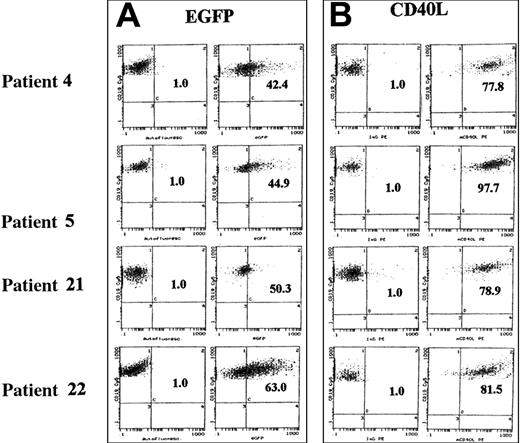
Freshly isolated CD5/CD19+ cells from patients with established diagnosis of B-CLL were infected with AAV/EGFP and AAV/CD40L at an MOI of 100 and assessed for transgene expression by flow cytometry after 48 hours. In Figure2, results with cells double-stained for the lineage marker CD19 and the transgene are shown for 4 representative patients (nos. 4, 5, 21, 22 in Table 1). Whereas green fluorescence was detected in only 1% of untransduced cells, expression increased to 42.4% (MFIR, 5.3), 44.9% (MFIR, 6.6), 50.3% (MFIR, 5.4), and 63.0% (MFIR, 15.2), respectively, after transduction with AAV/EGFP. Leukemic cells of the same patients were transduced with AAV/CD40L, and CD40L expression showed a marked shift to 77.8% (MFIR, 209.0), 97.7% (MFIR, 312.2), 78.9% (MFIR, 80.2), and 81.5% (MFIR, 164.9), respectively.
EGFP and murine CD40L expression after rAAV transduction (MOI 100).
Primary B-CLL cells derived from patients 4, 5, 21, and 22 (Table 1) were transduced at an MOI of 100 with rAAV vectors encoding EGFP (A) and CD40L (B) and analyzed for transgene by flow cytometry 48 hours later. The graphs on the left of each panel (control) represent the autofluorescence (EGFP) and isotype (CD40L) controls, respectively, and the graphs on the right of each panel show the cells transduced with rAAV vectors (AAV/EGFP, AAV/CD40L). Dot-plot analysis of the double staining for the B-cell lineage marker CD19 (Cy5-conjugated mAb; y-axis) and transgene expression (EGFP and PE-conjugated anti–murine CD40L; x-axis) is shown.
EGFP and murine CD40L expression after rAAV transduction (MOI 100).
Primary B-CLL cells derived from patients 4, 5, 21, and 22 (Table 1) were transduced at an MOI of 100 with rAAV vectors encoding EGFP (A) and CD40L (B) and analyzed for transgene by flow cytometry 48 hours later. The graphs on the left of each panel (control) represent the autofluorescence (EGFP) and isotype (CD40L) controls, respectively, and the graphs on the right of each panel show the cells transduced with rAAV vectors (AAV/EGFP, AAV/CD40L). Dot-plot analysis of the double staining for the B-cell lineage marker CD19 (Cy5-conjugated mAb; y-axis) and transgene expression (EGFP and PE-conjugated anti–murine CD40L; x-axis) is shown.
Cumulative data for 24 different patients with B-CLL studied for AAV infection are given in Table 1. The mean age of the study population was 62.3 years (SEM, 1.9 years) with a median of 62 years. Ten patients presented with early disease (Binet stage A) and 14 with advanced disease (Binet stage B, n = 4; Binet stage C, n = 10). Eighteen patients were studied for EGFP transduction; the mean percentage of EGFP-transduced cells was 30.3% (SEM, 3.6%). For CD40L-transduced samples (n = 15), 44.0% (mean) of CLL cells could be infected (SEM, 7.4%). Using the median 2-sample test, there was no correlation seen between transduction efficiency with AAV/EGFP or AAV/CD40L, respectively, and clinical stage of disease (P = .36 andP = .48), sex (P = .61 andP = .35), or age (P = .36 andP = .20). Furthermore, the association between the clinical stage of disease and susceptibility to AAV/EGFP or AAV/CD40L infection, respectively, was examined by the median 2-sample test using the Bonferroni method with adjustment for sex (male, female) and age (median age or younger, older than median age). Therefore,P < .0125 (0.05 divided by 4, due to 4 subgroups) would indicate a significant association at the .05 level. For all 4 subgroups (male, female, age ≤ 62 years, and > 62 years),P > .0125 was found, demonstrating no association between clinical stage and susceptibility to AAV/EGFP and AAV/CD40L infection, respectively.
Studying the patients' samples, which were both transduced for EGFP and CD40L (n = 9), values for transduction efficiency were not statistically different (t test for paired samples,P = .17); a mean percentage of 37.3% (SEM, 5.3%) of infected cells could be transduced by AAV/EGFP and 50.6% of CLL cells by AAV/CD40L (SEM, 11.3%). Transduction efficiencies of both viral vectors correlated in these patients with each other (Spearman correlation coefficient test: P = .01). Using the Bonferroni method for adjustment with respect to clinical stage and age of patients (adjustment for sex was not performed because of only one female patient studied both for EGFP and CD40L transduction), a significant P value in the Spearman correlation coefficient test (P < .0001) was only achieved for samples derived from patients with advanced clinical stage (Binet B or C).
Concentration and time kinetics of AAV transduction in CLL cells
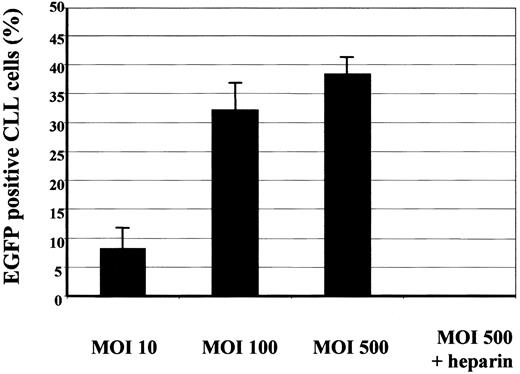
Primary B-CLL cells were infected with AAV/EGFP at different MOIs ranging from 10 to 500. Forty-eight hours after infection cells were analyzed for expression of EGFP by flow cytometry. At an MOI of 10, an average of 8.3% of cells (SEM, 3.9%) was positive for transgene expression. At an MOI of 100, a significant increase of the fraction of transgene-positive cells was observed (t test for paired samples: P = .02); an average of 32.2% of cells (SEM, 4.8%) showed an EGFP expression. At an MOI of 500, only a slight, but insignificant (t test, P = .31) increase in the fraction of EGPF+ cells was detectable (38.3%; SEM, 3.5%; Figure 3).
Concentration kinetics of AAV/EGFP.
Primary CLL cells of 4 patients (17-20; Table 1) were infected at an MOI of 10, 100, or 500 with rAAV vectors encoding EGFP. The cells were analyzed for expression of EGFP by flow cytometry 48 hours after infection. Shown is the mean percentage of transduced cells with SEM (error bars). Infected cells (MOI of 500) were incubated with 500 IU heparin and EGFP expression detected 48 hours later.
Concentration kinetics of AAV/EGFP.
Primary CLL cells of 4 patients (17-20; Table 1) were infected at an MOI of 10, 100, or 500 with rAAV vectors encoding EGFP. The cells were analyzed for expression of EGFP by flow cytometry 48 hours after infection. Shown is the mean percentage of transduced cells with SEM (error bars). Infected cells (MOI of 500) were incubated with 500 IU heparin and EGFP expression detected 48 hours later.
Recently, membrane-associated heparan sulfate proteoglycans (HSPGs) were identified as the primary receptors for AAV virions, thus mediating both AAV attachment to and infection of target cells.21 Inhibition of AAV attachment and infection was shown to be achieved in competition experiments using heparin, a molecule chemically very similar to heparan sulfate glycosaminoglycan thus functioning as a soluble receptor analog. Therefore, viral supernatants coding for EGFP (AAV/EGFP) were mixed with heparin and incubated with B-CLL cells. As seen in Figure 3, viral transduction of EGFP at a high MOI of 500 was significantly decreased by heparin (t test, P = .007) resulting in background level of EGFP expression; a mean percentage of only 0.05% of cells (SEM, 0.05%) were EGFP+.
Transgene expression was assessed over time using an MOI of 100. As seen in Figure 4, 12 hours after EGFP infection only small amounts of EGFP+ cells were detected by flow cytometry (mean, 0.4%; SEM, 0.1%). Twenty-four hours after transduction a mean of 4.6% of cells was positive (SEM, 1.3%). A significant (t test, P = .005) shift in the fraction of EGFP-expressing cells was seen after 48 hours (mean, 32.2%; SEM, 4.8%). A further although insignificant (ttest: P = .11) increase was detectable another 48 hours later (ie, 96 hours after infection) with an average EGFP+cell population of 53.0% (SEM, 8.6%). The mean percentage of EGFP+ cells was decreased 1 week after infection at significant levels in comparison to transduction data obtained 48 hours after infection (20.9%; SEM, 9.1%; t test:P = .04).
Time kinetics of AAV/EGFP.
CLL samples (patients 17-20; Table 1) were infected with AAV/EGFP (MOI of 100) and EGFP expression detected 12, 24, 48, 96, and 168 hours later. Shown is the mean percentage of the fraction of EGFP+ cells with corresponding SEM (error bars).
Time kinetics of AAV/EGFP.
CLL samples (patients 17-20; Table 1) were infected with AAV/EGFP (MOI of 100) and EGFP expression detected 12, 24, 48, 96, and 168 hours later. Shown is the mean percentage of the fraction of EGFP+ cells with corresponding SEM (error bars).
Up-regulation of CD80 on B-CLL cells after AAV/CD40L transduction
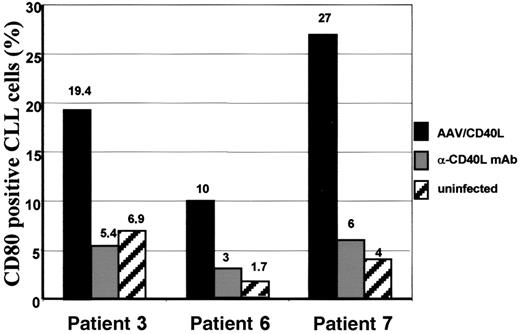
To prove that CLL cells transduced with CD40L became highly proficient antigen-presenting cells, expression of the costimulatory molecule CD80 was assessed before and 8 days after infection with AAV/CD40L. In CLL samples derived from 3 different patients, CD80 expression could be induced from 6.9% (MFIR, 1.6) to 19.4% (MFIR, 2.3) in patient 3, from 1.7% (MFIR, 1.2) to 10% (MFIR, 2.8) in patient 6, and from 4% (MFIR, 0.8) to 27% (MFIR, 2.4) in patient 7. CD80 expression on CD40L-transduced CLL cells (mean, 18.8%; SEM, 4.9%) was significantly higher in comparison to uninfected control CLL cells (mean, 4.2%; SEM, 1.5%; median 2-sample test,P = .03). Incubation with an anti–murine CD40L mAb could significantly inhibit CD80 up-regulation (mean, 4.8%; SEM, 0.9%) in these 3 patients with an expression of 5.4% (MFIR, 0.7), 3% (MFIR, 1.8), and 6% (MFIR, 1.9), respectively (median 2-sample test:P = .03; Figure 5). Primary CLL cells infected with AAV/EGFP did not result in any up-regulation of CD80 in comparison to uninfected controls (data not shown).
CD80 expression on B-CLL cells after CD40L transduction.
CD80 expression was assessed in samples derived from patients 3, 6, and 7 after infection with AAV/CD40L (black bars) in comparison to uninfected control CLL cells derived from the same patient (striped bars). Expression of CD80 was also detected after coincubation with specific anti–murine CD40L mAb (gray bars).
CD80 expression on B-CLL cells after CD40L transduction.
CD80 expression was assessed in samples derived from patients 3, 6, and 7 after infection with AAV/CD40L (black bars) in comparison to uninfected control CLL cells derived from the same patient (striped bars). Expression of CD80 was also detected after coincubation with specific anti–murine CD40L mAb (gray bars).
Transactivation of noninfected bystander leukemia cells by CD40L-transduced CLL cells
The transactivation capacity of AAV/CD40L-infected CLL cells was assessed after they were labeled with a green fluorescent dye (CellTracker) and used as stimulator cells for equal numbers of nonlabeled CLL B cells from the same patient. Uninfected nonlabeled bystander CLL cells of patient 16 (Table 1) were induced to express CD80 when cocultured with CD40L-infected CLL cells (29.4%; MFIR, 4.0), but not after coincubation with uninfected control CLL cells (1.3%; MFIR, 1.0). Similar results were obtained when naive CLL cells were stained and coincubated with CD40L-transduced nonlabeled leukemia cells; CD80 was up-regulated on naive bystander cells (29.7%; MFIR, 4.2), incubation with uninfected control CLL cells resulted in very low CD80 expression (0.3%; MFIR, 0.9). This stimulatory effect on bystander CLL cells could be abrogated by coincubation of stimulator cells with an anti–murine CD40L mAb (3.2%; MFIR 1.5). Furthermore, stimulation of naive CLL cells by mock-infected (wild-type AAV) CLL cells did not affect CD80 expression levels (0.7%; MFIR, 0.8). Specific up-regulation of CD80 on bystander CLL cells was also observed in 2 other patient samples studied (nos. 12 and 15; Figure6). CD80 expression on bystander cells after coincubation with CD40L-transduced CLL cells (mean, 18.4%; SEM, 6.1%) was significantly higher for all samples studied in comparison to data obtained after coincubation with wild-type AAV-infected (mean, 1.4%; SEM, 0.5%) or uninfected CLL cells (mean, 1.3%; SEM, 0.8%; median 2-sample test: P = .03).
Transactivation of bystander CLL cells after coincubation with CD40L-transduced CLL cells.
CLL cells (1 × 105) transduced with AAV/CD40L were coincubated with equal amounts of naive CLL cells labeled with a fluorescence dye (CellTracker). CD80 expression on naive bystander leukemic cells was detected 72 hours later by flow cytometry (black bars). In control assays anti–murine CD40L mAb was added to the mixture of transduced and bystander CLL cells before CD80 expression was detected 72 hours later (gray bars). As further controls uninfected (striped bars) or wild-type AAV-infected cells (open bars) were used for stimulation of bystander CLL cells.
Transactivation of bystander CLL cells after coincubation with CD40L-transduced CLL cells.
CLL cells (1 × 105) transduced with AAV/CD40L were coincubated with equal amounts of naive CLL cells labeled with a fluorescence dye (CellTracker). CD80 expression on naive bystander leukemic cells was detected 72 hours later by flow cytometry (black bars). In control assays anti–murine CD40L mAb was added to the mixture of transduced and bystander CLL cells before CD80 expression was detected 72 hours later (gray bars). As further controls uninfected (striped bars) or wild-type AAV-infected cells (open bars) were used for stimulation of bystander CLL cells.
CD40L-transduced CLL cells induce a proliferative T-cell response
We examined whether AAV/CD40L-infected CLL cells could stimulate allogeneic T cells in an HLA.A2-matched mixed lymphocyte reaction (MLR). For this purpose, we used highly purified allogeneic T cells from HLA.A2+ healthy donors and incubated them with γ-irradiated CLL cells from HLA.A2+ CLL patients. To minimize unspecific T-cell stimulatory effects by HeLa/SF-feeder cocultivation of infected CLL cells, transduced CLL cells were mixed 1:10 with naive bystander cells derived from the same CLL patient and used as mixture in allogeneic T-cell proliferation assays. On coculture with AAV/CD40L-infected CLL cells, allogeneic T cells were induced to undergo proliferation at rates significantly greater (mean, 13 274 cpm; SEM, 3493 cpm) than that observed with CLL cells infected with AAV/EGFP (mean, 2467 cpm; SEM, 142 cpm; median 2-sample test,P = .03; Figure 7). The experiments shown are representative for different allogeneic T-cell donors and different CLL samples examined.
Allogeneic, HLA-matched MLR.
HLA.A2+ CLL cells were infected with either AAV/CD40L or AAV/EGFP and incubated for 72 hours with naive CLL cells (1:10) derived from the same patient. Then 1 × 104 CLL cells irradiated with 200 Gy were cocultured with 1 × 105 purified healthy donor T cells (HLA.A2+) for another 96 hours, pulsed with [3H]-thymidine and T-cell proliferation determined 6 hours later (cpm). The data from one representative patient are depicted. Error bars represent SEM of triplicate measurements.
Allogeneic, HLA-matched MLR.
HLA.A2+ CLL cells were infected with either AAV/CD40L or AAV/EGFP and incubated for 72 hours with naive CLL cells (1:10) derived from the same patient. Then 1 × 104 CLL cells irradiated with 200 Gy were cocultured with 1 × 105 purified healthy donor T cells (HLA.A2+) for another 96 hours, pulsed with [3H]-thymidine and T-cell proliferation determined 6 hours later (cpm). The data from one representative patient are depicted. Error bars represent SEM of triplicate measurements.
Discussion
By use of a novel adenovirus-free packaging method we were able to generate very high-titer and pure viral preparations of rAAV. The infectious-to-particle titer achieved is quite favorable in comparison to other packaging procedures for AAV because eradication of contaminating helper virus particles is unnecessary.7Using this purified helper virus-free rAAV, we were able to transduce primary B-CLL cells at low MOIs with high efficiency. In comparison to adenoviral transgene vectors, rAAV vectors were efficient to transduce CLL cells at much lower MOIs.3 We have shown that the transduction potency of AAV vectors is independent of the transgene used. Differences observed between individual patients are independent of clinical stage of disease, age, or sex. We were able to provide evidence that the transduction of CLL cells by rAAV was specific and not due to a pseusotransduction because viral transduction could be significantly abrogated by heparin. Because membrane-associated HSPGs were identified as the primary receptors for AAV virions mediating both AAV attachment and infection, the blockade of infection by heparin, a chemical analog of heparan sulfate glycosaminoglycan, is thought to be specific for this type of vector transduction and has been demonstrated by others.21,24 After attachment of the virus it is assumed that AAV is internalized by clathrin-mediated endocytosis promoted by the interaction of the virus with the β5-subunit of αvβ5integrins.25 The role of αvβ5as functional receptor for AAV is not totally clarified because neither αvβ5, RGD peptides nor functional blocking mAb were able to block AAV-2 transduction as has been shown by others.26 Besides integrins, fibroblast growth factor receptor 1 (FGFR-1) has been implicated as coreceptor of AAV entry into target cells. Cells that do not express either HSPG or FGFR-1 fail to bind AAV and are resistant to AAV infection.27
Besides expression of specific receptors on target cells, transduction efficiency of AAV vectors correlates with the phosphorylation state of cellular proteins, especially the single-stranded D-sequence-binding protein (ssD-BP). This protein interacts with D(−) sequences within the inverted terminal repeat (ITR) of AAV.28,29 Efficient transgene expression requires dephosphorylation of ssD-BP, which is usually phosphorylated at tyrosine residues by the protein kinase activity of the cellular epidermal growth factor receptor (EGFR). Inhibition of the EGFR protein tyrosine kinase by the specific inhibitor tyrphostin results in dephosphorylation of ssD-BP and augmentation of AAV-mediated transgene expression.30 31
During AAV transduction primary B-CLL cells were incubated on a feeder layer expressing CD40L itself. By this CD40 activation, malignant B cells are shifted in a more proliferative status as has been shown previously.32 Based on cell cycle profile assessment using propidium iodide staining and flow cytometric analysis, Granziero and colleagues demonstrated an increase in the proliferative pool with 0.5% to 5.3% of CLL cells in S phase after 3 days of CD40 stimulation by human soluble CD40L.33 In our preliminary experiments we could show an increase in the fraction of CLL cells in S phase (< 5%) after cultivating them on CD40L-expressing feeder cells (data not shown). Therefore, besides an improved packaging protocol the preactivation of B cells by CD40 cross-linking remains a prerequisite for efficient transgene transduction by AAV. For adenoviral infection of primary CLL cells this CD40 stimulus was shown to improve transduction efficiency.3 A future goal for AAV-mediated transduction of CLL cells will be to replace this time-consuming cellular feeder system; besides AAV-2 other serotypes of AAV will be studied for their B-cell tropism. Serotypes 1, 3, 4, and 5 have been shown to transduce muscle cells more efficiently than recombinant AAV-2 particles.34 Furthermore, packaging of self-complementary rAAVs (scAAVs) could enable a feeder-independent transduction by rAAV. These are AAV vectors with about half the size of wild-type AAV and are therefore preferentially packaged to dimeric molecules, that is, scAAVs contain 2 complementary sequences of the genome in an inverted repeat configuration. Thus the limiting step of conversion of a single-stranded AAV vector to a double-stranded transgene sequence in the target cell is not necessary. In a murine model scAAV particles coding for erythropoietin showed a faster and higher transgene expression in comparison to monomeric AAV particles after intramuscular injection.35 Finally, retargeting of AAV vectors by modification of their capsid proteins could enable an improved transduction of malignant B cells. For recombinant AAV-2 vectors a region I-587 was recently defined at the capsid that allowed insertion of a peptide ligand thus allowing efficient retargeting of AAV vectors to cells with specific integrin receptors.19 Using an immunoglobulin-binding domain at this insertion site, B cell-specific antibodies could redirect via their Fc region AAV particles to B-CLL cells.
By infection with viral particles encoding murine CD40L, we modified primary human B-CLL cells to express a functional ligand for CD40. We used murine CD40 ligand because it was shown that recombinant soluble murine CD40L is able to bind human CD40 on B cells and induces a T-cell activation.36 Furthermore, this heterologous ligand could be distinguished from endogenously expressed human CD40L on infected human cells because no cross-reactivity of murine and human antibodies was observed. Therefore, it can be excluded that putatively unspecific stimulation of endogenous human CD40L during AAV infection or cytophilic soluble human CD40L made by the HeLa/SF feeder cells account for false-positive transduction signals. In a clinical phase 1 trial using murine CD40L, no unspecific immune reactions were observed in treated patients. CD40L transduction of B-CLL cells resulted not only in expression of the immune accessory molecule CD80 on infected cells, but also in activation of noninfected bystander leukemia B cells. Similar results with an up-regulation of CD54 and CD86 on leukemic bystander cells were observed previously using an adenoviral transfer system.3 We could show that this bystander effect was specific because incubation with an antibody directed against murine CD40L abrogated the induction of costimulatory molecules. Furthermore, coincubation of bystander cells with mock-infected or untransduced CLL cells did not affect the expression of CD80. Infection with AAV/CD40L significantly improves the functional antigen-presenting capacity of CLL cells in vitro because allogeneic T cells can be induced to proliferate at higher levels in comparison to mock-infected CLL cells. Similar results have also been shown for CLL cells transduced by adenoviral vectors coding for CD40L.3,4 In our experimental setting this T-cell proliferative response is mainly mediated by activated CLL bystander cells that were coincubated with CD40L-transduced CLL cells, thus resembling an in vivo vaccination situation where the number of resident nontransduced leukemia cells would greatly outnumber the infused CD40L-infected CLL cells.4 Having a nonimmunogenic vector transfer system like AAV in hand, it is now possible in future experiments to exclude any unspecific T-cell induction and define CLL-specific cytolytic T-cell responses using putative CLL-associated antigens.37 38
In conclusion, in the present study we have shown that an efficient transduction of primary B-CLL cells with the costimulatory molecule CD40L can be achieved by high-titer, adenovirus-free rAAV vectors. CD40L gene transfer resulted in specific up-regulation of the costimulatory molecule CD80 on infected B-CLL cells and on leukemic bystander cells and induced an allogeneic T-cell response. Therefore transduction of immunostimulatory molecules into CLL cells is now possible with these biologically active preparations of rAAV and enables vaccination strategies with this safe vector system in the near future.
The authors gratefully acknowledge the assistance and help of many colleagues who enabled the preparation of this report: Dr J. Samulski for providing us with the pXX6 plasmid and Dr G. Kröner-Lux for stimulating discussions; Dr A. Girod, F. Gerner, and M. Hutter for their instruction in the initial stage of this study and their support in vector preparations; and S. Anton for her technical assistance. We gratefully acknowledge our colleagues, especially Dr A. Gerl, Dr R. Forstpointner, and Dr M. Mempel, and the nursing staff from the Medical Clinic III at the KGMC who took care of the patients on the wards and in the outpatient clinic.
C.-M.W. and M.H. were supported in part by grants from the Deutsche Forschungsgemeinschaft (We 2346/1-1 and SFB 455) and the Matthias-Lackas-Stiftung.
C.-M.W. and D.M.K. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Michael Hallek, Medizinische Klinik III, Klinikum Grosshadern, Ludwig-Maximilians-Universität, Marchioninistr 15, D-81377 München, Germany; e-mail:michael.hallek@med3.med.uni-muenchen.de.



![Fig. 7. Allogeneic, HLA-matched MLR. / HLA.A2+ CLL cells were infected with either AAV/CD40L or AAV/EGFP and incubated for 72 hours with naive CLL cells (1:10) derived from the same patient. Then 1 × 104 CLL cells irradiated with 200 Gy were cocultured with 1 × 105 purified healthy donor T cells (HLA.A2+) for another 96 hours, pulsed with [3H]-thymidine and T-cell proliferation determined 6 hours later (cpm). The data from one representative patient are depicted. Error bars represent SEM of triplicate measurements.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/5/10.1182_blood.v100.5.1655.h81702001655_1655_1661/3/m_h81723031007.jpeg?Expires=1769102594&Signature=mEJRr5yO9aiyOl-QZb5KKzknZA7tnNK1fl091a7cbAtDO1WFvAkH0Z5Ky~9ZUhT7tHmCI8PWYbhdfVnfXrQqwZhMCqg~zG5sGYh7uaGdqmsSThShsXPv2bGE4gqAuUvv0mnjFGj8qCqeF9dCCxdytVzimKFSLi3LKp~cQkyI5m0KiOOm8YBjJqH-5wLHl~jlZa5So5UW4lUh7BoFgd-PKUbTx8et4242xW2ifUv7ZLOIm63N7XzOHae~H42YDuxY~3y5i4hXKQyX9keVxteTLuuvrxLo6TbVhgEo4dU87vuGr9ay72qkFpI-paIfg2ThL~wV7v6YlZxxpeJUctCaRw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal