The combination of interleukin 2 (IL-2) and antiretroviral therapy (ART) represents an emerging strategy in the treatment of patients infected with HIV. Aside from its immunomodulatory role, however, IL-2 may induce replication of human herpesvirus 8 (HHV-8)/Kaposi sarcoma (KS)–associated herpesvirus. We retrospectively evaluated HHV-8 plasma viremia and cellular load, as well as anti–HHV-8 antibody titers, in sequential samples from 84 patients receiving ART alone or in combination with IL-2. At baseline, HHV-8 plasma viremia was present only in 2 HHV-8–seropositive patients in whom KS subsequently developed during or immediately after termination of IL-2 therapy. The level of viremia increased during follow-up and peaked at the time of the clinical manifestation of KS. Moreover, transient peaks of HHV-8 viremia were temporally associated with administration of IL-2. HHV-8 plasma viremia was never detected in the other 47 patients receiving IL-2 nor in 35 controls treated only with ART. Thus, IL-2 therapy seems safe in most patients infected with both HIV and HHV-8, except for those with detectable HHV-8 viremia, who may not be eligible for IL-2 treatment.
Introduction
Although therapeutic regimens that combine low doses of interleukin 2 (IL-2) with antiretroviral therapy (ART) have been used without such major clinical concerns as high-grade toxicity or increased levels of HIV-1 viremia,1,2 the effects of IL-2 on replication of human herpesvirus 8 (HHV-8)/Kaposi sarcoma (KS)–associated herpesvirus and the development of KS, which is 20 000-fold more frequent in patients with AIDS than in the general population,3 must be evaluated carefully. In vitro, some IL-2-induced cytokines, such as IL-1β and interferon–γ (IFN-γ), increase the replication of HHV-8.4,5 Moreover, administration of IL-2 has been reported to exacerbate the clinical manifestations of epidemic KS.6 7
Because of the high seroprevalence of HHV-8 in the Italian population,8-11 particularly among patients with HIV infection,12 13 we assessed the effect of IL-2 therapeutic regimens on HHV-8 plasma and cellular load and correlated the latter with the development of KS. For this purpose, we investigated sequential plasma samples and peripheral blood mononuclear cell (PBMC) pellets from a cohort of HIV-1–infected patients enrolled in a randomized phase 2 trial of administration of IL-2 with ART. To provide a control, we studied a cohort of patients with recent HIV seroconversion who were treated with the same ART protocol and followed for a comparable length of time.
To measure HHV-8 DNA load in biologic samples, we developed a novel real-time quantitative polymerase chain reaction (PCR) system that combines TaqMan technology with use of a synthetic DNA calibrator molecule to normalize the intersample extraction recovery and monitor PCR artifacts. Using this new assay, we demonstrated, for the first time, a strict temporal correlation between multiple administrations of IL-2 and increases in HHV-8 viremia in patients with a detectable HHV-8 plasma load before the beginning of IL-2 administration.
Patients and methods
Patients and sample selection
The study was approved by the San Raffaele Hospital ethics committee, and all patients signed informed consent forms in accordance with the Declaration of Helsinki. Characteristics of the patients enrolled in the IL-2 plus ART protocol and details on the different regimens of IL-2 administration were reported elsewhere.2 Those in the group of ART-treated patients with recent HIV seroconversion had at least one HIV-seronegative sample within 6 months of the time the first seropositive sample was obtained. PBMCs from all patients were characterized at entry and throughout the study by their cell-surface expression of CD4 and CD8 on 2-color flow cytometry. HIV replication was monitored in plasma by an HIV-1 branched DNA signal-amplification assay (Chiron, Emeryville, CA) according to the manufacturer's instructions. Plasma and PBMC samples were obtained before and at the termination of the experimental protocol. Each sample was cryopreserved at −80°C in at least 2 aliquots that were never thawed before analysis.
Quantification of HHV-8 DNA by real-time quantitative PCR
Plasma (1 mL) was centrifuged at 1500g for 15 minutes at 4°C to remove cells and platelets and then subjected to high-speed centrifugation (26 000g for 120 minutes at 4°C) to concentrate virion particles. DNA extraction from the spun material and from PBMCs was performed by using a phenol-chloroform assay as described previously.14 HHV-8 DNA and human β-actin were quantified by using TaqMan technology and an ABI Prism device (7700 SDS; Applied Biosystems, Foster City, CA). A β-actin quantitative detection system (Applied Biosystems) was used to quantify human genomic DNA in both plasma and PBMCs. At least 1 μg genomic DNA recovered from each PBMC pellet was subjected to real-time PCR analysis. Primers and calibrator probe were specifically selected to avoid cross-hybridization with the HHV-8 and β-actin sequences. Details about the real-time PCR technique for HHV-8 are reported elsewhere (F.B., et al; manuscript submitted).
Serologic assays
A total of 170 plasma samples obtained from 84 HIV-infected patients were investigated for the presence of antibodies (Abs) against lytic and latent HHV-8 antigens. Abs to lytic antigens of HHV-8 were detected by using an immunofluorescent assay (IFA) based on the BCBL-1 cell line,15 whereas Abs against latent HHV-8 antigens were detected by using an IFA based on the BCP-1 cell line as described previously.16
Results and discussion
No major differences were observed in the baseline characteristics of the 2 groups of patients enrolled in the IL-2 protocol (the group treated with ART plus IL-2 and the control group treated only with ART), except for a slightly higher HIV-1 viral load in the group treated with the combination therapy (Table1). Not surprisingly, the group of ART-treated patients with recent HIV-1 seroconversion had higher mean CD4 cell counts and HIV-1 plasma loads and no intravenous drug users (Table 1). However, all patient groups were homogeneous with respect to treatment, age, sex, follow-up, and HHV-8 seroprevalence (Table 1). In agreement with previous observations,13 17 HHV-8 seroprevalence was remarkably high among homosexual men (21 of 38 [55%]).
Demographic, immunologic, and virologic characteristics of patients, according to therapy group
| Characteristic . | Type of therapy . | ||
|---|---|---|---|
| ART/RS (n = 20) . | ART (n = 15) . | ART + IL-2 (n = 49) . | |
| Risk factor | |||
| Heterosexual | 9 | 2 | 14 |
| Homosexual | 11 | 8 | 19 |
| IVDU | 0 | 5 | 16 |
| Sex | |||
| Male | 13 | 10 | 30 |
| Female | 7 | 5 | 19 |
| Mean age, y (range) | 38.6 (27-56) | 40.7 (35-52) | 39.1 (27-64) |
| Mean time since therapy began, mo (range) | 57.4 (25-69) | 55.4 (49-61) | 53.3 (49-61) |
| Mean CD4+cells/μL (range) | 667 (303-1 200) | 348 (201-490) | 353.5 (127-684) |
| Mean CD8+ cells/μL (range) | 1 053 (454-2 138) | 1 001 (470-2 490) | 763 (221-1 900) |
| Mean HIV-1 plasma load (genome equivalent/mL)* | 50 643 (500-325 000) | 3 392 (500-14 700) | 5 746 (500-39 810) |
| No. with plasma positive for HHV-8 | 0 | 0 | 2 |
| No. with PBMCs positive for HHV-8 | 0 | 0 | 4 |
| No. with anti–HHV-8 lytic/latent Abs (%) | 9 (45) | 5 (33) | 17 (37) |
| Characteristic . | Type of therapy . | ||
|---|---|---|---|
| ART/RS (n = 20) . | ART (n = 15) . | ART + IL-2 (n = 49) . | |
| Risk factor | |||
| Heterosexual | 9 | 2 | 14 |
| Homosexual | 11 | 8 | 19 |
| IVDU | 0 | 5 | 16 |
| Sex | |||
| Male | 13 | 10 | 30 |
| Female | 7 | 5 | 19 |
| Mean age, y (range) | 38.6 (27-56) | 40.7 (35-52) | 39.1 (27-64) |
| Mean time since therapy began, mo (range) | 57.4 (25-69) | 55.4 (49-61) | 53.3 (49-61) |
| Mean CD4+cells/μL (range) | 667 (303-1 200) | 348 (201-490) | 353.5 (127-684) |
| Mean CD8+ cells/μL (range) | 1 053 (454-2 138) | 1 001 (470-2 490) | 763 (221-1 900) |
| Mean HIV-1 plasma load (genome equivalent/mL)* | 50 643 (500-325 000) | 3 392 (500-14 700) | 5 746 (500-39 810) |
| No. with plasma positive for HHV-8 | 0 | 0 | 2 |
| No. with PBMCs positive for HHV-8 | 0 | 0 | 4 |
| No. with anti–HHV-8 lytic/latent Abs (%) | 9 (45) | 5 (33) | 17 (37) |
RS indicates patients with recent HIV seroconversion; and IVDU, intravenous drug user.
The lower limit of detection and quantification was 500 genome equivalents/mL.
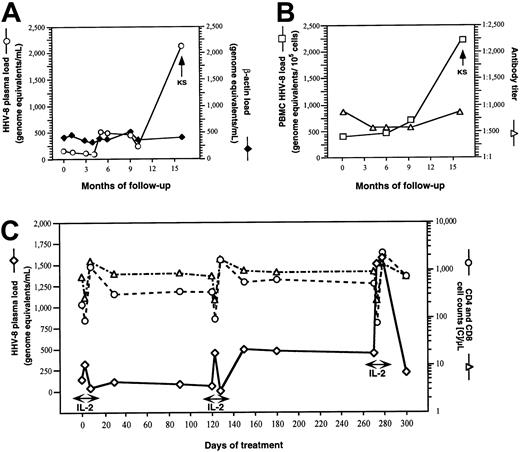
HHV-8 DNA was not detectable in any of the samples obtained from 53 HHV-8–seronegative patients (252 plasma and PBMC samples) or from 14 HHV-8–seropositive patients treated with ART (80 plasma and PBMC samples). Only in 4 patients treated with IL-2 plus ART was HHV-8 DNA detected in circulating mononuclear cells, and in only 2 of them (patient 70 [Figure 1B] and patient 72, 12 and 65 HHV-8 genome equivalents/105 cells, respectively) was it found repeatedly in all tested samples. The results for all PCR-positive PBMC samples (n = 8) were concordant with the antilytic but not the antilatent Ab test results. HHV-8 plasma viremia was detectable only in the same 2 patients (patient 70 [Figure 1A and 1C] and patient 72, 100 and 80 HHV-8 genome equivalents/mL, respectively), both of whom had subsequent development of KS (during IL-2 treatment in patient 72 and 5 months after the end of the 1-year period of IL-2 administration in patient 70). In both patients, HHV-8 viremia was also present at baseline, before the beginning of IL-2 administration.
Dynamics of HHV-8 plasma viremia and human DNA plasma contamination, PBMC HHV-8 load and anti–HHV-8 lytic Ab titers, and dynamics of HHV-8 replication and CD4 and CD8 T-cell counts in one IL-2–treated patient in whom KS developed.
(A,B) Plasma load of HHV-8 DNA (○) and β-actin (♦), HHV-8 load of PBMCs (■), and anti–HHV-8 lytic antigens Ab titer (▵), in patient 70. IL-2 was administered subcutaneously every 8 weeks for a total of 6 cycles (5 days twice a day for each cycle). KS lesions developed after 12 months of IL-2 therapy, 5 months after the last administration of IL-2. (C) Dynamics of HHV-8 plasma viremia (⋄) and CD4 (○) and CD8 (▵) T-cell counts during IL-2 therapy in patient 70.
Dynamics of HHV-8 plasma viremia and human DNA plasma contamination, PBMC HHV-8 load and anti–HHV-8 lytic Ab titers, and dynamics of HHV-8 replication and CD4 and CD8 T-cell counts in one IL-2–treated patient in whom KS developed.
(A,B) Plasma load of HHV-8 DNA (○) and β-actin (♦), HHV-8 load of PBMCs (■), and anti–HHV-8 lytic antigens Ab titer (▵), in patient 70. IL-2 was administered subcutaneously every 8 weeks for a total of 6 cycles (5 days twice a day for each cycle). KS lesions developed after 12 months of IL-2 therapy, 5 months after the last administration of IL-2. (C) Dynamics of HHV-8 plasma viremia (⋄) and CD4 (○) and CD8 (▵) T-cell counts during IL-2 therapy in patient 70.
Although large cohort studies have demonstrated that HHV-8 seropositivity predicts progression to KS in HIV-positive homosexual men,17-19 only HHV-8 plasma viremia was clearly associated with the development of KS in our cohort. Neither the anti–HHV-8 Ab titer nor the detection of HHV-8 sequences in circulating PBMCs could unambiguously identify patients at risk for subsequent development of KS. To date, none of the other patients seropositive for HHV-8 have had onset of KS, even though some HHV-8–seropositive patients had a second round of IL-2 plus ART. Because both patients in whom KS developed were homosexuals, we calculated the incidence of KS among our homosexual patients (n = 38). The incidence was 11.4 cases/1000 person-years; this did not differ significantly (on 2-tailed Fisher exact test) from the overall incidence of KS observed in Italian HIV-seropositive homosexuals.13
We analyzed the dynamics of HHV-8 plasma viremia and cellular load in one patient (patient 70) for whom a considerable number of sequential samples was available (Figure 1A and 1B). HHV-8 plasma load was low during the first 4 months of observation (57-138 genome equivalents/mL). It then increased significantly during IL-2 therapy, ranging from 219 to 492 genome equivalents/mL, and peaked at the time of the clinical manifestation of KS (2097 genome equivalents/mL). Similarly, the PBMC viral load (Figure 1B) increased from the beginning to the end of the IL-2 therapy (from 180 to 538 genome equivalents/105 cellular genomes), reaching a maximum of 2194 genome equivalents at the time of KS manifestation. The lytic Ab titers measured at the same time points did not vary significantly (Figure 1B). A low but constant level of cellular DNA contamination was observed in all plasma samples (between 210 and 410 cellular genome equivalents/mL), thus ruling out the possibility that the variations in HHV-8 plasma load were due to a different rate of destruction of latently infected cells during manipulation of samples (Figure1A).
We then correlated the degree of IL-2–induced immune reconstitution with the dynamics of HHV-8 viremia by analyzing plasma samples obtained from patient 70 during 3 cycles (the first, third, and last) of IL-2 administration (Figure 1C). Three time points were analyzed in each cycle: baseline (just before the first IL-2 injection), the third day (after 5 injections of IL-2), and the 8th day (2 days after the last IL-2 injection of the cycle). HHV-8 viremia progressed steadily, despite a significant increase in CD4 cell count (from 192 to 722 cells/μL; Figure 1B) and a progressive normalization of the ratio of CD4 to CD8 cells (from 0.26 to 1.02; data not shown). CD4 and CD8 cell counts decreased dramatically on the third day of IL-2 administration. This was followed by a quick recovery of CD8 cells and a sharp increase in the number of circulating CD4 cells (Figure 1C). Interestingly, the HHV-8 viremia had an opposite pattern; it increased with each IL-2 injection and returned quickly to the baseline level shortly after the last IL-2 administration, although the peak of viremia was more prolonged during the last cycle of treatment. The increase in HHV-8 viremia during IL-2 administration was also observed in the few plasma samples available from the other patient who had appearance of KS lesions during IL-2 treatment (baseline, < 10 genome equivalents/mL; third day, 80 genome equivalents/mL; and 8th day, < 10 genome equivalents/mL).
Although in vitro IL-2 does not seem to induce HHV-8 replication in chronically infected, primary effusion lymphoma (PEL)–derived cell lines,4 IL-2–induced inflammatory cytokines can alone sustain production of HHV-8 viral particles in primary blood-derived mononuclear cells and in a PEL-derived B-cell line, BCBL-1.4,5 Moreover, the observed temporal relation between the peaks of HHV-8 viremia and the drastic reduction in circulating CD4 and CD8 cells suggests that some of the pathological mechanisms involved in the IL-2–induced vascular leak syndrome20 may also contribute to the spread of HHV-8 infection and the appearance of KS lesions. Indeed, productively HHV-8–infected mononuclear cells could adhere to cytokine-activated endothelial cells21 and propagate the infection to the microvascular endotelium.22 Alternatively, infected cells could migrate into the tissue and differentiate into spindlelike endothelial cells on exposure to IFN-γ23 produced by the extravasated CD8 and natural killer cells.24 25
Several epidemiologic studies have reported a direct correlation between low CD4 cell counts and manifestations of KS.12,17 18 In our patients, however, KS occurred despite a progressive reconstitution of CD4 T-cell counts, suggesting that more subtle qualitative defects of the immune system may account for the appearance of KS. Indeed, in these patients, a defective or incomplete restoration of the specific anti–HHV-8 immune response in the context of a more reactive, cytokine-boosted immune system might represent a favorable milieu for the development of KS.
In this study, we observed the first evidence that in vivo administration of IL-2 is temporally associated with transient increases in HHV-8 viremia and, more importantly, that persistent HHV-8 replication is required for subsequent development of KS lesions. However, the finding that KS occurred only in patients in whom HHV-8 replication was already detectable (albeit at a lower level) before initiation of the IL-2 regimen suggests that IL-2–based therapeutic regimens can be safely implemented in most patients with both HIV and HHV-8 infection. Indeed, the incidence of KS in the homosexual patients studied was not significantly greater than the overall KS incidence among Italian homosexuals infected with HIV-1. Special caution regarding safe use of IL-2–based approaches is necessary only in patients who have signs of pre-existing active HHV-8 infection or HHV-8–associated disease. Therefore, specific assays for detecting active HHV-8 infection might represent a useful clinical management tool in dually infected patients, especially in populations at higher risk of development of KS.
We thank Dr Priscilla Biswas for critically reviewing the manuscript and Ms. Stefania Laus for editorial assistance.
Supported by grants from the II Italian National AIDS Project, Ministry of Health, Rome.
M.M. and F.B. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Mauro S. Malnati, Unit of Human Virology, Via Olgettina 58, 20132 Milan, Italy; e-mail: malnati.mauro@hsr.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal