Single-center experiences have shown that intensified treatments with autologous transplantation are a promising therapeutic strategy for patients with high-risk follicle-center lymphoma (FCL) at diagnosis, whereas data from prospective multicenter trials are still lacking. This paper describes the results of a prospective multicenter study of an intensified purging-free high-dose sequential (i-HDS) chemotherapy schedule with peripheral blood progenitor cell (PBPC) autografting. The main feature of this program is harvesting stem cells after intensified chemotherapeutic debulking, with no ex vivo manipulation of PBPCs. Ninety-two previously untreated patients aged 60 or younger with advanced-stage FCL were enrolled by 20 Italian centers and evaluated on an intention-to-treat basis. i-HDS proved feasible with limited toxicity (87% patients completed the planned treatment schedule). i-HDS led to a complete remission rate of 88%. The projected overall survival and disease-free survival (DFS) were, respectively, 84% and 67% at 4 years. Centralized molecular analysis showed that polymerase chain reaction–negative harvests could be collected in 47% of cases. Following autograft, 65% of molecularly evaluable patients achieved clinical and molecular remission. The projected DFS at 4 years of this subgroup is 85%. This result emphasizes the importance of achieving maximal tumor reduction in these patients. In conclusion, our data show that highly effective intensified treatments can now be routinely offered to young patients with poor-risk FCL even at small institutions, with no need for sophisticated and expensive cell manipulation procedures.
Introduction
Several studies have investigated the role of intensified chemotherapy followed by autologous transplantation in the management of relapsed follicle-center lymphoma (FCL).1-7Results were encouraging with high rates of complete remission (CR) and molecular remission.1-10 The latest findings from the Dana Farber Cancer Institute show that molecular remission is associated with an extremely low relapse rate and a more than 80% projected freedom from relapse at 12 years.7 Autologous transplantation may thus possess a curative potential in this otherwise incurable disease.11,12 Similar approaches have been less frequently used at diagnosis.13-16 In fact, a recent retrospective study from Stanford University showed that patients treated with autologous transplantation as first-line treatment have a better outcome compared to those treated with conventional chemotherapy.16
Three important issues, however, still need to be addressed in evaluation of the real role of intensified approaches in FCL. First, there have been no multicenter prospective trials. A single-center trial carries the risk of overestimation of outcomes due to selection biases, and only highly qualified clinical teams may be able to achieve similar results with high-dose programs. Second, most autografting programs require ex vivo purging procedures, which are cumbersome, expensive, and difficult to reproduce.7,17-20 Third, the most promising results have been obtained only in small groups of patients.16
Promising results have recently been provided by using an intensified high-dose sequential chemotherapy (i-HDS) program as front-line therapy for high-risk FCL patients.15,21 This involves the collection of peripheral blood progenitor cells (PBPCs) following a prolonged chemotherapeutic debulking to obtain an in vivo purging effect.15 The i-HDS does not include any ex vivo purging procedure. In a single-center experience, polymerase chain reaction (PCR)–negative harvests were collected in 68% of patients and approximately half of them achieved persistent clinical and molecular remission following autologous transplantation.21 22
A multicenter, prospective trial was therefore launched in 1996 by 20 hematologic centers affiliated with the Gruppo Italiano Trapianto Midollo Osseo (GITMO) to evaluate applicability and efficacy of the i-HDS regimen in 92 patients with FCL. Its results were similar to those observed in previous single-center pilot trials. They show that an ex vivo purging-free autografting procedure is feasible with limited toxicity, induces high rates of CR, and leads to persistent molecular remissions in a good proportion of patients. Thus, high-dose chemotherapy treatments aimed to maximally cytoreduce and possibly cure patients with FCL can be easily performed at both small and large institutions.
Patients and methods
Inclusion criteria
Patients were eligible if they were aged between 18 and 60 and had Ann Arbor stage III or IV FCL as defined by the International Working Formulation (WF B, C, or D)23 or Revised European and American Lymphoma classification (REAL grade I, II, or III).24 Patients should have received no previous chemotherapy or extended-field radiotherapy and have one or more of the following adverse prognostic features: bulky disease (> 5 cm), high serum lactic dehydrogenase (LDH) level, disease-related compression symptoms, systemic “B” symptoms, Eastern Cooperative Oncology Group performance status (ECOG PS) of at least 2, or bone marrow (BM) invasion more than 20%. Absence of concurrent heart, kidney, lung, and liver disease was also required, as well as hepatitis B surface (HBs) antigen and hepatitis C virus (HCV) antibody negativity. Informed consent was obtained and the institutional review boards of all the participating centers approved the study.
Patient characteristics
Between December 1996 and February 1999, 92 patients (median age, 46 years; range, 28-60 years) were treated at 20 Italian hematologic centers affiliated to the GITMO. Patient characteristics are described in Table 1. Eighty-four percent had Ann Arbor stage IV disease. BM involvement was present in 80%, whereas extranodal sites of disease other than BM were present in 55%. Fifty-one percent had a bulky mass and 37% had an elevated serum LDH concentration. “B” symptoms were present in 30% and leukemic disease (peripheral blood lymphocytes > 12 000/mm3) in 12%. Thirty-seven percent had an age-adjusted International Prognostic Index (aaIPI) score of 2 or higher.25 26
Patient characteristics at study entry
| . | No. . | % . |
|---|---|---|
| Total | 92 | 100 |
| Male/female ratio | 42/50 | |
| Median age (range), y | 46 (28-60) | |
| Stage IV | 77 | 84 |
| Bulky mass (> 5 cm) | 47 | 51 |
| High serum LDH | 34 | 37 |
| “B” symptoms | 28 | 30 |
| BM involvement | 74 | 80 |
| Extranodal sites (other than BM) | 51 | 55 |
| Leukemic disease (lymphocytes > 12 000/mm3) | 11 | 12 |
| ECOG PS ≥ 2 | 8 | 9 |
| aaIPI ≥ 2 | 34 | 37 |
| . | No. . | % . |
|---|---|---|
| Total | 92 | 100 |
| Male/female ratio | 42/50 | |
| Median age (range), y | 46 (28-60) | |
| Stage IV | 77 | 84 |
| Bulky mass (> 5 cm) | 47 | 51 |
| High serum LDH | 34 | 37 |
| “B” symptoms | 28 | 30 |
| BM involvement | 74 | 80 |
| Extranodal sites (other than BM) | 51 | 55 |
| Leukemic disease (lymphocytes > 12 000/mm3) | 11 | 12 |
| ECOG PS ≥ 2 | 8 | 9 |
| aaIPI ≥ 2 | 34 | 37 |
The median number of patients treated at each center was 3 (range, 1-15). The annual reports of the GITMO national registry show that the 20 units performed a median number of 31 (range, 8-94) autologous transplantations per year in 1997-1998. Thirty-six patients (39%) were treated at small institutions performing 31 or fewer autografts per year for the treatment of hematologic malignancies; 56 (61%) were treated at larger institutions.
Treatment schedule
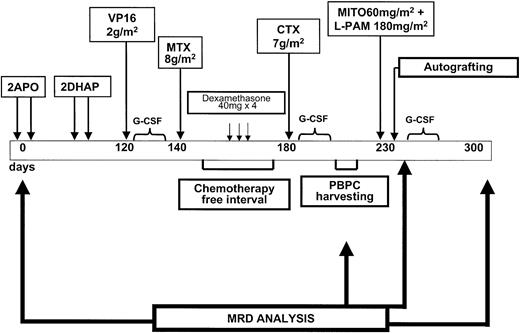
The i-HDS regimen has already been described.15,27Briefly, it consists of intensive debulking prior to the high-dose phase, including 2 complete, full-dose APO (doxorubicin, vincristine, prednisone) courses, totaling four 75-mg/m2 doxorubicin administrations.28 Patients not achieving CR following these courses received 2 additional DHAP (Ara-C, cisplatin, dexamethasone) courses.29 The high-dose phase consisted of etoposide (VP16) 2 g/m,2 followed by methotrexate (MTX) 8 g/m2 and cyclophosphamide (CTX) 7 g/m2. PBPC collection was scheduled after the last course to exploit the “in vivo purging effect” operated by high-dose chemotherapy.15 A chemotherapy-free interval of 40 days was scheduled prior to hd CTX 7 g/m2, to allow optimal PBPC mobilization.30 Three high-dose dexamethasone courses (dexamethasone at 40 mg/d for 4 consecutive days) were administered every 10 days during this interval. A minimum of 5 × 106CD34+ cells/kg was required for autologous transplantation with PBPCs only. Patients failing to meet this minimum were taken off therapy. The conditioning regimen for autologous transplantation consisted of mitoxantrone (MITO) 60 mg/m2 on day −5 and melphalan (L-PAM) 180 mg/m2 on day −2.31PBPCs were reinfused on day 0. Granulocyte colony-stimulating factor (G-CSF; filgrastim or lenograstim) was given at 5 μg/kg daily following VP16, CTX, and autograft. Radiotherapy was scheduled on bulky sites or on residual masses approximately 2 months after autograft. The whole i-HDS program is summarized in Figure1.
Schematic representation of the treatment schedule used in this patient series.
APO course consisted of doxorubicin 75 mg/m2 days 1 and 22, vincristine 1.2 mg/m2 days 1, 8, and 22, and prednisone 50 mg/m2 days 1 to 22. DHAP course consisted of cisplatin 100 mg/m2 day 1, Ara-C 4 g/m2 day 2, dexamethasone 40 mg days 1 to 4.
Schematic representation of the treatment schedule used in this patient series.
APO course consisted of doxorubicin 75 mg/m2 days 1 and 22, vincristine 1.2 mg/m2 days 1, 8, and 22, and prednisone 50 mg/m2 days 1 to 22. DHAP course consisted of cisplatin 100 mg/m2 day 1, Ara-C 4 g/m2 day 2, dexamethasone 40 mg days 1 to 4.
Evaluation and statistics
Clinical response was assessed by complete restaging at 2 months after autograft, thereafter at 3-month intervals for the first year and then at 6-month intervals. According to the Cheson criteria,32 CR was defined by the absence of any clinical sign of disease, whereas partial remission (PR) was defined by a 50% or more tumor reduction. Patients achieving less than PR were considered as having stable disease.32 Progression was defined as a 50% or more tumor increase or by the appearance of new lesions.32 All patients started on treatment were considered evaluable for response and outcome on an intention-to-treat basis. Overall survival (OS) was measured from the start of therapy up to the date of death or last follow-up alive.32Progression-free survival (PFS) for all patients was taken from the start of therapy until disease progression or death from lymphoma.32 Disease-free survival (DFS) for patients in CR was measured from the first recording of a CR to the date of progression.32 Event-free survival (EFS) was calculated from the start of therapy up to the first adverse event, that is, relapse or progression, secondary malignancy, treatment-related death, or last follow-up alive. The closing date for analysis was December 31, 2001. OS, DFS, PFS, and EFS were calculated according to the Kaplan-Meier method.33 The log-rank test was used to compare survival curves.34
Minimal residual disease assessment by nested PCR
All patients with an available tumor specimen were initially screened for the presence of the Bcl-2 translocation on diagnostic tissues (ie, lymph node or BM). Nested PCR amplification for both the major breakpoint region and minor cluster region was carried out as originally described by Gribben et al.8,35 When the Bcl-2 translocation could not be amplified, an alternative tumor marker was sought by amplifying and sequencing the immunoglobulin heavy-chain (IgH) gene rearrangement.36,37 This method gave a tumor-specific forward primer derived from the second complementarity-determining region and a reverse tumor-specific primer derived from the third complementarity-determining region.37 PCR detection of minimal residual disease (MRD) was then performed as previously described.37
Time points chosen for molecular analysis are shown in Figure 1. PCR analysis was performed at diagnosis, on PBPC and BM samples obtained before autologous transplantation, and then at 6-month intervals following autologous transplantation. Patients were considered as having PCR− harvests if at least one PBPC or BM harvest was PCR−. Molecular remission was defined as absence of molecular disease in 2 consecutive BM samples (spaced by at least 6 months) in a patient showing evidence of CR by means of standard radiologic and histologic analysis.
Results
Treatment feasibility and clinical response
Treatment feasibility and responses are illustrated in Tables2 and 3. The regimen proved feasible at the multicenter level (Table 2). Eighty patients (87%) completed the program. Interruptions were due to toxic deaths (2%), disease progressions (3%), grade IV toxicity (1%), consent withdrawal (3%), and insufficient PBPC mobilization (3%). There was no difference in feasibility between small and large institutions (data not shown; P = .89).
i-HDS feasibility in 92 evaluable patients (100%)
| Toxic deaths | 2 (2%) |
| Treatment withdrawals | 3 (3%) |
| Not given transplants | 4 (4%) |
| Low mobilization | 3 (3%) |
| Toxicity | 1 (1%) |
| Progressions | 3 (3%) |
| Patients successfully given transplants | 80 (87%) |
| Median CD34+ cells × 106/kg mobilized (range) | 10.4 (0.6-81.6) |
| Toxic deaths | 2 (2%) |
| Treatment withdrawals | 3 (3%) |
| Not given transplants | 4 (4%) |
| Low mobilization | 3 (3%) |
| Toxicity | 1 (1%) |
| Progressions | 3 (3%) |
| Patients successfully given transplants | 80 (87%) |
| Median CD34+ cells × 106/kg mobilized (range) | 10.4 (0.6-81.6) |
Response to i-HDS in 92 evaluable patients (100%)
| Toxic deaths | 2 (2%) |
| Progressions | 3 (3%) |
| Partial responses | 6 (6%) |
| Complete responses | 81 (88%) |
| Toxic deaths | 2 (2%) |
| Progressions | 3 (3%) |
| Partial responses | 6 (6%) |
| Complete responses | 81 (88%) |
The most frequent violations to the treatment schedule were delays due to shortage of hospital beds. The overall delay exceeded 3 months (range, 2-6 months) in 12% of patients. In addition, 9 patients eligible for postgraft radiotherapy did not receive it. One patient was switched to allogeneic transplantation while she was in PR at the end of the high-dose phase. Follow-up for this patient was stopped at this time.
Eighty-one patients (88%) achieved CR (Table 3), 49 at the end of the high-dose phase and 32 following autologous transplantation. Despite the intensive program, 3 patients (3%) had disease progression under treatment (Table 3). These 3 patients underwent salvage programs with multiple regimens including fludarabine and rituximab with poor response. The 2 patients (2%) who died of treatment-related toxicity were in clinical remission when the fatal toxic episode occurred.
Early and late toxicity
Two toxic deaths were reported; one patient died of ventricular fibrillation associated with myocardial infarction on day +10 following autologous transplantation; the second developed severe cytomegalovirus pneumonia 15 days after high-dose CTX and died of respiratory failure on day +21. Hematopoietic recovery and transfusion requirements following high-dose VP16 and high-dose CTX and following MITO/L-PAM are summarized in Table 4. Grades III to IV extrahematologic early nonfatal toxicity (other than oral and gastrointestinal mucositis during the myeloablative phase) included ischemic stroke at the end of the high-dose phase (1%), sepsis (2%), pneumonia (3%), hepatitis due to HBV reactivation (2%), gallbladder empyema (1%), acute heart infarction (1%), pulmonary embolism (1%), and gastric hemorrhage following the initial APO course in a patient with gastric localization (1%). Thirty-one percent of these side effects were recorded during the debulking phase with conventional chemotherapy, 38% during the high-dose phase, and 31% during the final myeloablative phase. No difference in toxicity was observed between patients treated at small and large institutions (P = .99; data not shown). All patients recovered from these acute episodes except the patient experiencing ischemic stroke who had persistence of neurologic defects. Because this patient was already in CR, the final autografting phase was omitted (Table2).
Hematologic toxicity and transfusional requirement following high-dose etoposide, high-dose CTX, and autograft
| Parameters . | Hd VP16 . | Hd-CTX . | MITO/L-PAM . |
|---|---|---|---|
| Days with WBC < 0.5 × 109/L | 3 (0-8) | 5 (0-9) | 8 (3-14) |
| Days with platelets < 20 × 109/L | 3 (0-7) | 5 (0-10) | 10 (5-20) |
| Median no. of platelet transfusions (range) | 0 (0-3) | 1 (0-6) | 3 (0-8) |
| Median no. of RBC transfusions (range) | 0 (0-4) | 1 (0-9) | 2 (0-8) |
| Parameters . | Hd VP16 . | Hd-CTX . | MITO/L-PAM . |
|---|---|---|---|
| Days with WBC < 0.5 × 109/L | 3 (0-8) | 5 (0-9) | 8 (3-14) |
| Days with platelets < 20 × 109/L | 3 (0-7) | 5 (0-10) | 10 (5-20) |
| Median no. of platelet transfusions (range) | 0 (0-3) | 1 (0-6) | 3 (0-8) |
| Median no. of RBC transfusions (range) | 0 (0-4) | 1 (0-9) | 2 (0-8) |
Hd indicates high-dose; WBC, white blood cells; RBC, red blood cells.
With a median follow-up of 40 months, the following late toxic episodes were recorded: herpes zoster reactivation (3%) always responding to acyclovir, autoimmune thrombocytopenia (1%) that resolved spontaneously, and congestive heart failure (3%; New York Heart Association class I and II) effectively controlled by therapy. Myelodysplastic syndrome (MDS) and secondary myeloid leukemia occurred in 4 patients (4%). One was in CR. The other events occurred following repeated courses of salvage chemotherapy due to relapsed or resistant FCL. Another patient developed T-cell acute lymphoblastic leukemia (T-ALL) while in CR at 48 months after autografting. Two of these 5 patients have already died (1 with myeloid leukemia and 1 with T-ALL); 3 are presently alive (2 without treatment).
Clinical outcome
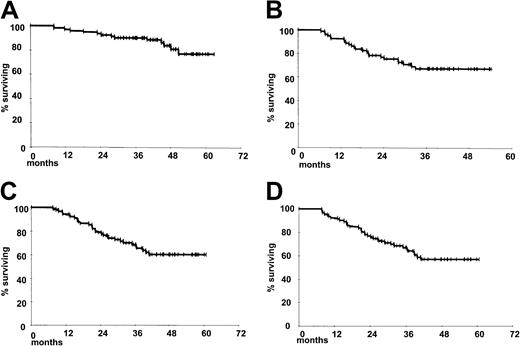
The survival projections are shown in Figure2. Among the 81 patients in CR at the end of the treatment, there have been 24 relapses; 5 relapses occurred among the 6 patients in PR. At present 56 patients are alive in continuous CR at a median follow-up of 43 months (range, 24-61 months), one with secondary untreated MDS. The 4-year DFS and PFS projections are 67% and 60%, respectively (Figure 2B,C). Of the 29 patients who had relapses, 21 are alive at a median follow-up of 44.4 months, 4 with no need for additional treatment. Salvage treatments were heterogeneous; in most cases patients were treated with rituximab-containing conventional or intensified schedules. Twelve patients achieved a second CR, 11 by means of a rituximab-containing regimen and 1 by means of radiotherapy alone. Thus, at present 78 of 92 (85%) patients are alive. At a median follow-up of 43 months, the estimated 4-year OS projection is 84% (Figure 2A). Overall, 56 (55 in CR and 1 in PR) patients are alive, with no sign of disease progression and no severe late complications, with a 4-year EFS projection of 57% (Figure 2D).
Survival estimates.
Kaplan-Meier estimate of probability of OS (A), DFS (B), PFS (C), and EFS (D) for the 92 patients evaluated in the study. Data were evaluated on an intention-to-treat basis.
Survival estimates.
Kaplan-Meier estimate of probability of OS (A), DFS (B), PFS (C), and EFS (D) for the 92 patients evaluated in the study. Data were evaluated on an intention-to-treat basis.
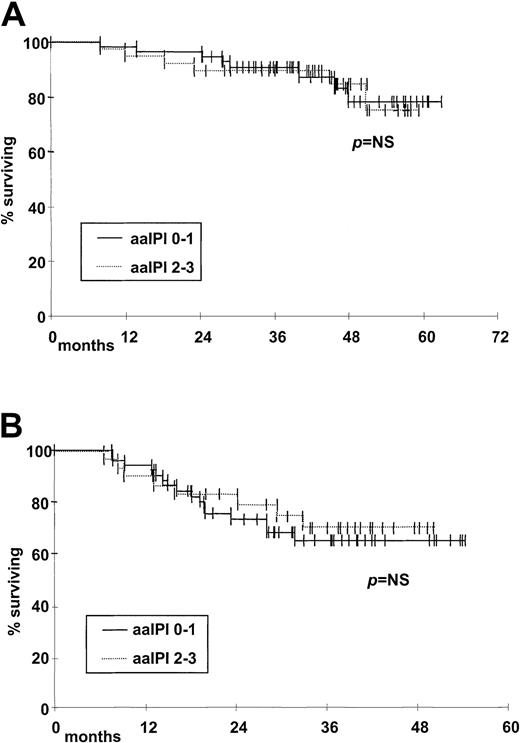
The outcome has been also evaluated according to the aaIPI score.25 26 There were no significant differences in OS and DFS between patients with aaIPI of 0 to 1 and those with aaIPI of 2 to 3 (Figure 3A,B).
Kaplan-Meier estimate of probability of OS and DFS according to aaIPI score.
(A) OS and (B) DFS for patients with low (0 or 1) aaIPI score (n = 58, solid line) versus patients with high (2 or 3) aaIPI score (n = 34, dotted line); P = NS.
Kaplan-Meier estimate of probability of OS and DFS according to aaIPI score.
(A) OS and (B) DFS for patients with low (0 or 1) aaIPI score (n = 58, solid line) versus patients with high (2 or 3) aaIPI score (n = 34, dotted line); P = NS.
PCR analysis of stem cell harvests
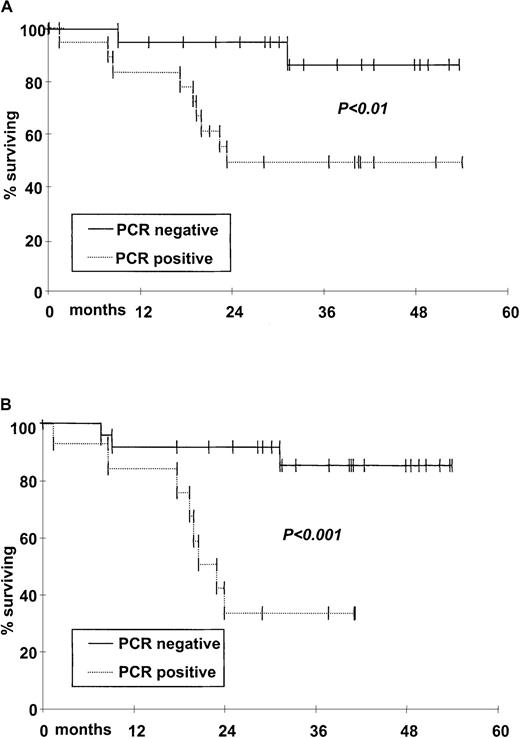
As summarized in Table 5, a molecular marker was obtained from the diagnostic tissue in 42 (76%) of 55 patients tested molecularly. The tumor marker was the Bcl-2/IgH translocation in 36 patients (65%). In addition a molecular marker derived from the IgH sequence was obtained in 6 (31%) of 19 patients lacking a Bcl-2/IgH translocation (Table 5). A total of 126 pretransplantation stem cell harvests were analyzed. Fifty-nine (47%) were PCR−. Twenty (48%) of 42 evaluable patients obtained one or more PCR− harvests; 18 are in continuous CR and only 2 had disease recurrence. Thirteen (59%) of the 22 patients collecting only PCR+ harvests had relapse (P < .01). DFS curves of the 2 populations are shown in Figure 4A. The outcome of the 6 patients collecting both PCR− and PCR+ harvests was similar to that of patients collecting only PCR− harvests (data not shown). Patients in whom the diagnostic sample was not available had a similar clinical behavior in terms of OS, PFS, DFS, and EFS compared to those studied molecularly (data not shown).
Results on PCR-based analysis of MRD
| . | No. . | Samples tested . | % . |
|---|---|---|---|
| Patients with a molecular marker | 42 | 55 | 76 |
| Bcl-2+ | 36 | 55 | 65 |
| IgH+ | 6 | 19 | 31 |
| PCR−harvests5-150 | 59 | 126 | 47 |
| Patients in molecular remission5-150 | 24 | 37 | 65 |
| . | No. . | Samples tested . | % . |
|---|---|---|---|
| Patients with a molecular marker | 42 | 55 | 76 |
| Bcl-2+ | 36 | 55 | 65 |
| IgH+ | 6 | 19 | 31 |
| PCR−harvests5-150 | 59 | 126 | 47 |
| Patients in molecular remission5-150 | 24 | 37 | 65 |
In some patients in whom a molecular marker was available, follow-up samples were not available.
Kaplan-Meier estimate of probability of DFS according to PCR status of harvests and molecular follow-up.
(A) DFS for patients whose harvests were PCR− (n = 20, solid line) versus patients whose harvests were PCR+(n = 22, dotted line); P < .01. (B) DFS for patients achieving a molecular remission (n = 24, solid line) versus patients with PCR+ follow-up (n = 13, dotted line);P < .001.
Kaplan-Meier estimate of probability of DFS according to PCR status of harvests and molecular follow-up.
(A) DFS for patients whose harvests were PCR− (n = 20, solid line) versus patients whose harvests were PCR+(n = 22, dotted line); P < .01. (B) DFS for patients achieving a molecular remission (n = 24, solid line) versus patients with PCR+ follow-up (n = 13, dotted line);P < .001.
Molecular follow-up
Molecular monitoring was performed on postgraft BM samples. Twenty-four (65%) of 37 evaluable patients achieved molecular remission, 22 immediately following autologous transplantation, and 2 at 6 and 12 months, following an initial detection of PCR+results on 1 or 2 samples. All these patients were also in CR. Six patients autografted with PCR+ PBPC became PCR− during the molecular follow-up.
Only 3 relapses (12%) occurred among patients achieving molecular remission after autografting. One was a localized retro-orbital relapse with persistent PCR negativity at BM level. This patient achieved second CR with radiotherapy alone and he is in persistent molecular remission. The second occurred in a patient who displayed 2 consecutive PCR− results at 6 and 12 months from autografting. This relapse was heralded by recurrence of PCR positivity at 18 months from autografting while the patient still had no sign of clinical relapse. A third patient had a relapse at 12 months from transplantation as diffuse large cell lymphoma. Unfortunately, we could not perform IgH sequencing on the relapse sample to rule out the occurrence of a second lymphoma as already reported.38 In contrast, 10 relapses were noted in the 13 patients who failed to achieve molecular remission (77%). DFS of patients achieving postgraft molecular remission compared to those remaining PCR+ is shown in Figure 4B (P < .001).
Discussion
This paper illustrates the results of a multicenter prospective study using i-HDS, an ex vivo purging-free intensified approach with PBPC autografting, in a series of 92 previously untreated patients, aged 60 or younger, with advanced FCL. Results show that i-HDS is a feasible approach that can be performed with acceptable toxicity at both small and large institutions. Response and outcome were similar to those reported in previous single-center experiences and are promising, particularly for patients with aaIPI 2 or higher.14-16Centralized molecular analysis showed that PCR− harvests can be collected using a chemotherapy-mediated in vivo purging approach. Finally, the observation of a high proportion of patients in prolonged clinical and molecular remission suggests that at least some of them might have been cured of their disease.
Feasibility is a major issue in the setting of intensified regimens in FCL, especially due to the need to obtain PCR− collections for autografting.7-12,21,22 This is critical in FCL as opposed to other neoplasms such as multiple myeloma, where transplantation is not delivered with curative intent,39,40 and diffuse large cell lymphoma, where tumor contamination of stem cell harvests is infrequent. Conventional autografting approaches such as those used by the Dana Farber Cancer Institute7,8,14 and the Stanford University16groups successfully clear MRD from stem cell harvests by ex vivo manipulation. However, this strategy is expensive, time-consuming, and too sophisticated for the small and medium-sized institutions that currently treat most patients with FCL. This probably explains why no multicenter trial has been so far published using these strategies. Indeed, most centers participating to our study (16 of 20) do not currently perform ex vivo manipulation procedures. Nevertheless, all centers were able to perform the whole schedule. The chemotherapy program was completed in most patients enrolled and no differences were observed in terms of feasibility between small and large centers.
Toxicity is another important issue for patients with FCL treated with autografting programs. Early toxicity was not excessive, although 2 toxic deaths were reported. This is in line with the treatment-related mortality (TRM) expected with the use of intensive chemotherapy with autograft.41,42 The TRM of 2% is, in fact, analogous or even lower than that reported in single-center experiences with autograft in FCL at diagnosis.14-16 Additional major toxic episodes were successfully managed with appropriate treatment and did not show evidence of clustering in any treatment phase. Thirty-one percent occurred during the early conventional phase, suggesting that a significant proportion of them would also have occurred if patients had only received a CHOP-like (CTX, doxorubicin, vincristine, prednisone) regimen.
The occurrence of 4 cases of secondary MDS is of some concern, particularly because it cannot be excluded that additional episodes will occur during the long-term follow-up. However, it should be noted that 3 of 4 instances of MDS occurred in patients who received additional treatment due to relapse. Although our treatment is already free of total body irradiation, additional steps should probably be undertaken to reduce the risk of second tumors. One possibility would be to replace high-dose VP16 with a less leukemogenic drug such as Ara-C.43,44 A more intensified etoposide-free program has proved feasible and effective for patients with mantle cell lymphoma and relapsed FCL.45-47 In addition, new nonchemotherapeutic drugs, such as anti-CD20 rituximab, are suitable for inclusion in the i-HDS schedule to reduce the risk of recurrence.45 46 This might reduce the need for salvage chemoradiotherapy and lower the risk of secondary neoplasms.
The efficacy of i-HDS in FCL was confirmed in this multicenter study. The 88% CR rate is analogous to that reported in the previous single-center pilot study.15 Thus, the promising results observed at the single-center level do not reflect selection biases or availability of particularly experienced teams. In addition, results of centralized PCR-based analysis were consistent with a potent antilymphoma activity of i-HDS. Approximately 60% of patients evaluable for MRD reached a persistent PCR− status following autologous transplantation. These patients had an extremely low risk of relapse. Thus, a good proportion of FCL patients undergoing i-HDS at diagnosis experiences a prolonged clinical and molecular remission. It is conceivable that these patients might have been cured of their disease, as already suggested in previous experiences using intensive approaches.7,12 21
The most significant results with the use of high-dose chemoradiotherapy and autograft in FCL patients at diagnosis have been obtained at the Dana Farber Cancer Institute and at Stanford University.14,16 Our patient characteristics were quite similar. They were selected for age 60 or younger, advanced disease, and one or more adverse prognostic features, according to the criteria available at the time of the study. We observed an 84% survival projection at 4 years. This is lower than the OS reported by the 2 American groups. It should be noted that in their studies only patients responsive to conventional induction therapy were considered for the high-dose program, whereas our analysis was made on an intention-to-treat basis and the outcome of all enrolled patients was evaluated.14,16 In addition, the differences in OS may in part reflect a better handling of disease recurrence for patients enrolled in single-center compared to multicenter programs. In fact, our PFS and DFS projections were comparable to those reported by the Stanford and Dana Farber groups.14 16 Our results demonstrate that approximately 60% of patients are disease-free survivors as in the single-center studies.
Our study was not designed to demonstrate the superiority of i-HDS compared to conventional chemotherapy and thus any conclusion on this issue should be suspended until the results of currently ongoing prospective randomized trials are available. However, the observation that following i-HDS we failed to see any difference in outcome between patients with aaIPI 2 or higher and those with aaIPI less than 2 is particularly intriguing. Indeed, these results suggest that an intensified treatment might be beneficial for patients with poor prognosis according to the aaIPI score, whereas any benefit for patients with less aggressive disease would be extremely difficult to prove, even in large randomized trials.
We are witnessing a very exciting age in the treatment of FCL because novel treatment approaches are dramatically changing its natural history. Several new molecularly targeted therapeutic approaches are now entering the clinical arena, such as naked and radiolabeled monoclonal antibodies, vaccination strategies, and antisense oligonucleotides.48-55 There is little doubt that intensified chemotherapies may appear rather obsolete by comparison. Nevertheless, it should be noted that autografting-containing regimens were one of the most effective in the era before monoclonal antibodies. This treatment was the first proving able to modify the natural evolution of FCL as outlined by the high incidence of prolonged clinical and molecular remission observed in a high proportion of patients.7,12,15,16 It is now clear that rituximab and perhaps other innovative drugs can be easily integrated within autografting-containing regimens.45 46 Thus, intensified treatments should still be considered as effective therapeutic weapons worthwhile of being evaluated in combination with novel drugs. To verify this hypothesis a randomized trial comparing rituximab-supplemented i-HDS versus rituximab-supplemented CHOP has been recently launched by the GITMO group for FCL patients with an aaIPI score of 2 or higher.
Investigators from the following Institutions in Italy contributed to the trial: Divisione Universitaria di Ematologia, Cattedra di Ematologia (Torino): M. Ladetto, S. Vallet, M. Astolfi, D. Drandi, A. Pileri, C. Tarella, I. Ricca, S. Sametti, F. Volpato, M. Boccadoro; Bone Marrow Transplantation Unit, Istituto Scientifico H. S. Raffaele (Milano): P. Corradini, A. Pescarollo, C. Voena, M. Bregni; Divisione Universitaria di Ematologia, Policlinico Borgo Roma (Verona): F. Benedetti, M. Sorio, G. Pizzolo; Divisione Ospedaliera di Ematologia, AO S. Giovanni Battista (Torino): U. Vitolo, C. Boccomini, E. Gallo; Dipartimento di Biotecnologie Cellulari ed Ematologia, Università La Sapienza (Roma): M. Martelli, M. T. Petrucci, A. Pulsoni, F. Mandelli; Dipartimento di Ematologia, AO Bianchi-Melacrino-Morelli (Reggio Calabria): M. Brugiatelli, G. Messina, F. Nobile; Divisione di Ematologia, AO S. Maurizio (Bolzano/Bozen): P. Coser, N. Pescosta; Divisione Universitaria di Ematologia, AO S. Eugenio, Università Tor Vergata (Roma): A. Perrotti, S. Amadori; Divisione di Ematologia, AO V. Cervello (Palermo): I. Majolino, C. Patti, S. Mirto; Divisione Universitaria di Ematologia, AO Spirito Santo (Pescara): G. Fioritoni, F. Angrilli; Divisione di Ematologia-CTMO, Ospedale Maggiore (Cremona): S. Morandi, C. Bergonzi; Divisione di Oncoematologia e TMO, Ospedale La Maddalena (Palermo): M. Musso; Divisione di Ematologia, AO S. Bortolo (Vicenza): R. Zambello, F. Rodeghiero; Divisione di Ematologia, Ospedali Riuniti SS. Giovanni e Paolo, (Venezia): T. Chisesi; Divisione di Ematologia, AO Casa Sollievo della Sofferenza (S. Giovanni Rotondo): N. Di Renzo, M. Carella; Divisione di Ematologia, AO S. Chiara (Trento): P. Vivaldi; Divisione di Medicina Generale, Ospedale S. Giovanni Vecchio antica sede (Torino): A. De Crescenzo; Divisione di Ematologia, AO S. Croce (Cuneo): A. Gallamini, C. Castellino; Divisione di Ematologia, AO SS. Antonio e Biagio (Alessandria): F. Salvi, A. Levis; Dipartimento di Ematologia, AO S. Martino (Genova): G. Santini.
Prepublished online as Blood First Edition Paper, June 7, 2002; DOI 10.1182/blood-2002-02-0621.
Supported in part by Associazione Italiana Ricerca sul Cancro, Milano, Italy; by Compagnia di San Paolo, Torino, Italy; and by Regione Piemonte. D.D. is a recipient of a fellowship from Associazione Italiana Ricerca sul Cancro.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Marco Ladetto, Cattedra di Ematologia, Via Genova 3, 10126 Torino, Italy; e-mail: marco.ladetto@unito.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal