The chemokine stromal cell–derived factor 1 (SDF-1) is essential for perinatal viability, B lymphopoiesis, and bone marrow myelopoiesis, and is a potent monocyte and T-lymphocyte chemoattractant. Interactions of SDF-1 with its receptor CXCR4 have been implicated in CD34+ cell migration and homing. Here it is shown that human SDF-1β (hSDF-1β) alone secreted by hSDF-1β–transduced tumor cells promotes efficacious antitumor responses. The murine C1498 leukemia and B16F1 melanoma models have been studied. For expression of hSDF-1β by tumor cells (SDF-tumor cells), packaging cell lines secreting retroviruses encoding hSDF-1β have been used. The results demonstrate that 50% (B16F1) and 90% (C1498) of naive mice injected with SDF-tumor cells reject their tumors. Prophylactic vaccination of naive mice with irradiated SDF-tumor cells leads to systemic immunity, and therapeutic vaccination leads to cure of established tumors. Mice that previously rejected live SDF-tumor cells are immune to the rejected tumor but susceptible to another tumor and have in vitro tumor-specific cytotoxic T lymphocyte (CTL) activity. SDF-tumor cells are not rejected by immunodeficientscid mice. Immunohistochemistry shows significant infiltration of SDF-1 tumors by T cells, and in vivo T-cell depletion studies indicate that CD4+ T cells are required for SDF-mediated tumor rejection. In conclusion, the present data suggest that SDF-1/CXCR4 interactions have the potential to regulate efficacious antitumor immune responses; exploitation of these interactions may lead to novel therapeutic interventions.
Introduction
Stromal cell–derived factor 1 (SDF-1) is a member of the CXC subfamily of chemokines that was originally identified as a product of murine and human bone marrow stromal cells and as a growth factor for B-cell progenitors.1-3 In contrast to other chemokines that are induced by inflammation, SDF-1 is constitutively produced by stromal cells and is essential during embryonic life for B lymphopoiesis, bone marrow myelopoiesis, neuronal patterning, and cardiovascular development.4-6 Two alternatively spliced variants (SDF-1α and SDF-1β) of a single gene have been described, which are identical, except that SDF-1β has an additional 4 amino acid residues in the carboxyl terminus.7 No differences have been identified between SDF-1α– and SDF-1β–regulated expression and function. Most recently, the cloning and characterization of SDF-1γ, a novel SDF-1 transcript with developmentally regulated expression in the nervous system has been reported.8 Possibly related to its absolute requirement for embryogenesis, SDF-1 is highly conserved among species and there is 99% amino acid homology between mouse and human SDF-1 (hSDF-1).
The CXC chemokine receptor 4 (CXCR4) is the only known receptor for SDF-1, is broadly expressed in cells of both the immune and the central nervous systems, and has been recently found to be a key coreceptor required for the infection of T-tropic HIV-1 strain of CD4+T lymphocytes.9-11 Over the last 5 years, an increasingly essential role of SDF-1/CXCR4 interactions in various physiologic processes has been demonstrated. Thus, SDF-1 plays a key role in regulating transendothelial migration and homing of hematopoietic stem and progenitor cells,12-16 is a potent chemotactic factor for T and pre-B lymphocytes17,18 and dendritic cells (DCs),19 and has an effect on T-cell rolling and tight adhesion to activated endothelial cells.20 SDF-1 also induces in vitro and in vivo angiogenic activity,21 and is a costimulatory factor for CD4+ T-cell activation.22 Most recently, SDF-1/CXCR4 interactions have been implicated in the accumulation of CD4+ T cells within inflamed rheumatoid arthritis synovium, thus leading to the identification of SDF-1 as a key regulator of the local inflammation.23
Based on the reported T-cell chemotactic and costimulatory properties of SDF-1, it is reasonable to speculate that the presence of this chemokine in the tumor microenvironment may actuate immune responses capable of modifying the pattern of in vivo tumor growth. Indeed, Nomura et al have recently reported on the antitumor activity of SDF-1α in 2 murine models.24 In their study, although the immunogenic MethA fibrosarcoma and HM-1 ovarian carcinoma models secreting high levels of SDF-1α (90 ng/mL and 55 ng/mL, respectively) had been used, tumor regression could only be achieved when tumors were transfected to coexpress interleukin 2 (IL-2) or granulocyte-macrophage colony-stimulating factor (GM-CSF) as well as SDF-1α. In this report, we have studied the antitumor activity of hSDF-1β in 2 poorly immunogenic models, the C1498 myeloid leukemia and the B16F1 melanoma. Our results demonstrate that hSDF-1β–transduced C1498 and B16F1 cells, secreting low levels of the chemokine (1.6 ng/mL and 2.6 ng/mL, respectively) as compared to the levels reported by Nomura et al,24 are rejected in 50% (B16F1) and 90% (C1498) of the mice, and that tumor rejection leads to tumor-specific memory responses. We also provide evidence that therapeutic vaccination could cure tumor-bearing animals when only low numbers of irradiated, hSDF-1β–transduced tumor cells were used. The importance of the SDF-1 levels secreted locally by transduced tumor cells is discussed.
Materials and methods
Mice
Female C57BL/6, female C57BL/6 scid, and female C57BL/6 B-cell–deficient (homozygous for the Igh-6tm1Cgn) mice (6-8 weeks old), were purchased from Jackson Laboratories (Bar Harbor, ME). The animals were kept at the animal facility of Genetics Institute (Andover, MA) according to the Institute's guidelines.
Tumor models
The C1498 leukemia and B16F1 melanoma cell lines were purchased from American Type Culture Collection (ATCC; Rockville, MD) and were maintained in vitro at 37°C in Dulbecco modified Eagle medium (DMEM) containing 10% fetal calf serum (FCS), 2% glutamine, and 1% penicillin-streptomycin. For the establishment of tumors, 2 × 105 C1498 cells were injected intravenously in the tail vein of C57BL/6 mice, and 105 B16F1 cells were injected intradermally in the flank of C57BL/6 mice. Tumor-bearing animals either died within 20 to 35 days after tumor inoculation (C1498) or were killed when tumors reached a size of approximately 400 to 600 mm2 (B16F1).
Expression of hSDF-1β by tumor cells
For the expression of hSDF-1β by tumor cells (SDF-tumor cells), supernatants from PT67 amphotropic packaging cells (Clontech, Palo Alto, CA) secreting murine stem cell virus (MSCV)/hSDF-1β retrovirus were used. The retroviral vector backbone has been previously described.25 Briefly, the vector uses the long terminal repeat (LTR) of the MSCV and contains a selectable neo gene under the control of an encephalomyocarditis virus (EMCV) internal ribosome entry site (IRES). Producer cells secreting mock virus were used for infection of control cells. Packaging cells were maintained at 37°C in DMEM containing 10% FCS, 2% glutamine, 1% penicillin-streptomycin, and 1 μg/mL (active) G418 (Gibco BRL, Gaithersburg, MD). Infection of tumor cells with recombinant viruses has been previously described.26 Briefly, wild-type tumor cells (5-7 × 105/mL) were exposed 2 or 3 times to viral supernatant for 4 to 6 hours in the presence of 8 μg/mL polybrene. Designated numbers of G418-selected, SDF-tumor cells or control tumor cells were used for in vivo injections.
Human SDF-1 ELISA
The P67-SDF packaging cells and tumor cells (106cells/mL) were cultured for 24 hours and levels of hSDF-1 in culture supernatants were determined by sandwich enzyme-linked immunosorbent assay (ELISA; Endogen, Woburn, MA). The sensitivity of the assay is 5 pg/mL.
51Cr release CTL assays
Spleens were collected from mice that had rejected live SDF-tumor cells and single-cell suspensions were prepared. Splenocytes (5 × 106) were cocultured with irradiated (7335 cGy) wild-type tumor cells (1 × 105) in 2 mL complete RPMI/well of a 24-well tissue culture plate (Costar, Cambridge, MA). Six days later, splenocytes were harvested and used as effector cells in cytotoxic T lymphocyte (CTL) assays. B16F1 and C1498 cells (H-2d) or control allogeneic mammary adenocarcinoma TSA (H-2b) tumor cells (2 × 106) were labeled with 7.4 MBq (200 μCi) 51Cr (New England Nuclear, Boston, MA) for 90 minutes, washed twice, and used as targets (5000/well) in the CTL assays. The standard 4-hour CTL assays were set up with various effector-to-target (E/T) ratios as previously described.26
In vivo T-cell subset depletions
The monoclonal antibodies (mAb's) GK1.5 (rat antimouse CD4) and 53-6.7 (rat antimouse CD8; ATCC, Manassas, VA) were used for in vivo T-cell subset depletions. The mAb's were produced and purified by standard techniques at Genetics Institute. For in vivo depletion experiments the mice were injected intraperitoneally on 3 consecutive days with mAb (0.5 mg/injection). Depletion of CD4+ or CD8+ T cells was verified 3 days after the last injection by flow cytometric analysis of spleen cells. The analysis showed that more than 95% depletion of the appropriate subset was achieved with normal levels of the other subset (data not shown). Three days after the last injection the mice were inoculated with live SDF-tumor cells and antibody injections continued every 5 days for 3 weeks.
Immunohistochemistry
C57BL/6 mice were injected intradermally with 105live wild-type or SDF-B16F1 cells, tissues were collected on days 3, 7, and 14 (10 mice/time point/cell type) after tumor inoculation, and tumor cell and immune cell infiltrates (ICIs) were evaluated. Tissues were bisected and one half was cryopreserved in optimum cutting temperature (OCT; Tissue-Tec, Sakura Finetek, Torrance, CA) by liquid nitrogen–cooled isopentane method, and the other half was fixed in 10% neutral-buffered formalin. For histologic evaluation, 5-μm sections from paraffin-embedded tissues were stained with hematoxylin and eosin. For CD4 (L3T4), CD8a (53-6.7), CD11c (HL3), and Gr-1 (RB6-8C50) (Pharmingen, San Diego, CA) immunohistochemistry, cryopreserved samples were cryosectioned onto capillary gap micro slides and fixed in acetone before storing at −20°C. Immediately prior to staining, stored cryosections were fixed in cold acetone for 5 minutes, air-dried, blocked with avidin-biotin block (Zymed Laboratories, South Francisco, CA) and finally washed in phosphate-buffered saline (PBS). For CD45R/B220 (RA3-6B2, Pharmingen), CD3 (CD3-12, Serotec, Oxford, England), and von Willebrand factor (von Willebrand Factor, Dako, Carpinteria, CA) immunohistochemistry, paraffin-embedded sections were used, and either proteinase K or microwave antigen retrieval was used. Serial sections stained with the appropriate isotype-matched immunoglobulins were used as negative controls. An indirect streptavidin-peroxidase method was used with DAB (3,3′-diaminobenzidine tetrahydrochloride; Research Genetics, Huntsville, AL) as the color chromogen.
Statistical analysis
Individual experiments consisted of 10 mice per treatment group. The statistical survival analysis was performed using the standard Mantel-Cox log-rank test. Proliferation results are mean ± SD. The statistical significance between various groups was analyzed by the Student t test.
Results
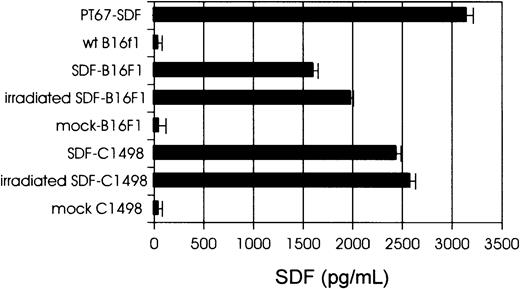
Expression of hSDF-1 by tumor cells
Tumor cells were transduced with PT67-hSDF-1β or PT67-mock retroviral supernatants as described in “Materials and methods.” The levels of hSDF-1 secreted by packaging cell lines, SDF-tumor cells, and control (mock) cells were determined by hSDF-1 ELISA (Figure1). The in vitro growth rate of SDF-tumor cells was similar to the growth rate of control cells (data not shown). Immunostaining and flow cytometric analysis revealed enhanced leukocyte function-associated antigen (LFA-1; CD11a/CD18) expression by SDF-tumor cells compared to control cells, but no differences in expression of CD80, CD86, or major histocompatibility complex (MHC) class I when SDF-tumor and control cells were compared (data not shown). Expression of SDF-1 by B16F1 cells resulted in apparent morphologic changes in the form of long cytoplasmic projections and greater adherence to plastic (data not shown).
Expression of hSDF-1β by tumor cells.
Levels of hSDF-1 secreted by PT67-SDF packaging cells, SDF-tumor cells, and control (mock) cells, as determined by hSDF-1 ELISA. Cell lines (106 cells/mL) were cultured for 24 hours and supernatants were collected, filtered (0.45-μm pore-size filter) and assayed for hSDF-1 levels.
Expression of hSDF-1β by tumor cells.
Levels of hSDF-1 secreted by PT67-SDF packaging cells, SDF-tumor cells, and control (mock) cells, as determined by hSDF-1 ELISA. Cell lines (106 cells/mL) were cultured for 24 hours and supernatants were collected, filtered (0.45-μm pore-size filter) and assayed for hSDF-1 levels.
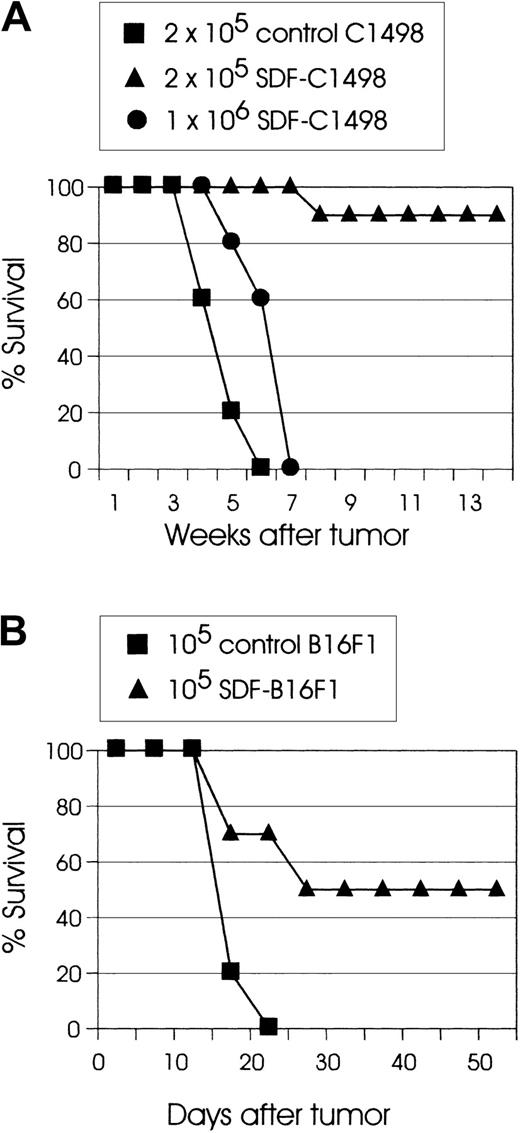
Tumorigenicity of SDF-tumor cells
To directly assess the effect of the chemokine SDF-1 on the tumorigenicity of tumor cells, live SDF-transduced cells were inoculated into syngeneic mice. In these experiments, SDF-C1498 leukemia cells had reproducibly the lowest tumorigenicity. In several experiments, injection of C57BL/6 mice with 2 × 105 live SDF-C1498 cells resulted in 90% to 100% tumor rejection. However, although mice that were injected with 1 × 106 live SDF-C1498 cells had some tumor growth delay as compared to control animals (P < .005), eventually all these mice developed lethal leukemia (Figure 2A). In the B16F1 model, 50% to 60% of mice injected with SDF-B16F1 cells reproducibly showed delayed tumor growth, but ultimately developed lethal tumors. In this model, although some mice (10%-20%) had eventually small palpable tumors, these tumors did not progress, and finally they regressed. Thus, as shown in Figure 2B, 50% of mice injected with SDF-B16F1 cells had long-term, tumor-free survival (P = .0014). In all experiments, mice that had rejected their tumors did not develop any clinical signs of toxicity and remained alive and tumor-free for several weeks or months after tumor inoculation. These results support the idea that local secretion of SDF-1 by transduced tumor cells leads to the development of effector antitumor responses and the elimination of clinically detectable tumors.
SDF expression significantly reduces the in vivo tumorigenicity of tumor cells.
(A) C57BL/6 mice (10 mice/group) were injected intravenously with the indicated numbers of live SDF-C1498 cells or control C1498 cells. Mice injected with 1 × 106 SDF-C1498 (●) had some tumor growth delay as compared to control (▪) mice (P < .005), but eventually developed lethal leukemia, whereas 90% of the mice injected with 2 × 105 SDF-c1498 cells (▴) rejected their leukemia. This graph is representative of 4 independent experiments. (B) C57BL/6 mice (10 mice/group) were injected intradermally in the flank with control B16F1 (▪) or SDF-B16F1 (▴) cells. All control mice developed lethal tumors; 50% of the SDF-B16F1 mice remained tumor free (P = .0014). This graph is representative of 3 independent experiments.
SDF expression significantly reduces the in vivo tumorigenicity of tumor cells.
(A) C57BL/6 mice (10 mice/group) were injected intravenously with the indicated numbers of live SDF-C1498 cells or control C1498 cells. Mice injected with 1 × 106 SDF-C1498 (●) had some tumor growth delay as compared to control (▪) mice (P < .005), but eventually developed lethal leukemia, whereas 90% of the mice injected with 2 × 105 SDF-c1498 cells (▴) rejected their leukemia. This graph is representative of 4 independent experiments. (B) C57BL/6 mice (10 mice/group) were injected intradermally in the flank with control B16F1 (▪) or SDF-B16F1 (▴) cells. All control mice developed lethal tumors; 50% of the SDF-B16F1 mice remained tumor free (P = .0014). This graph is representative of 3 independent experiments.
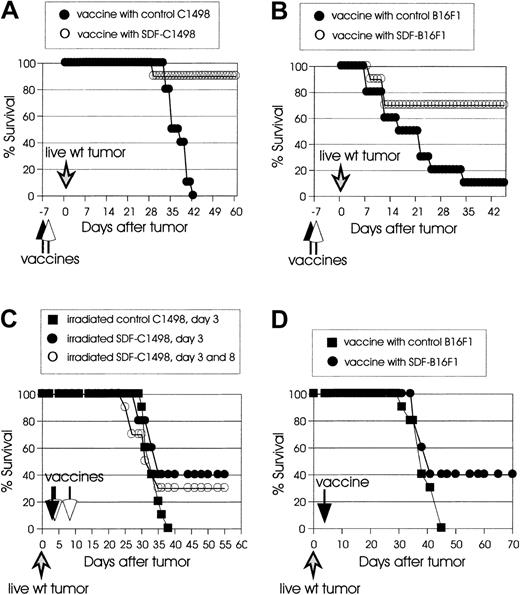
Irradiated SDF-tumor cells induce prophylactic and therapeutic immunity
We next evaluated the capacity of vaccinations with irradiated SDF-tumor cells to protect mice from live wild-type tumor challenge (prophylactic immunity), or to cure mice with already established tumors (therapeutic immunity). As shown in Figure3A, vaccination with 2 × 105 irradiated SDF-C1498 cells on day −7 resulted in 90% protection against challenge with 2 × 105 live wild-type C1498 cells, whereas no protection was observed in mice vaccinated with irradiated control C1498 cells. In the B16F1 model (Figure 3B), 10% of the mice that were vaccinated with 105irradiated control B16F1 cells on day −7 and challenged at a distant site (opposite flank) with 105 live wild-type cells on day 0 were protected, whereas 70% of the mice vaccinated with 105 irradiated SDF-B16F1 cells were protected (P = .018).
Irradiated SDF-tumor cells support the induction of systemic prophylactic and therapeutic immunity.
(A) C57BL/6 mice (10 mice/group) were vaccinated intravenously with irradiated 2 × 105 control C1498 (●) or SDF-C1498 (○) cells and challenged a week later with live 2 × 105 wild-type C1498 cells. Vaccination with SDF-C1498 cells resulted in 90% protection and resistance to wild-type tumor challenge; all the mice in the control group developed lethal leukemia. The graph is representative of 2 independent experiments. (B) C57BL/6 mice (10 mice/group) were vaccinated intradermally in one flank with irradiated 105 control B16F1 (●) or SDF-B16F1 (○) cells and challenged a week later in the opposite flank with live 105 wild-type B16F1 cells. Vaccination with SDF-B16F1 cells resulted in 70% protection of the mice and resistance to wild-type tumor challenge; vaccination with wild-type B16F1 only protected 10% of the mice. The graph is representative of 2 independent experiments. (C) C57BL/6 mice (10 mice/group) were injected intravenously on day 0 with live 2 × 105 wild-type C1498 cells. On day 3, they were vaccinated intravenously with irradiated 105 control (▪) or SDF-C1498 (●) cells. A third group of mice was vaccinated twice (day 3 and 8 after live wild-type tumor inoculation) with 105 SDF-C1498 cells (○). One vaccine on day 3 resulted in 40% leukemia-free survival; vaccines on day 3 and 8 resulted in 30% leukemia-free survival. These results were replicated in a second experiment. (D) C57BL/6 mice (10 mice/group) were injected intradermally on day 0 with live 105 wild-type B16F1 cells and vaccinated on day 3 with irradiated 105 control (▪) or SDF-B16F1 (●) cells. Vaccination with SDF-B16F1 cells resulted in 40% cure of tumors and long-term tumor-free survival. The graph is representative of 2 independent experiments.
Irradiated SDF-tumor cells support the induction of systemic prophylactic and therapeutic immunity.
(A) C57BL/6 mice (10 mice/group) were vaccinated intravenously with irradiated 2 × 105 control C1498 (●) or SDF-C1498 (○) cells and challenged a week later with live 2 × 105 wild-type C1498 cells. Vaccination with SDF-C1498 cells resulted in 90% protection and resistance to wild-type tumor challenge; all the mice in the control group developed lethal leukemia. The graph is representative of 2 independent experiments. (B) C57BL/6 mice (10 mice/group) were vaccinated intradermally in one flank with irradiated 105 control B16F1 (●) or SDF-B16F1 (○) cells and challenged a week later in the opposite flank with live 105 wild-type B16F1 cells. Vaccination with SDF-B16F1 cells resulted in 70% protection of the mice and resistance to wild-type tumor challenge; vaccination with wild-type B16F1 only protected 10% of the mice. The graph is representative of 2 independent experiments. (C) C57BL/6 mice (10 mice/group) were injected intravenously on day 0 with live 2 × 105 wild-type C1498 cells. On day 3, they were vaccinated intravenously with irradiated 105 control (▪) or SDF-C1498 (●) cells. A third group of mice was vaccinated twice (day 3 and 8 after live wild-type tumor inoculation) with 105 SDF-C1498 cells (○). One vaccine on day 3 resulted in 40% leukemia-free survival; vaccines on day 3 and 8 resulted in 30% leukemia-free survival. These results were replicated in a second experiment. (D) C57BL/6 mice (10 mice/group) were injected intradermally on day 0 with live 105 wild-type B16F1 cells and vaccinated on day 3 with irradiated 105 control (▪) or SDF-B16F1 (●) cells. Vaccination with SDF-B16F1 cells resulted in 40% cure of tumors and long-term tumor-free survival. The graph is representative of 2 independent experiments.
Next we examined the effect of irradiated SDF-tumor cells on the growth of already established tumors. In the first series of experiments, mice were injected with live wild-type C1498 cells (2 × 105cells/mouse) or live wild-type B16F1 cells (105cells/mouse), and 3 days later were injected with irradiated SDF-C1498 cells or SDF-B16F1 (106 cells/mouse). Control mice were injected with irradiated control tumor cells. In this protocol, although SDF vaccines resulted in delayed growth of established tumors as compared to control vaccines, they were unable to inhibit the development of lethal tumors (data not shown). Based on the failure of the protocol to cure any mice and on a recent report that SDF-1 has a concentration-dependent bifunctional effect on T-cell chemotaxis (attracting at low concentration and repulsing at high concentration),27 we speculated that the failure of the vaccines might have been the use of high numbers of irradiated tumor cells. Therefore, in a second series of experiments, mice were vaccinated with 105 irradiated SDF-C1498 cells, either once (day 3 after tumor inoculation) or twice (day 3 and 8 after tumor inoculation). As shown in Figure 3C, 40% of mice that had received one vaccination developed therapeutic immunity and rejected their leukemia (P = .09 versus control mice). The repeat of vaccination on day 8 did not enhance antitumor responses, because only 30% of the mice vaccinated twice rejected their tumors. Similar results were obtained in the B16F1 model, in which vaccines with irradiated 105 SDF-B16F1 cells on day 3 after tumor inoculation resulted in 40% tumor rejection (Figure 3D). Overall, these results demonstrate that SDF-mediated immune responses led to both protective and therapeutic systemic immunity; however, experiments on therapeutic vaccines suggest that these immune responses might be tightly regulated by the local SDF-1 levels.
SDF-tumor rejection supports the development of antitumor memory T cells
It has been previously shown that the mechanisms responsible for tumor rejection in cytokine gene immunotherapy may not be the same as those required for immune memory.28-30 Furthermore, the immune mechanisms necessary for maintenance of the antitumor CTL memory are not clearly established. To address the issue of CTL memory development and persistence in our SDF-1 studies, we challenged C57BL/6 mice with wild-type C1498 cells 3 or 4 months after the rejection of live SDF-C1498 cells. As shown in Figure4A, 40% (3 months) and 50% (4 months) of mice that rejected SDF-secreting tumors had generated a sufficient memory response to resist this tumor challenge (P = .0001 versus control mice). The resistance to challenge was C1498 leukemia-specific, because when the resistant mice were challenged with live wild-type B16F1 melanoma cells, all mice developed lethal tumors (data not shown). CTL memory responses were also studied in the B16F1 model, in which 40% of mice that had rejected SDF-B16F1 cells were able to reject a live wild-type challenge given a month later (data not shown).
CTL memory development and persistence.
(A) SDF-tumor rejection supports the development of antitumor memory T cells. C57BL/6 mice (10 mice/group) were challenged intravenously with 2 × 105 wild-type C1498 cells 3 (●) or 4 months (▴) after the rejection of live SDF-C1498 cells. Naive C57BL/6 mice were used as controls (▪). Both groups had delayed tumor growth, as compared to control animals, and 40% (3 months) and 50% (4 months) of the mice in the 2 groups were resistant to the challenge (P = .0001 versus control). The graph is representative of 2 independent experiments. (B) 51Cr release CTL assays. Spleens were collected from mice 11 weeks after SDF-B16F1 tumor inoculation/rejection and splenocytes were cocultured with irradiated B16F1 cells as described in “Materials and methods.” Six days later, splenocytes were harvested and used as effector cells in CTL assays. 51Cr-labeled B16F1 (H-2d) or control allogeneic TSA (H-2b) tumor cells were used as targets in the standard 4-hour CTL assays. Splenocytes from mice that had rejected SDF-B16F1 tumors lysed syngeneic B16F1 but not allogeneic TSA cells. The results are representative of 2 independent experiments. (C) CD4+ cells are indispensable for SDF-mediated tumor rejection. C57BL/6 mice were depleted of CD4+ (●) or CD8+ (▴) T cells, as described in “Materials and methods.” Control mice were treated with PBS (▪). Three days after the last injection the mice were injected intravenously with 2 × 105 live SDF-C1498 cells and antibody injections continued every 5 days for 3 weeks. All the mice treated with PBS and 80% of the mice treated with anti-CD8+ mAb rejected the SDF-C1498 cells and did not develop any signs of leukemia. Depletion of CD4+ T cells resulted in 100% lethal leukemia (P = .0001 versus control PBS).
CTL memory development and persistence.
(A) SDF-tumor rejection supports the development of antitumor memory T cells. C57BL/6 mice (10 mice/group) were challenged intravenously with 2 × 105 wild-type C1498 cells 3 (●) or 4 months (▴) after the rejection of live SDF-C1498 cells. Naive C57BL/6 mice were used as controls (▪). Both groups had delayed tumor growth, as compared to control animals, and 40% (3 months) and 50% (4 months) of the mice in the 2 groups were resistant to the challenge (P = .0001 versus control). The graph is representative of 2 independent experiments. (B) 51Cr release CTL assays. Spleens were collected from mice 11 weeks after SDF-B16F1 tumor inoculation/rejection and splenocytes were cocultured with irradiated B16F1 cells as described in “Materials and methods.” Six days later, splenocytes were harvested and used as effector cells in CTL assays. 51Cr-labeled B16F1 (H-2d) or control allogeneic TSA (H-2b) tumor cells were used as targets in the standard 4-hour CTL assays. Splenocytes from mice that had rejected SDF-B16F1 tumors lysed syngeneic B16F1 but not allogeneic TSA cells. The results are representative of 2 independent experiments. (C) CD4+ cells are indispensable for SDF-mediated tumor rejection. C57BL/6 mice were depleted of CD4+ (●) or CD8+ (▴) T cells, as described in “Materials and methods.” Control mice were treated with PBS (▪). Three days after the last injection the mice were injected intravenously with 2 × 105 live SDF-C1498 cells and antibody injections continued every 5 days for 3 weeks. All the mice treated with PBS and 80% of the mice treated with anti-CD8+ mAb rejected the SDF-C1498 cells and did not develop any signs of leukemia. Depletion of CD4+ T cells resulted in 100% lethal leukemia (P = .0001 versus control PBS).
The persistence of memory CTLs was also evaluated with in vitro51Cr release assays. Spleens from C57BL/6 mice that had rejected SDF-B16F1 or SDF-C1498 cells 3 months earlier were removed and assayed for in vitro tumor-specific CTL activity. In both models, splenocytes from immunized but not naive mice generated a cytolytic response on stimulation with wild-type tumor cells. As shown in the B16F1 model in Figure 4B, the response was B16F1 (H-2b)-specific because the same cells did not lyse alloantigen-presenting TSA (H-2d) cells. These results suggest that the immune mechanisms mediating rejection of live SDF-tumor cells can support the development of long-lived tumor-specific memory cells.
CD4+ cells are required for SDF-mediated tumor rejection
Previous work has demonstrated that rejection of tumor cells requires both CD4+ and CD8+ T cells or CD4+ cells alone.30-33 To delineate the T-cell subsets involved in SDF-mediated priming and effector phase of antitumor responses, we inoculated SDF-C1498 cells into mice depleted in vivo of CD4+ or CD8+ T cells as described in “Materials and methods.” The C1498 tumor model was selected because of the reproducibly high percentage (90%-100%) of animals rejecting tumors following injection with tumor cells expressing SDF-1. As shown in Figure 4C, 100% of the mice treated with 1 × PBS and 80% of the mice treated with anti-CD8+ mAb rejected the SDF-C1498 cells and did not develop any signs of leukemia. In contrast, the depletion of CD4+ T cells resulted in lethal leukemia in 100% of the mice injected with SDF-C1498 (P = .0001 versus control PBS). Although this result does not address the issue of the importance of CD4+ T cells in the generation and maintenance of antitumor memory CTL, it clearly demonstrates that CD4+ cells are absolutely necessary during the priming/effector phase of antitumor T-cell responses.
Scid mice do not reject SDF-tumors
In addition to in vivo depletion experiments, we studied the pattern of in vivo SDF-tumor growth in immunodeficient mice. In these experiments, SDF-C1498 (Figure 5A) or SDF-B16F1 (Figure 5B) cells were injected into C57BL/6 mice carrying the scid (no T cells or B cells) mutation. In both tumor models, SDF-transduced and control cells grew in all scidanimals, indicating that T cells are indispensable for the elimination of SDF-secreting tumors. Because SDF-1β was originally described as B-cell growth factor,2 and the humoral immune response to tumor-associated antigens has been demonstrated in animals and humans, we tested the growth of SDF-tumor cells in B-cell–deficient mice. Experiments in both models demonstrated that 70% to 80% of B-cell–deficient mice injected with either control or SDF-tumor cells rejected their tumors (data not shown). These results are consistent with earlier observations that B cells can inhibit the generation of T-cell–dependent antitumor responses,34 and also demonstrate that B cells are not required for the rejection of SDF-tumor cells.
Scid mice do not reject SDF-tumors.
(A) C57BL/6 scidmice (10 mice/group) were injected intravenously with 2 × 105 control C1498 (▪) or SDF-C1498 (✖) cells. Naive C57BL/6 mice injected with 2 × 105 control C1498 (●) or SDF-C1498 (▴) cells were used as control. Naive mice injected with SDF-C1498 cells rejected their tumor; all other groups of mice developed lethal leukemia. The results are representative of 2 separate experiments. (B) C57BL/6 scid mice (10 mice/group) were injected intradermally with 105 control B16F1 (▪) or SDF-B16F1 (●) cells. Control naive C57BL/6 mice were injected with 105 SDF-B16F1 (▴) cells. Forty percent of naive animals rejected SDF-B16F1 tumors and remained tumor-free. Both groups ofscid animals developed lethal tumors. Similar results were obtained in a second experiment.
Scid mice do not reject SDF-tumors.
(A) C57BL/6 scidmice (10 mice/group) were injected intravenously with 2 × 105 control C1498 (▪) or SDF-C1498 (✖) cells. Naive C57BL/6 mice injected with 2 × 105 control C1498 (●) or SDF-C1498 (▴) cells were used as control. Naive mice injected with SDF-C1498 cells rejected their tumor; all other groups of mice developed lethal leukemia. The results are representative of 2 separate experiments. (B) C57BL/6 scid mice (10 mice/group) were injected intradermally with 105 control B16F1 (▪) or SDF-B16F1 (●) cells. Control naive C57BL/6 mice were injected with 105 SDF-B16F1 (▴) cells. Forty percent of naive animals rejected SDF-B16F1 tumors and remained tumor-free. Both groups ofscid animals developed lethal tumors. Similar results were obtained in a second experiment.
T cells infiltrate in vivo growing SDF-B16F1 but not wild-type B16F1 tumors
To further characterize the immune cells that participate in tumor rejection, we did a series of histology-immunohistochemistry studies during the in vivo growth of wild-type B16F1 and SDF-B16F1, as described in “Materials and methods.” As shown previously in Figure2B, injection of C57BL/6 mice with SDF-B16F1 cells reproducibly resulted in 40% to 50% long-term, tumor-free survival. In the histology study, no animals in the wild-type and SDF-1 groups had clinically palpable tumors on day 3 after tumor inoculation, and microscopically, small clusters of tumor cells were detectable in both groups. SDF-1 tumors had mild ICIs, consisting of lymphocytes and plasma cells, whereas wild-type tumors had minimal ICIs. In both tumors, scattered inflammatory cells (neutrophils, macrophages) were detected (data not shown). On day 7, tumors were not clinically detectable in either group. Microscopically, wild-type tumors had minimal ICIs, whereas SDF-1 tumors had significant ICIs and were infiltrated primarily by CD3+, CD4+, and CD8+ T cells (Figure 6). No major differences in other cell types (B cells, DCs, neutrophils, macrophages) or in the tumor vasculature (as determined by staining for von Willebrand factor) could be identified between wild-type and SDF-1 tumors (data not shown). On day 14, all control animals but few animals with SDF-1 tumors had palpable tumors. Histologically, wild-type animals had very large tumors consisting of numerous tumor cells and minimal ICIs, whereas most SDF-1 animals had only scattered tumor cells or small cell clusters and significant ICIs (data not shown). Interestingly, inflammatory cells (neutrophils and macrophages) were detectable in both groups with relatively fewer cells found in the control animals. Collectively, these studies with tissue collection until day 14 after tumor inoculation found that wild-type tumors grew faster and considerably larger than SDF-1 tumors and that SDF-1 tumors but not wild-type B16F1 tumors were infiltrated by T cells during the early stages of tumor development. The altered pattern of in vivo SDF-tumor growth/rejection could be linked to the degree of T-cell infiltration.
T cells infiltrate SDF-B16F1 but not wild-type B16F1 tumors.
C57BL/6 mice were injected intradermally with wild-type B16F1 or SDF-B16F1 cells and tissues were collected for histology/immunohistochemistry, as described in “Materials and methods.” Tissue sections from samples collected at day 7 after tumor inoculation are shown here. Immune cell infiltrates (CD3, CD4, and CD8 T cells; arrows) are observed prominently in SDF-tumors, but not in wild-type tumors (original magnification × 10).
T cells infiltrate SDF-B16F1 but not wild-type B16F1 tumors.
C57BL/6 mice were injected intradermally with wild-type B16F1 or SDF-B16F1 cells and tissues were collected for histology/immunohistochemistry, as described in “Materials and methods.” Tissue sections from samples collected at day 7 after tumor inoculation are shown here. Immune cell infiltrates (CD3, CD4, and CD8 T cells; arrows) are observed prominently in SDF-tumors, but not in wild-type tumors (original magnification × 10).
Discussion
In this report we demonstrate that the human chemokine SDF-1, secreted at the tumor site by genetically modified murine tumor cells, regulates the in vivo priming and effector phase of immune responses required for successful rejection of tumors and also supports the development of long-lived tumor-specific CTL responses.
Although considerable research has focused on SDF-1/CXCR4 signaling pathways15,16,35,36 and their role in T-cell chemotaxis and stem cell homing, little is known on the role of SDF-1/CXCR4 interactions in regulating T-cell–mediated immune responses. Recently, growing evidence indicates that, in addition to being a T-cell chemoattractant, SDF-1 may also have more fundamental immunoregulatory properties, including enhancement of T-cell proliferation and cytokine secretion,22 and inhibiting activation-induced apoptosis of T cells.23 The results in our study reveal the potential of SDF-1 alone to regulate a complicated network of in vivo immune mechanisms underlying tumor growth and tumor immunosurveillance. Several interesting observations emerge from our experiments. Tumor cells in both models studied had reduced in vivo tumorigenicity, indicating that relatively low levels of SDF-1 are sufficient, or even required, to fuel antitumor immune responses. It is noteworthy that in the study by Nomura et al24 in which 2 immunogenic tumor models were used, transfected tumor cells secreting high levels of SDF-1α were rejected only when they coexpressed either IL-2 or GM-CSF. The notion that high local levels of SDF-1 may actually result in inhibition of T-cell chemoattraction is supported by our observation that vaccinations with low numbers, but not with high numbers of irradiated SDF-tumor cells, were able to cure already established tumors. Further support is provided by previously reported observations that there is a concentration-dependent bidirectional movement of T cells in an in vitro response to SDF-1, and that high levels of SDF-1 reverse the in vivo antigen-induced T-cell migration.27 Here we provide the first evidence that, in addition to T-cell migration, in vivo effector T-cell function, that is, antitumor cytotoxicity, is tightly regulated by local levels of SDF-1.
It is generally accepted that unmodified tumor cells are poor antigen-presenting cells (APCs) because they lack appropriate expression of costimulatory molecules, absolutely required for effective antigen-specific T-cell activation. Immunogenicity of tumor cells can be restored by genetic modification of the cells to express either B7 costimulatory molecules, or potent immunomodulatory cytokines like GM-CSF or IL-12.26,33,37,38 In these studies, rejection of live modified tumors supported the development of long-lived memory responses in 100% of the animals; similarly, vaccinations with irradiated modified tumors resulted in 100% protection against live wild-type challenge, demonstrating that both effector and memory CTL responses were mediated by adaptive immunity. In this report, although 100% of SDF-tumors grew out inscid mice, indicating the pivotal role of T cells in immune mechanisms underlying tumor rejection, rejection of live SDF-tumor cells by immunocompetent mice only conferred 40% to 50% systemic immunity and resistance to wild-type challenge. It is also noteworthy that 100% of CD4+-depleted animals developed lethal tumors (indicating an indispensable role of CD4+ cells during the priming and effector phase of immune responses leading to rejection of live SDF-tumors), whereas only 20% of CD8+-depleted animals developed lethal tumors. The results from both the in vivo depletion and the in vivo challenge (ie, 40%-50% of mice that had rejected SDF-tumors had systemic immunity) raise the possibility that both adaptive (CD4+ T cells, and to a lesser extent CD8+ T cells) and innate (CD4+ natural killer [NK] T cells) immunity may be part of the antitumor immune mechanisms. To our knowledge, there is no information as yet on CXCR4 expression regulation or on the role of SDF-1/CXCR4 interactions in murine CD4+NK T cells. These cells are known to react with the MHC class I–like molecule CD1d, and have been reported as playing a crucial role in the early stages of protective immunity against parasites, and as stimulators of IL-12 production by APCs in the innate immune system.39,40 In addition to our results, 2 recent reports support a putative role of CD4+ NK T cells in the present study. First, LFA-1 (significantly up-regulated on SDF-tumor cells) and intercellular adhesion molecule 1 (ICAM-1) interactions play a pivotal role in CD4+NK T trafficking and homing.41 Second, it has been demonstrated that only transforming growth factor β (TGF-β), known to be secreted by several types of tumor cells, including melanoma cells,42but not other cytokines or chemokines tested, up-regulated expression of CXCR4 on human NK cells.43 Thus, the indispensable role of CD4+ T cells as determined by the in vivo depletion with the use of an anti-CD4 mAb, may be a consequence of eliminating both CD1-restricted CD4+NK T and CD4+ T cells. Future studies with CD1-deficient mice, which also lack CD1-restricted CD4+NK T cells will address the issue of the role of these cells in tumor rejection.
It has been shown that SDF-1 is involved in trafficking and recruitment of several types of DCs, including immature DCs,44 mature DCs,45 and plasmacytoid DCs.46 Whereas immature DCs traffic from blood to tissues where they capture antigens, mature DCs prime naive T cells in draining lymphoid organs. Although our immunohistochemistry did not demonstrate increased numbers of CD11c+ cells in SDF-1 tumors as compared to control tumors, this cannot rule out that DCs may have a certain role in augmenting immune responses against SDF-tumor cells. It is also noteworthy that although SDF-1 has been implicated in regulating angiogenesis21,47 and possibly tumor progression,48 we did not have immunohistochemical evidence for increased numbers of endothelial cells in SDF-B16F1 tumors, which would have been indicative of enhanced SDF-1–induced neovascularization. This may be a result of the specific type of the tumor and strain of mouse (C57BL/6) being studied, as well as the nature of the local immune response.
Previously, it has been reported that recombinant SDF-1 increases activation marker expression by anti-CD3–activated CD4+ T cells in vitro and enhances their proliferation and cytokine production, including IL-2 and interferon γ.22 We have observed in in vitro experiments that SDF-tumor cells, but not control tumor cells, significantly stimulate proliferation of naive syngeneic T cells (K.D.-J., unpublished observations, February 2001). Extrapolation of these in vitro results to an in vivo setting could mean that, in addition to T-cell chemoattractant properties, local levels of SDF-1β in the tumor microenvironment may have an important role in regulating T-cell proliferative responses. A putative beneficial effect of SDF/CXCR4 interactions on T cells at the tumor site is further supported by a recent report demonstrating that, although signaling of most chemokines induces only transient responses of short duration in human T cells, signaling through CXCR4 is distinct by its capacity to support sustained activation of the kinases ERK-2, phosphatidylinositol 3-kinase, and protein kinase B.35 These kinases have been implicated in cell proliferation, survival, and differentiation.49 50
Thus far, most emphasis in the literature has been directed toward the physiologic role of SDF-1 in migration and homing of hematopoietic progenitor cells. Our results on the potential of SDF-1β alone to regulate in vivo antitumor immunity, together with recent evidence indicating a key role of SDF-1 on regulating rheumatoid inflammation,23 reveal that SDF-1 is a chemokine with a broad range of immunoregulatory properties. Further exploring and understanding the mechanisms controlling tissue-specific CXCR4/SDF-1 interactions and their role in physiologic and disease conditions may lead to novel therapeutic interventions.
The authors thank Teresa S. Hawley for help with retroviral constructs, Dr Joseph Carroll in packaging line development, Laboratory Animal Research staff of Genetics Institute for technical assistance, and Dr Bethany M. Goad for reviewing histology slides.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Kyriaki Dunussi-Joannopoulos, 200 Cambridge Park Dr, Cambridge, MA 02140.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal