Cytokine-mobilized peripheral blood is increasingly used instead of bone marrow as the source of cells for allogeneic transplantation. Although cells lead to faster hematologic recovery, their effects on graft-versus-host disease, relapse, and survival are less certain. Between January 1996 and February 2000, 228 patients with chronic myeloid leukemia, acute myeloid leukemia, or myelodysplasia were randomized to receive either bone marrow or peripheral blood allografts from HLA-matched siblings. All patients received busulfan and cyclophosphamide as conditioning chemotherapy and cyclosporine and methotrexate as graft-versus-host disease prophylaxis. We compared the times to neutrophil and platelet recovery, acute and chronic graft-versus-host disease, relapse, and overall survival between the groups. The median times to neutrophil recovery were 19 days and 23 days and the times to platelet recovery were 16 days and 22 days in the peripheral blood and bone marrow groups, respectively (P < .0001 for both comparisons). The cumulative incidence of grades II to IV acute graft-versus-host disease 100 days after transplantation was 44% in both groups (hazard ratio, 0.99; 95% confidence interval, 0.66-1.49; P > .9), and the incidence of extensive chronic graft-versus-host disease at 30 months after transplantation was 40% with peripheral blood and 30% with bone marrow (hazard ratio, 1.23; 95% confidence interval, 0.78-1.96; P = .37). There was no statistically significant difference in the probability of relapse of the underlying disease between the groups. The probabilities of survival at 30 months after transplantation were 68% and 60% in the peripheral blood and bone marrow groups, respectively (hazard ratio, 0.62; 95% confidence interval, 0.39-0.97; P = .04). In patients with chronic myeloid leukemia, acute myeloid leukemia, and myelodysplasia undergoing allogeneic transplantation from matched siblings, the use of peripheral blood instead of bone marrow leads to faster hematologic recovery, similar risk of graft-versus-host disease, and improved survival.
Introduction
Traditionally, cells for allografting have been harvested directly from the pelvis of donors under general anesthesia. Hematopoietic progenitor cells also circulate in the peripheral blood, and their numbers are increased by administration of cytokines such as granulocyte colony-stimulating factor (G-CSF), allowing collection by leukapheresis. In autologous transplantation, such mobilized blood cells have largely replaced bone marrow as the source of cells for transplantation because their use leads to more rapid neutrophil and platelet recovery1,2 and faster immune reconstitution.3 However, enthusiasm for the adoption of mobilized blood cells for allogeneic transplantation has been tempered by 3 main considerations.4 First, there was concern that the use of cytokines such as G-CSF may cause complications in healthy donors. Second, the peripheral blood harvest contains 10-fold more T cells than bone marrow,5-7 which may be harmful because T cells are the predominant effector cells of graft-versus-host disease (GVHD). Finally, the peripheral blood harvest may contain predominantly committed progenitors and lack the pluripotent stem cells required for long-term hematopoietic engraftment.
These concerns were allayed by initial reports of transplantations using allogeneic peripheral blood cells that indicated that donors tolerated G-CSF well and that patients had sustained neutrophil and platelet recovery with no increase in acute GVHD.8-10These reports have been supported by subsequent studies. Generally, G-CSF has been well tolerated by healthy donors, and long-term complications have not been reported.11,12 However, there have been recent reports of nonfatal splenic rupture in 2 healthy donors receiving G-CSF.13,14 In the first 119 allogeneic peripheral blood transplantations reported, 46 patients (39%) developed grades II to IV acute GVHD.15-19 This incidence is similar to that expected after marrow transplantation. In a large randomized study,20 the incidence of grades II to IV acute GVHD was also similar in the peripheral blood and bone marrow arms (64% versus 57%; P = .36).
Chronic GVHD has been reported to be higher in some but not all studies of allogeneic peripheral blood transplantation.15,18,20-25However, randomized trials20,24-27 have not been powered to detect a difference. A retrospective analysis of 288 peripheral blood and 536 bone marrow human leukocyte antigen (HLA)–identical sibling transplantations reported by the International Bone Marrow Transplant Registry and the European Group for Blood and Marrow Transplantation28 described significantly more chronic GVHD among peripheral blood recipients at 1 year (65% versus 53%, P = .02).
We report the results of a randomized, multicenter study comparing bone marrow and peripheral blood in recipients of matched sibling allogeneic transplants for myeloid malignancies. The study was designed so that the only difference between the arms was the infusion of bone marrow or peripheral blood cells on day 0. All patients received the same conditioning chemotherapy and GVHD prophylaxis regimen. The primary objective of the study was to compare the time to neutrophil recovery in the 2 groups.
Materials and methods
Study design
The study was a randomized phase 3 trial conducted at 8 bone marrow transplantation (BMT) centers in Canada and New Zealand. The trial was approved by the institutional review board at each center, and patients and their donors gave informed consent before randomization. Eligible patients had chronic myeloid leukemia (CML) in chronic or accelerated phase, acute myeloid leukemia (AML) in first or subsequent remission, or myelodysplastic syndrome (MDS) and had an HLA-matched sibling donor. Patients were required to be between the ages of 16 and 65 years old, have an Eastern Cooperative Oncology Group performance status of 0 or 1, and have adequate cardiac function (ejection fraction greater than 50% by radionuclide scan), pulmonary function (carbon monoxide diffusing capacity greater than 50% predicted), and renal function (measured creatinine clearance greater than 60 mL/min). Donors were 5/6 or 6/6 HLA-matched siblings medically fit to undergo bone marrow harvest and apheresis. Patients and donors were excluded if they were positive for HIV antibody or hepatitis B surface antigen or otherwise did not meet the transplantation criteria of the BMT center. Eligible patient–donor pairs were randomized centrally in permuted blocks of 4. Pairs were stratified before randomization by disease (CML, AML, or MDS) and center.
Conditioning regimen and GVHD prophylaxis
Conditioning chemotherapy was administered according to the lesser of the patient's actual or ideal body weight. Patients received busulfan (1 mg/kg orally every 6 hours for 16 doses, day −7 to day −4) followed by cyclophosphamide (60 mg/kg intravenously for 2 days, day −3 and day −2). Phenytoin was given to prevent busulfan-induced seizures, and hyperhydration was given to prevent cyclophosphamide-induced hemorrhagic cystitis.
All patients received cyclosporine and methotrexate as GVHD prophylaxis. Cyclosporine (12.5 mg/kg orally or 5 mg/kg intravenously each day in 2 divided doses) was begun on day −2 and adjusted to maintain trough whole-blood cyclosporine levels between 200 and 400 ng/mL. Methotrexate was administered intravenously on days +1 (15 mg/m2), +3, +6, and +11 (10 mg/m2), with protocol-specified dose reductions for direct hyperbilirubinemia, decreased calculated creatinine clearance, Bearman29 grade 2 or 3 oral mucositis, pleural effusions, or ascites.
Bone marrow and peripheral blood harvest
Donors randomized to bone marrow harvest underwent this procedure on day 0. Under general or regional anesthesia, bone marrow was aspirated from the posterior iliac crests until more than 2 × 108 nucleated cells per kilogram patient weight but less than 22 mL/kg donor weight of bone marrow was obtained. The bone marrow harvest was depleted of red cells by apheresis when there was a major ABO incompatibility between donor and patient and infused on day 0.
Donors randomized to peripheral blood harvest received G-CSF (filgrastim) subcutaneously for 4 consecutive days (days −5 to −2) according to their weight (less than 60 kg, 300 μg/d; 60-90 kg, 480 μg/d; more than 90 kg, 600 μg/d). On days −2 and −1, donors underwent 10- to 12-L aphereses using peripheral venous access where possible. Red cell depletion was not carried out. The apheresis products were stored at 4°C without agitation, and both products were infused into the patient on day 0. If fewer than 2.5 × 106 CD34+ cells/kg patient weight were collected after 2 aphereses, donors also underwent a bone marrow harvest on day 0. In these cases, both the peripheral blood collections and the bone marrow cells were infused on day 0.
Supportive care
While in the hospital, patients were cared for in single rooms equipped with high-efficiency particulate air filtration from day 0 until neutrophil recovery. Patients received transfusions of irradiated red blood cells and platelets to maintain their hemoglobin above 80 g/L and platelets above 10 × 109/L, respectively. Patients seronegative for cytomegalovirus (CMV) antibody whose donors were CMV-seronegative received CMV-negative red blood cell and platelet transfusions. Patients received low-dose standard heparin (100 U · kg−1 · d−1 either as a continuous intravenous infusion or in 2 divided subcutaneous doses every 12 hours) starting prior to the first dose of conditioning chemotherapy and continuing until day +28 or first hospital discharge as hepatic veno-occlusive disease prophylaxis.30
Broad-spectrum antibiotics were initiated at the first episode of neutropenic fever. Prophylactic ciprofloxacin (500 mg orally twice daily) during neutropenia was used at some centers according to local institutional policy. Patients at risk of herpes simplex virus infection received prophylactic low-dose acyclovir (400 mg orally or 80 mg intravenously twice daily from day 0 until day +28). Posttransplantation antifungal prophylaxis and growth factors to promote hematologic recovery were not routinely used. All patients received trimethoprim–sulfamethoxazole as Pneumocystis carinii pneumonia (PCP) prophylaxis from neutrophil recovery until at least 6 months after transplantation. Patients unable to tolerate trimethoprim–sulfamethoxazole received alternate PCP prophylaxis according to local institutional policy. Patients at risk for CMV disease underwent either preemptive therapy (surveillance bronchoscopy at days +35 and/or +49, followed by treatment with ganciclovir for patients whose bronchoalveolar lavage was positive for CMV by shell vial culture) or prophylactic therapy (ganciclovir from neutrophil recovery until day +100 for all patients at risk of CMV disease). At one center, some patients received conditioning chemotherapy, transplantation, and initial posttransplantation care on an outpatient basis.
Laboratory analysis
The nucleated cells, CD34+ cells, and T cells (CD3+) of each bone marrow and peripheral blood collection were measured and expressed per kilogram patient weight. The nucleated cells were enumerated by automated cell counter or manually using a counting chamber, CD34+ cells were enumerated according to the International Society of Hematotherapy and Graft Engineering methodology,31 and T cells were enumerated by flow cytometry.
Study end points
The primary end point was the time to neutrophil recovery. Neutrophil recovery was defined as the second of 2 consecutive days with an absolute neutrophil count greater than 0.5 × 109/L. The null hypothesis assumed no difference in time to neutrophil recovery between the treatment groups. We sought to reject this hypothesis in favor of the alternative hypothesis of a 7-day difference in neutrophil recovery for patients assigned to peripheral blood compared with those assigned to bone marrow. It was assumed that the median time to neutrophil recovery for patients assigned to bone marrow was 20 days. Furthermore, using a 2-sided alpha of 0.05 with 0.80 power, and assuming an accrual period of 1 year and a follow-up period of 100 days, 178 patients allocated in a 1:1 ratio were required to detect this difference.32 No interim analysis was planned. After the study began, the sample size was increased to have sufficient power to detect a 20% absolute difference in the incidence of chronic GVHD. Following publication of the preliminary results of the randomized study by Bensinger et al,33 an interim analysis was undertaken using a Pocock stopping boundary,34 and accrual to the study was stopped in February 2000. This report includes data on all 228 patients enrolled to February 2000 with follow-up data to February 2001.
Secondary end points of the study included time to platelet recovery, outcomes related to hematologic recovery, acute GVHD, chronic GVHD, relapse, and survival. Platelet recovery was defined as the third of 3 consecutive days with a platelet count greater than 20 × 109/L and independence of platelet transfusion for 7 days. Outcomes related to hematologic recovery were number of red blood cell and platelet transfusions during the first 60 days after transplantation, number of febrile days during the first 30 days after transplantation, number of days on nonprophylactic antibiotics from day 0 until first discharge, number of days in the hospital from day 0 until first discharge, and number of days in the hospital during the first 100 days after transplantation. Acute GVHD was evaluated according to standard criteria.35 Patients who survived until at least day +14 were evaluable for acute GVHD. Chronic GVHD was evaluated according to standard criteria.36 Deaths were classified as due to either relapse of the underlying disease or nonrelapse causes.
Statistical analysis
The cumulative incidences of neutrophil and platelet recovery, grades II to IV and grades III to IV acute GVHD, overall and extensive chronic GVHD, relapse of disease, and nonrelapse mortality were computed according to the method described by Kalbfleisch and Prentice.37 Estimates of overall survival were calculated using the method of Kaplan and Meier.38 The 2 treatment groups were assessed for statistically significant differences of these end points using the likelihood ratio χ2 statistic derived from stratified (by disease type and center) Cox proportional hazards models.39 Hazard ratios from these models and their 95% confidence intervals were used to describe the relative effectiveness of the 2 treatment groups. Differences in day +30 and day +100 mortality between the treatment groups were assessed for statistical significance using a z statistic with correction for continuity.40 The statistical significance of the difference in CD34+cells/kg collected during the first and second apheresis was evaluated using the Wilcoxon signed rank-sum test.40 Differences in outcomes between the treatment groups related to hematologic recovery were assessed using the Wilcoxon rank sum test. Where appropriate, all statistical comparisons used the intention-to-treat principle. All reported P values are 2-sided.
Results
Two hundred twenty-eight patient–donor pairs were randomized between January 1996 and February 2000 at 7 BMT centers in Canada and 1 in New Zealand. One pair was ineligible and excluded from analysis because the donor was the father of the patient. The remaining 227 pairs are the subject of this report. One patient who did not undergo transplantation was lost to follow-up within 1 week of randomization. The minimum follow-up of all remaining surviving patients was 12 months from randomization, with a median follow-up of 32.8 months (range, 12-61 months).
Of the 227 eligible patients, 109 (48%) were randomized to peripheral blood and 118 (52%) were randomized to bone marrow. The treatment groups were well balanced with respect to baseline characteristics of patients and donors (Table 1).
Donor and patient characteristics according to transplantation arm
| . | Bone marrow (n = 118) . | Peripheral blood (n = 109) . |
|---|---|---|
| Donor age, y | 44 (13-74) | 43 (17-67) |
| Patient age, y | 44 (19-64) | 45 (19-64) |
| CMV-seronegative patient and donor | 40 (34%) | 30 (28%) |
| CML | 55 (47%) | 54 (49%) |
| CP-1 | 49 (42%) | 46 (42%) |
| > CP-1, AP | 6 (5%) | 8 (7%) |
| AML | 43‡ (36%) | 39‡ (36%) |
| CR-1 | 33 (28%) | 29 (27%) |
| > CR-1 | 9 (8%) | 9 (8%) |
| MDS | 20 (17%) | 16 (15%) |
| Early disease* | 87 (74%) | 78 (72%) |
| Advanced disease† | 31 (26%) | 31 (28%) |
| . | Bone marrow (n = 118) . | Peripheral blood (n = 109) . |
|---|---|---|
| Donor age, y | 44 (13-74) | 43 (17-67) |
| Patient age, y | 44 (19-64) | 45 (19-64) |
| CMV-seronegative patient and donor | 40 (34%) | 30 (28%) |
| CML | 55 (47%) | 54 (49%) |
| CP-1 | 49 (42%) | 46 (42%) |
| > CP-1, AP | 6 (5%) | 8 (7%) |
| AML | 43‡ (36%) | 39‡ (36%) |
| CR-1 | 33 (28%) | 29 (27%) |
| > CR-1 | 9 (8%) | 9 (8%) |
| MDS | 20 (17%) | 16 (15%) |
| Early disease* | 87 (74%) | 78 (72%) |
| Advanced disease† | 31 (26%) | 31 (28%) |
Data are presented as median (range) or n (%).
CP-1 indicates first chronic phase; AP, accelerated phase; and CR-1, first complete remission.
Early disease: AML CR-1, CML CP-1, refractory anemia, and refractory anemia with ringed sideroblasts.
Advanced disease: AML beyond CR-1, CML beyond CP-1 and AP, refractory anemia with excess blasts, and refractory anemia with excess blasts in transformation.
One patient with AML for whom disease status at transplantation was not known.
Peripheral blood and bone marrow harvests
The characteristics of the peripheral blood and bone marrow harvests are shown in Table 2. Most patients randomized to peripheral blood (95 of 109; 87%) received peripheral blood alone. One patient randomized to peripheral blood received bone marrow alone and 2 did not undergo transplantation. Eleven patients randomized to peripheral blood received peripheral blood and bone marrow, 10 because fewer than 2.5 × 106CD34+ cells/kg were collected with 2 aphereses, as specified in the protocol. One patient received peripheral blood and bone marrow even though more than 2.5 × 106CD34+ cells/kg were collected with 2 aphereses; this was a protocol violation.
Bone marrow and peripheral blood harvest characteristics according to transplantation arm
| . | Bone marrow (n = 118) . | Peripheral blood (n = 109) . | ||
|---|---|---|---|---|
| First collection . | Second collection . | Total . | ||
| Volume, mL | 1000 | 138 | 131 | 283 |
| (50-1616) | (50-1150) | (37-381) | (115-1334) | |
| CD34+ cells/kg patient | 2.4 | 2.5 | 3.7 | 6.4 |
| weight × 106 | (0.1-14.3) | (0.1-16.1) | (0.1-18.7) | (0.7-32.0) |
| CD3+ cells/kg patient | 0.3 | 1.9 | 1.7 | 3.7 |
| weight × 108 | (0.01-1.6) | (0.3-27.1) | (0.02-6.0) | (1.2-30.8) |
| . | Bone marrow (n = 118) . | Peripheral blood (n = 109) . | ||
|---|---|---|---|---|
| First collection . | Second collection . | Total . | ||
| Volume, mL | 1000 | 138 | 131 | 283 |
| (50-1616) | (50-1150) | (37-381) | (115-1334) | |
| CD34+ cells/kg patient | 2.4 | 2.5 | 3.7 | 6.4 |
| weight × 106 | (0.1-14.3) | (0.1-16.1) | (0.1-18.7) | (0.7-32.0) |
| CD3+ cells/kg patient | 0.3 | 1.9 | 1.7 | 3.7 |
| weight × 108 | (0.01-1.6) | (0.3-27.1) | (0.02-6.0) | (1.2-30.8) |
Data are presented as median (range).
Among the 10 donors from whom fewer than 2.5 × 106CD34+ cells/kg were collected by apheresis, the median number of cells collected was 1.9 × 106CD34+ cells/kg (range, 0.7-2.4 × 106). The median CD34+cells/kg collected with the first apheresis was 2.5 × 106 CD34+ cells/kg (range, 0.1-16.1 × 106), compared with 3.7 × 106CD34+ cells/kg (range, 0.1-18.7 × 106) with the second apheresis (P < .0001). In 48 of 93 (52%) peripheral blood donors, more than 2.5 × 106CD34+ cells/kg were collected during the first apheresis. Three donors required placement of a central venous catheter to facilitate the peripheral blood collections. All donors tolerated the apheresis procedures well, with mild to moderate bone pain and flulike symptoms. None experienced a serious adverse event or required hospitalization.
Most patients randomized to bone marrow (115 of 118; 97%) received bone marrow alone. Two patients randomized to bone marrow received peripheral blood alone, and one did not undergo transplantation.
Hematologic recovery
The median times to neutrophil recovery were 19 days (range, 12-35 days) and 23 days (range, 13-68 days) in the peripheral blood and bone marrow groups, respectively (hazard ratio, 0.45; 95% confidence interval, 0.33-0.62; P < .0001) (Figure1A). Eight patients died prior to neutrophil recovery, 3 in the peripheral blood group and 5 in the bone marrow group. The median times to platelet recovery were 16 days (range, 0-100 days) and 22 days (range, 0-100 days) in the peripheral blood and bone marrow groups, respectively (hazard ratio, 0.46; 95% confidence interval, 0.34-0.62; P < .0001) (Figure 1B). Seventeen patients died prior to platelet recovery, 4 in the peripheral blood group and 13 in the bone marrow group. There were statistically significant differences in the number of platelet transfusions, days on nonprophylactic antibiotics during the first hospitalization, and duration of the first hospitalization favoring the peripheral blood group (Table 3).
Hematologic recovery by transplantation arm.
(A) Neutrophil recovery. (B) Platelet recovery.
Hematologic recovery by transplantation arm.
(A) Neutrophil recovery. (B) Platelet recovery.
Secondary outcomes related to hematologic recovery according to transplantation arm
| . | Bone marrow (n = 118) . | Peripheral blood (n = 109) . | P3-150 . |
|---|---|---|---|
| Red blood cell transfusions3-151 | 6 (0-74) | 4 (0-53) | .23 |
| Platelet transfusions3-151 | 6 (0-107) | 3 (0-74) | < .0001 |
| Febrile days during first 30 days after transplantation | 4 (0-30) | 3 (0-23) | .66 |
| Days on nonprophylactic antibiotics from day 0 to first discharge | 17 (1-127) | 14 (3-59) | .001 |
| Days in hospital from day 0 to first discharge | 28 (13-156) | 25 (9-82) | .0006 |
| Days in hospital during first 100 days after transplantation | 32 (0-100) | 28 (16-90) | .09 |
| . | Bone marrow (n = 118) . | Peripheral blood (n = 109) . | P3-150 . |
|---|---|---|---|
| Red blood cell transfusions3-151 | 6 (0-74) | 4 (0-53) | .23 |
| Platelet transfusions3-151 | 6 (0-107) | 3 (0-74) | < .0001 |
| Febrile days during first 30 days after transplantation | 4 (0-30) | 3 (0-23) | .66 |
| Days on nonprophylactic antibiotics from day 0 to first discharge | 17 (1-127) | 14 (3-59) | .001 |
| Days in hospital from day 0 to first discharge | 28 (13-156) | 25 (9-82) | .0006 |
| Days in hospital during first 100 days after transplantation | 32 (0-100) | 28 (16-90) | .09 |
Data are presented as median (range).
Wilcoxon rank sum test.
Number of transfusions during the first 60 days after transplantation. One red blood cell transfusion refers to one unit of packed red blood cells. One platelet transfusion refers to 4 to 6 pooled random donor units or one apheresis unit.
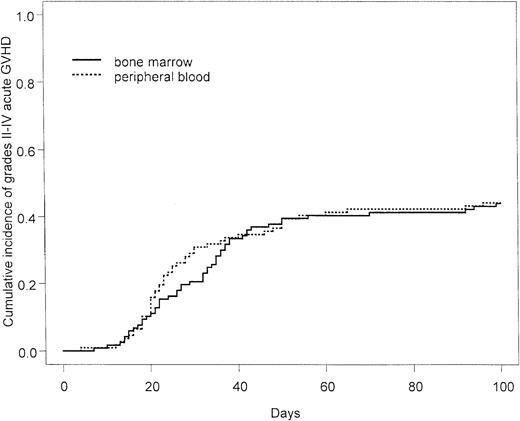
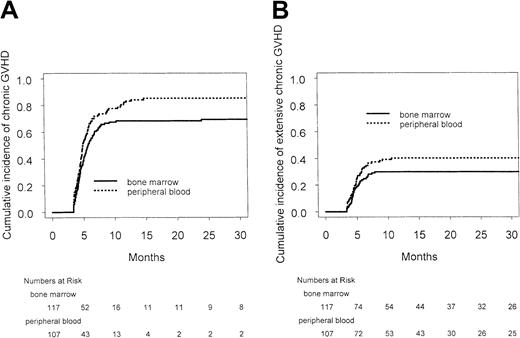
There was no difference in the cumulative incidence or severity of acute GVHD. The cumulative incidences of grades II to IV acute GVHD at day +100 after transplantation were 51 of 117 (44%) and 47 of 107 (44%) in the peripheral blood and bone marrow groups, respectively (hazard ratio, 0.99; 95% confidence interval, 0.66-1.49;P > .9) (Figure 2), and the cumulative incidences of grades III to IV acute GVHD at day +100 after transplantation were 28 of 107 (26%) and 21 of 117 (18%) in the peripheral blood and bone marrow groups, respectively (hazard ratio, 1.48; 95% confidence interval, 0.83-2.62; P = .18). The cumulative incidences of chronic GVHD at 30 months after transplantation were 85% and 69% in the peripheral blood and bone marrow arms, respectively, and the corresponding cumulative incidences of extensive chronic GVHD at 30 months after transplantation were 40% and 30% in the peripheral blood and bone marrow arms, respectively (Figure 3). Although there was a trend to more overall and extensive chronic GVHD among patients randomized to peripheral blood, this was not statistically significant (hazard ratio for overall chronic GVHD, 1.09; 95% confidence interval, 0.79-1.49;P = .62; hazard ratio for extensive chronic GVHD, 1.23; 95% confidence interval, 0.78-1.96; P = .37).
Rates of chronic GVHD by transplantation arm.
(A) Chronic GVHD by transplantation arm. (B) Extensive chronic GVHD by transplantation arm.
Rates of chronic GVHD by transplantation arm.
(A) Chronic GVHD by transplantation arm. (B) Extensive chronic GVHD by transplantation arm.
Nonrelapse mortality, relapse, and survival
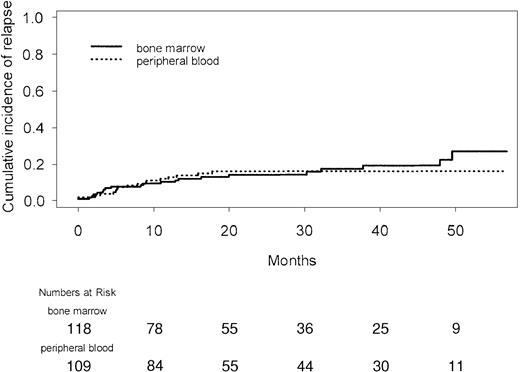
Overall survival was improved in recipients of peripheral blood transplants. This benefit was seen early; the actuarial probability of death at day +30 was 2.8% for patients randomized to peripheral blood and 7.6% for those randomized to bone marrow (P = .18). At day +100, the actuarial probabilities of death were 7.4% and 16.1%, respectively (P = .07). The benefit in overall survival was due to a reduction in nonrelapse deaths (Figure4) in the peripheral blood arm, with no difference between the groups in early or late relapses (Figure 5) or deaths in relapse (Table 4).
Relapse and nonrelapse mortality according to transplantation arm
| . | Bone marrow (n = 118) . | Peripheral blood (n = 109) . |
|---|---|---|
| Total number of deaths | 49 | 33 |
| Number of deaths in relapse | 11 | 10 |
| Number of nonrelapse deaths | 38 | 23 |
| Days 0-30 | 9 | 3 |
| Days 31-100 | 7 | 4 |
| After day 100 | 22 | 16 |
| . | Bone marrow (n = 118) . | Peripheral blood (n = 109) . |
|---|---|---|
| Total number of deaths | 49 | 33 |
| Number of deaths in relapse | 11 | 10 |
| Number of nonrelapse deaths | 38 | 23 |
| Days 0-30 | 9 | 3 |
| Days 31-100 | 7 | 4 |
| After day 100 | 22 | 16 |
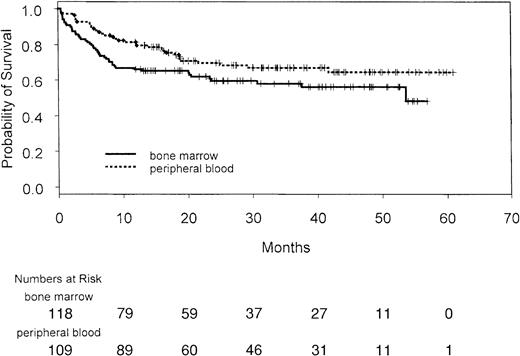
With a median follow-up of 32.8 months (range, 12-61 months), the overall survival of patients randomized to peripheral blood was statistically significantly better than for those randomized to bone marrow (Figure 6). The estimated probability of survival at 30 months after transplantation was 68% in the peripheral blood group and 60% in the bone marrow group (hazard ratio, 0.62; 95% confidence interval, 0.39-0.97;P = .04). Although the study was not powered for subgroup analysis, among the 3 disease groups for which there had been prospective stratification, there was a benefit in overall survival favoring peripheral blood for patients with CML (Figure7A) and a trend favoring peripheral blood for patients with MDS (Figure 7C), but not for those with AML (Figure7B). The interaction between disease type and treatment was not statistically significant (P = .18). In a post hoc analysis, patients were grouped retrospectively into those with early disease (first chronic phase CML, first remission AML, refractory anemia, and refractory anemia with ringed sideroblasts) and those with advanced disease. The overall survival of patients with early disease was not different between the groups (Figure 7D); however, there was an overall survival benefit in patients with advanced disease favoring the peripheral blood group (Figure 7E). The interaction between disease stage and treatment was not statistically significant (P = .11). There was no disease subgroup for which peripheral blood transplantation was associated with poorer overall survival.
Overall survival of all patients by treatment arm. Vertical lines indicate individual patients.
Overall survival of all patients by treatment arm. Vertical lines indicate individual patients.
Overall survival by treatment arm according to disease subgroups.
Vertical lines indicate individual patients. (A) CML. (B) AML. (C) MDS. (D) Early disease: first chronic phase CML, first remission AML, refractory anemia, refractory anemia with ringed sideroblasts. (E) Advanced disease: CML beyond first chronic phase, AML beyond first remission, refractory anemia with excess blasts, refractory anemia with excess blasts in transformation.
Overall survival by treatment arm according to disease subgroups.
Vertical lines indicate individual patients. (A) CML. (B) AML. (C) MDS. (D) Early disease: first chronic phase CML, first remission AML, refractory anemia, refractory anemia with ringed sideroblasts. (E) Advanced disease: CML beyond first chronic phase, AML beyond first remission, refractory anemia with excess blasts, refractory anemia with excess blasts in transformation.
Discussion
In this trial of allografting for myeloid malignancies, patients randomized to receive peripheral blood had significantly better overall survival compared with those randomized to receive bone marrow. This benefit was due to lower nonrelapse mortality. Similar to results in autologous transplantation1,2 and other randomized allogeneic studies,20 24-27 the use of peripheral blood cells led to faster neutrophil and platelet recovery. Some statistically significant differences in secondary outcomes related to hematologic recovery were seen, but more important, the faster hematologic recovery probably accounts for the lower early (before day 30) nonrelapse mortality in the peripheral blood group. Interestingly, we noted a trend toward lower nonrelapse mortality in the 30- to 100-day period as well as beyond 100 days among patients randomized to receive peripheral blood. Given that the cumulative incidence of acute GVHD was similar in the 2 groups, the reduction in nonrelapse deaths is most likely due to the effects of earlier hematologic recovery and/or earlier immune reconstitution. Faster hematologic recovery may lead to an earlier return to health and allow patients to withstand subsequent complications, thereby reducing late mortality.
Compared with bone marrow, peripheral blood harvests contain approximately 10-fold more T cells, which are important effectors of GVHD. Our study confirms the observation made by Bensinger et al20 that this does not lead to more acute GVHD. The observation that acute GVHD is not increased despite the 10-fold higher number of T cells in peripheral blood may be related to the use of G-CSF. G-CSF directs activated T cells to a Th2 type (secreting interleukin 4 [IL-4] and IL-10) and away from a Th1 response (secreting IL-2 and γ-interferon) that promotes acute GVHD.41,42 An alternative theory is that while a threshold dose of T cells is required for acute GVHD to develop, additional T cells beyond this level do not lead to a further increase in acute GVHD. The observation that allogeneic peripheral blood transplants are not associated with more acute GVHD may not extend to chronic GVHD. In accord with other studies,15,18,20 23 we noted a trend toward a higher cumulative incidence of chronic GVHD among peripheral blood recipients. Chronic GVHD is a serious long-term consequence of allogeneic transplantation, and further follow-up of patients in this and other randomized studies will be necessary to determine how this affects late mortality and quality of life.
We did not observe a difference in relapse in the 2 treatment groups, despite a trend toward an increased incidence of chronic GVHD among peripheral blood recipients. In the hypothesis-generating subgroup analysis, there was a survival benefit of peripheral blood transplantation for patients with CML and those with advanced disease. Among the myeloid malignancies, CML is most likely to benefit from a graft-versus-leukemia effect, and therefore the survival benefit gained by the use of peripheral blood cells in this subgroup might have been expected to result from fewer relapse deaths. However, this was not the case, and the benefit resulted from fewer nonrelapse deaths. The difference in survival between the treatment arms was most striking for patients with advanced disease. Bensinger et al43 have also reported an overall survival benefit of peripheral blood transplantation in patients with advanced disease. Patients with advanced disease have greater difficulty tolerating the early complications of allografting and appear to benefit from the faster hematologic recovery associated with the use of blood cells. However, it is not clear why patients with CML had lower mortality with peripheral blood transplantation but patients with AML did not.
Our study differs from other reported randomized trials in 2 important respects. First, all patients received the same conditioning chemotherapy and GVHD prophylaxis. Second, donors received only 4 days of low-dose G-CSF prior to leukapheresis. The median dose of G-CSF in this study was 6.2 μg/kg/d for 4 days, compared with 10 to 16 μg/kg/d for 5 days used in other randomized studies. All donors in this study were required to undergo 2 apheresis procedures, and on the first day of apheresis, donors had received only 3 doses of G-CSF. This probably explains why the median CD34+ cell counts in this study are less than those reported by others. Although only a randomized study can compare the effects of different mobilization strategies on donors and patients, the approach used in this study was well tolerated by donors and permitted the collection of an adequate harvest in most cases. Although the failure to collect more than 2.5 × 106 CD34+ cells/kg with 2 aphereses in 10% of donors may be considered high, this threshold would have been achieved in most donors with a third apheresis. Alternatively, a lower CD34+ threshold could have been accepted.
In conclusion, our study demonstrates that the use of allogeneic peripheral blood cells rather than bone marrow leads to better overall survival in recipients of matched sibling allografts for myeloid malignancies. Patients assigned to receive peripheral blood had faster hematologic recovery, and there was no subgroup of patients for whom peripheral blood transplantation was associated with increased mortality compared with bone marrow. However, statistically significant improvement in survival was restricted to patients with CML and those with advanced disease. A potential disadvantage of the use of peripheral blood allografts may be an increased likelihood of chronic GVHD, and, in this regard, long-term follow-up is required. Further work is needed to determine which particular groups of patients benefit from this approach to allografting.
This study was undertaken under the auspices of the Canadian Bone Marrow Transplant Group (CBMTG). We gratefully acknowledge the patients, donors, and staff of the BMT Programs who participated in this study. We thank Ms Anthea Lau for excellent coordination of data management and Ms Isabel Cameron for her secretarial assistance.
Prepublished online as Blood First Edition Paper, May 17, 2002; DOI 10.1182/blood-2002-01-0048.
S.C. and D.R.S. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Stephen Couban, Rm 417, Bethune Bldg, Queen Elizabeth II Health Sciences Centre, 1278 Tower Rd, Halifax, Nova Scotia, Canada B3H 2Y9; e-mail: scouban@is.dal.ca.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal