The ets transcription factor, TEL, undergoes chromosomal rearrangements with the tyrosine kinase JAK2. TEL-JAK2 is constitutively active, confers cell line factor independence, and activates signal transducer and activator of transcription-1 (STAT1), STAT3, and STAT5. Data from bone marrow transplantation models suggest that STAT5 activation does not account for the entire disease phenotype induced by TEL-JAK2. This study examined additional signaling pathways that are activated by TEL-JAK2. TEL-JAK2 expression in Ba/F3 cells results in constitutive association and tyrosine phosphorylation of Shc and Ship-1 and, consequently, recruitment of Grb2 to TEL-JAK2. Direct Grb2 recruitment is also possible because a putative Grb2 binding site, Tyr314, is present on TEL-JAK2(5-19) and TEL-JAK2(5-12). Studies with a TEL-JAK2(5-19)Tyr314Phe mutant support a role for Tyr314 in Grb2 recruitment, because Grb2 association with TEL-JAK2(5-19)Tyr314Phe is significantly reduced. Interestingly, TEL-JAK2(5-19)Tyr314Phe shows reduced Ras activation when compared with TEL-JAK2(4-17), TEL-JAK2(5-12), and TEL-JAK2(5-19). Analysis of extracellular signal–regulated kinase-1/2 (ERK1/2), stress-activated protein/Jun kinase (SAPK/JNK), and p38 demonstrates the activation of SAPK/JNK and phosphorylation of p38 by all TEL-JAK2 isoforms. TEL-JAK2(5-12) and TEL-JAK2(5-19) preferentially phosphorylate ERK2, whereas TEL-JAK2(4-17) phosphorylated ERK2 at lower levels. Inhibition studies demonstrated that ERK1/2 activation was necessary for Ba/F3 factor independence mediated by TEL-JAK2(5-19), while inhibition of SAPK/JNK or p38 activity had no effect. Our data reveal the requirement of ERK activation by TEL-JAK2(5-19) in Ba/F3 cells and suggest that TEL-JAK2 leukemogenic potential may be mediated in part through ERK1/2.
Introduction
The activation of signaling pathways elicits a variety of biologic responses, including cell cycle progression, cellular differentiation, and apoptosis. The deregulation of certain signaling pathways may cause malignant transformation. One mechanism of deregulation results from a chromosomal rearrangement leading to the generation of activated tyrosine kinase fusion proteins. For example, the fusion of the oligomerization domain from BCR to the tyrosine kinase domain of ABL generates an oncoprotein possessing constitutive enzymatic activity. BCR-ABL is associated with chronic myelogenous leukemia.1 Expression of BCR-ABL results in the activation of a number of signaling pathways, including signal transducer and activator of transcription-1 (STAT1),2-4STAT3,4 STAT5,2-5 Ras,6stress-activated protein/Jun kinase (SAPK/JNK),7 and phosphatidylinositol-3 kinase (PI-3K) pathways.8Furthermore, the activation of Ras,6 Raf-1,9PI-3K,8 protein kinase B (PKB),8 and the transcription factors Myc10 and c-Jun7 have been shown to be required for BCR-ABL–mediated transformation. In addition to BCR-ABL, other chromosomal translocations that generate activated tyrosine kinase fusions have been described and implicated in leukemogenesis. A significant number of these rearrangements fuse theets transcription factor, TEL, with receptor and nonreceptor tyrosine kinases, including TEL-PDGFβR,11 TEL-ABL,12,13TEL-ARG,14 TEL-TRKC,15,16 and TEL-JAK2.17 18
Many cytokine and growth factor receptors, upon ligand binding, directly recruit the Grb2-Sos complex, thereby localizing the receptor complex within proximity of Ras. Sos facilitates the exchange of GDP for GTP on Ras, leading to its activation. In its GTP-bound form, Ras can elicit a variety of cellular responses by activating downstream signaling pathways, including the extracellular signal–regulated kinase-1 (ERK1) and ERK2 cascade and the PI-3K pathway. Alternatively, the Grb2-Sos complex can be brought to an activated receptor by indirect recruitment through the inositol phosphatase, Ship-1,19 the adaptor molecule, Shc,20,21 or the tyrosine phosphatase, Shp2.21,22 The BCR-ABL fusion protein can directly recruit Grb2-Sos via tyrosine 177 (Tyr177) on BCR-ABL23-25 or indirectly through Shc,24,25 Ship-1,26Shp2,27 or Cbl.28-32 Recently, data emerging from BCR-ABL bone marrow transplantation models suggest an important role for Tyr177 in myeloid disease progression.33-35 The requirement for Ras activation in BCR-ABL–mediated transformation has also been demonstrated from studies utilizing dominant negative Ras overexpression.6
Several other activated tyrosine kinase fusions, including TEL-ABL, TEL-PDGFβR, and TEL-JAK2 also activate STAT transcription factors. More specifically, STAT1 and STAT5 activation has been frequently implicated in BCR-ABL,2-5 TEL-ABL,36TEL-PDGFβR,37,38 and TEL-JAK2 18,39,40signaling, while STAT3 activation has been observed in cells expressing BCR-ABL or TEL-JAK2.4,40 The importance of STAT5 activation has been addressed for the TEL-JAK2 fusion protein in a bone marrow transplantation model using STAT5a and STAT5b (STAT5a/b)–deficient bone marrow. Wild-type mice, undergoing transplantation with bone marrow expressing TEL-JAK2, developed a fatal mixed myeloproliferative and lymphoproliferative disease.39,41 Transplantation of bone marrow cells deficient in STAT5a/b and transduced with TEL-JAK2 protected recipient mice from disease, thereby demonstrating the requirement for STAT5a/b in TEL-JAK2–mediated leukemogenesis. However, the activation of STAT5a/b is not sufficient for the development of the mixed myeloproliferative and lymphoproliferative disease because transplantation with bone marrow cells expressing constitutively active STAT5a resulted in only a myeloproliferative phenotype.41In addition, overexpression of the STAT5 target, oncostatin M, resulted in a myelofibrotic phenotype, indicating that reconstitution of one STAT5a/b target gene is insufficient to induce leukemia induced by TEL-JAK2 in this model system.41
These transplantation models demonstrate that STAT5a/b activation does not recapitulate the entire disease phenotype induced by TEL-JAK2. Because many growth factors, cytokines, and tyrosine kinase fusions, like BCR-ABL, recruit Grb2-Sos, we were interested in determining whether Grb2-Sos could be recruited to TEL-JAK2 and in examining the activation of downstream mitogen-activated protein kinase (MAPK) family members.
Materials and methods
Generation of TEL-JAK2 constructs
Three TEL-JAK2 isoforms were constructed utilizing sequences from TEL (kindly provided by Dr Gary Gilliland, Harvard Institutes of Medicine, Boston, MA) and JAK2 (kindly provided by Dr Peter Marynen, Catholic University of Leuven, Belgium) by standard molecular biologic techniques. Both strands of TEL and JAK2 subjected to polymerase chain reaction were sequenced to confirm the fidelity of the sequence and conservation of reading frame at the site of fusion.
Tyrosine 314 (Tyr314) was mutated to phenylalanine using the QuikChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA) as specified by manufacturer protocol.
Cell lines and culture
Ba/F3 cells were cultured as described.40Ba/F3 cell lines expressing pcDNA3-TEL-JAK2 constructs were maintained in 1 mg/mL G418 (Gibco). Stable expression of TEL-JAK2 constructs was obtained by electroporation of TEL-JAK2 constructs into Ba/F3 cells.39 Individual G418-resistant subclones were isolated by limiting dilution. Expression of TEL-JAK2 was confirmed by immunoprecipitation (IP) and immunoblot analysis.
Antibodies
Antibodies against c-Myc, phosphorylation-specific ERK1/2, Grb2, glutathione S-transferase (GST), Ras, and Ship-1 were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). A Shc antibody was obtained from BD Transduction Laboratories (Mississauga, Ontario, Canada), and ERK1/2 and monoclonal antiphosphotyrosine (pTyr) antibodies were purchased from Upstate Biotechnology (Lake Placid, NY). Phosphorylation-specific antibody against p38 was obtained from New England Biolabs (Beverly, MA). A pan-JAK antibody was generated as described.41 An anti-SAPK/JNK antibody has been previously reported.42
IP, in vitro mixing, and Western blotting
Ba/F3 and Ba/F3 TEL-JAK2–expressing cells were depleted of cytokine and stimulated in the presence or absence of interleukin-3 (IL-3).40 Cell lysis was performed in ice-cold lysis buffer containing 50 mM Tris-HCl (pH 8.0), 150 mM NaCl, 1.0% Triton X-100, and a cocktail of protease and phosphatase inhibitors.19 40
For IPs, an optimal amount of antibody was added to 1 to 2 mg of lysate and incubated for 1 hour at 4°C before the addition of 50 μL of protein A–Sepharose 4B beads (Amersham Pharmacia Biotech, Baie d'Urfe, Quebec, Canada) for rabbit polyclonal antibodies or protein G–Sepharose beads (Amersham Pharmacia Biotech) for goat polyclonal antibodies.
A total of 5 μg GST fusion protein bound to glutathione Sepharose beads (Amersham Pharmacia Biotech) was added to 1 to 2 mg of lysate and incubated for 2 hours at 4°C to conduct in vitro mixing experiments. The beads were washed 3 times in ice-cold lysis buffer before elution by boiling in Laemmli sample buffer containing 100 mM dithiothreitol. Protein complexes were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride for immunoblotting (IB). Western blotting was performed as previously described.19 40
Ras assay
TEL-JAK2–expressing cells were washed and replated in media without IL-3 and either 10% fetal bovine serum or 1% bovine serum albumin. Ba/F3-TEL-JAK2 subclones were harvested after overnight incubation without IL-3. For controls, pcDNA3-transfected Ba/F3 cells were stimulated for 10 minutes in media containing no cytokine or IL-3. Lysates were prepared in RIPA buffer containing protease and phosphatase inhibitors described above.
GST-Ras binding domain (RBD) fusion protein (provided by Dr Judy L. Meinkoth, University of Pennsylvania) was incubated with glutathione-agarose beads for 30 minutes and then washed 3 times in RIPA buffer; 1 mg of cell lysate was incubated with GST-RBD glutathione-agarose–conjugated beads for 30 minutes at 4°C. Beads were collected by centrifugation and washed with RIPA buffer. Following SDS-PAGE and transfer to nitrocellulose, a Western blot was performed with the rat monoclonal Ras antibody, Y13-259, followed by horseradish peroxidase antirat immunoglobulin G.
XTT assay
Cell proliferation assays were performed utilizing 2,3-bis (2-methoxy-2-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT) as previously described.42 U0126, SP600125, or SB203580 was added at increasing concentrations to examine effects on IL-3– and factor-independent proliferation.
SAPK/JNK in vitro kinase assay
SAPK/JNK (hereafter referred to as SAPK) was immunoprecipitated from 500 μg of lysate with an antibody against SAPK. Beads were washed 2 times in ice-cold lysis buffer before a final wash in kinase buffer containing 35 mM HEPES (pH 7.4), 20 mM magnesium chloride, 0.1 mM sodium orthovanadate, 2 mM dithiothreitol, and 20 μM adenosine triphosphate. Beads were incubated with 40 μL kinase buffer containing 5 μCi (185 MBq) [γ-32P]–adenosine triphosphate and 3 μg GST–c-Jun at 30°C for 30 minutes. Kinase reactions were resolved by SDS-PAGE and transferred onto nitrocellulose. Radiolabeled proteins were detected by PhosphorImager analysis (Amersham Biosciences, Piscataway, NJ).
Results
TEL-JAK2 fusion proteins are constitutively tyrosine phosphorylated
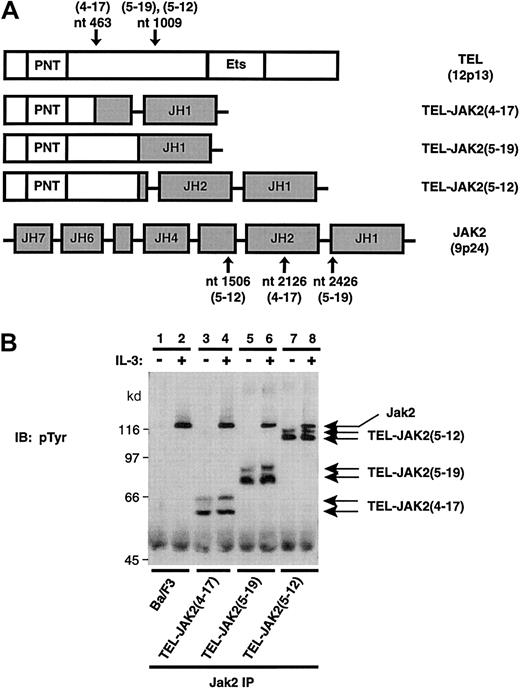
Three TEL-JAK2 fusion proteins that have been characterized from patients who presented with leukemia17,18 (Figure1A) were constructed and expressed in the murine IL-3–dependent hematopoietic cell line, Ba/F3. Subclones expressing each TEL-JAK2 isoform were selected via limiting dilution. The tyrosine phosphorylation status of each TEL-JAK2 fusion was analyzed by performing IPs with a pan-JAK specific antibody43 and phosphotyrosine analysis of Ba/F3 subclones expressing the fusion protein (Ba/F3-TEL-JAK2) (Figure 1B). TEL-JAK2 proteins were constitutively tyrosine phosphorylated in the absence of IL-3 stimulation (Figure 1B, lanes 3, 5, and 7). Furthermore, the levels of TEL-JAK2 tyrosine phosphorylation were not affected following IL-3 stimulation (Figure 1B, lanes 4, 6, and 8). Phosphotyrosine analysis also demonstrated that the expression of TEL-JAK2 in Ba/F3 cells did not affect the inducible phosphorylation of endogenous JAK2 (Figure 1B, lanes 4, 6, and 8). Transfection of the TEL-JAK2 constructs into Ba/F3 cells resulted in the translation of 2 isoforms for each fusion protein. Translation of the smaller forms was likely initiated from an internal translational start site found 127 base pairs downstream of the first translational start site, thereby generating a protein that was approximately 4 kd smaller than the full-length protein.44 Furthermore, Ba/F3-TEL-JAK2 subclones were capable of IL-3–independent growth (data not shown). The expression of TEL-JAK2 fusion proteins resulted in constitutive tyrosine phosphorylation of the fusion proteins and conferred IL-3 independence to Ba/F3 cells.
TEL-JAK2 fusion proteins are constitutively tyrosine phosphorylated in Ba/F3 cells.
(A) The characterized TEL-JAK2 fusions and the wild-type forms of TEL and JAK2. The breakpoints involved in the TEL-JAK2 chromosomal translocations are indicated by arrows. The TEL-JAK2(4-17) translocation fused nucleotide (nt) 463 of TEL to nt 2126 of JAK2, whereas TEL-JAK2(5-19) and TEL-JAK2(5-12) resulted in the fusion of TEL nt 1009 to JAK2 nt 2426 and JAK2 nt 1506, respectively. The 3 fusion proteins also contain a Myc-tag at the carboxy (C-) terminus. (B) Ba/F3 cells expressing the indicated TEL-JAK2 constructs were depleted of cytokine and then stimulated in the presence (+) or absence (−) of 10 ng/mL IL-3. IPs were performed with a pan-JAK antibody, and tyrosine-phosphorylated proteins were detected by IB with pTyr antibody.
TEL-JAK2 fusion proteins are constitutively tyrosine phosphorylated in Ba/F3 cells.
(A) The characterized TEL-JAK2 fusions and the wild-type forms of TEL and JAK2. The breakpoints involved in the TEL-JAK2 chromosomal translocations are indicated by arrows. The TEL-JAK2(4-17) translocation fused nucleotide (nt) 463 of TEL to nt 2126 of JAK2, whereas TEL-JAK2(5-19) and TEL-JAK2(5-12) resulted in the fusion of TEL nt 1009 to JAK2 nt 2426 and JAK2 nt 1506, respectively. The 3 fusion proteins also contain a Myc-tag at the carboxy (C-) terminus. (B) Ba/F3 cells expressing the indicated TEL-JAK2 constructs were depleted of cytokine and then stimulated in the presence (+) or absence (−) of 10 ng/mL IL-3. IPs were performed with a pan-JAK antibody, and tyrosine-phosphorylated proteins were detected by IB with pTyr antibody.
TEL-JAK2 fusion proteins constitutively tyrosine-phosphorylate Ship-1 and associate with the SH2 domain of Ship-1
Growth factors and cytokines activate Ras through the recruitment of Grb2-Sos. For example, BCR-ABL has also been shown to recruit Grb2 through Tyr177,23 and phosphorylation of this residue is necessary for the in vivo development of myeloid leukemia.33-35 Furthermore, studies in our laboratory have demonstrated indirect mechanisms for recruitment of Grb2-Sos to the erythropoietin receptor via the Src homology domain 2 (SH2) inositol phosphatase, Ship-1,19 or the adaptor protein, Shc.21 Thus, the ability of TEL-JAK2 to recruit and tyrosine phosphorylate Ship-1 was examined.
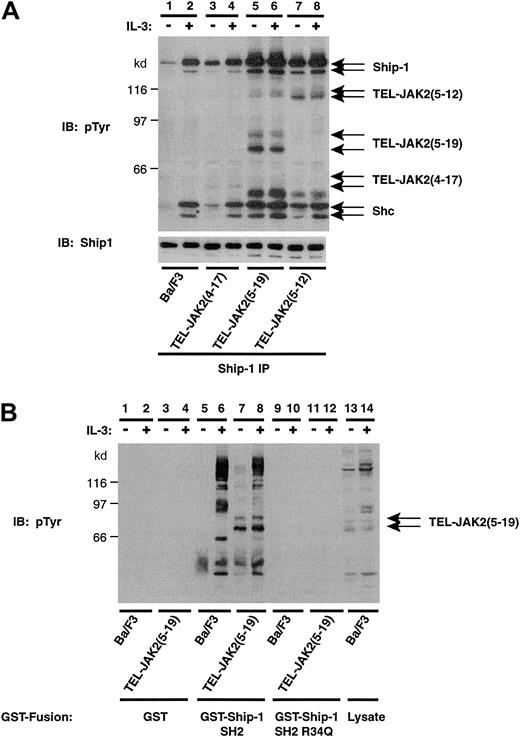
Ship-1 is a 145-kd enzyme that affects inositol lipid turnover.20,26 45-47 This protein contains putative SH3 binding sequences and 2 phosphotyrosine binding (PTB) sites that are recognized by the PTB domain of Shc. The status of Ship-1 tyrosine phosphorylation was analyzed in Ba/F3 cells expressing TEL-JAK2 (Figure2A). IL-3 stimulated the tyrosine phosphorylation of Ship-1 and, when phosphorylated, the 52- and 46-kd isoforms of Shc coimmunoprecipitated with Ship-1 (Figure 2A, lane 2). Ship-1 migrated as 145- and 130-kd proteins, which results from alternative translational initiation. Expression of TEL-JAK2(4-17) (Figure 2A, lane 3), TEL-JAK2(5-19) (Figure 2A, lane 5), or TEL-JAK2(5-12) (Figure 2A, lane 7) resulted in constitutive tyrosine phosphorylation of Ship-1 and association of Shc. Importantly, TEL-JAK2(5-19) (Figure 2A, lanes 5 and 6) and TEL-JAK2(5-12) isoforms (Figure 2A, lanes 7 and 8) coimmunoprecipitated with the Ship-1/Shc complex. Longer exposures also revealed the association of TEL-JAK2(4-17) in this experiment (data not shown). Equivalent Ship-1 levels were observed in all cell lines as determined by reprobing with Ship-1 antibody (Figure 2A, lower panel).
TEL-JAK2 expression results in constitutive tyrosine phosphorylation of Ship-1 and association with the SH2 domain of Ship-1.
(A) Ba/F3 and Ba/F3-TEL-JAK2 cells lines were depleted of cytokine and then stimulated in the presence (+) or absence (−) of IL-3. IPs were performed using a Ship-1 antibody, and tyrosine-phosphorylated proteins were detected by pTyr IB (upper panel). Total Ship-1 was detected by reprobing with a Ship-1 antibody (lower panel). (B) Ba/F3 and Ba/F3-TEL-JAK2(5-19) cells were depleted of cytokine and then stimulated in the presence (+) or absence (−) of IL-3. GST in vitro mixes were performed using 5 μg GST (lanes 1-4), GST–Ship-1 SH2 domain (lanes 5-8), or GST–Ship-1 Arg34Gln (lanes 9-12). Ba/F3 lysates are shown (lanes 13, 14). Tyrosine-phosphorylated proteins were detected by IB with a pTyr antibody.
TEL-JAK2 expression results in constitutive tyrosine phosphorylation of Ship-1 and association with the SH2 domain of Ship-1.
(A) Ba/F3 and Ba/F3-TEL-JAK2 cells lines were depleted of cytokine and then stimulated in the presence (+) or absence (−) of IL-3. IPs were performed using a Ship-1 antibody, and tyrosine-phosphorylated proteins were detected by pTyr IB (upper panel). Total Ship-1 was detected by reprobing with a Ship-1 antibody (lower panel). (B) Ba/F3 and Ba/F3-TEL-JAK2(5-19) cells were depleted of cytokine and then stimulated in the presence (+) or absence (−) of IL-3. GST in vitro mixes were performed using 5 μg GST (lanes 1-4), GST–Ship-1 SH2 domain (lanes 5-8), or GST–Ship-1 Arg34Gln (lanes 9-12). Ba/F3 lysates are shown (lanes 13, 14). Tyrosine-phosphorylated proteins were detected by IB with a pTyr antibody.
To address whether the interaction between Ship-1 and TEL-JAK2 was SH2 dependent, GST in vitro mixing experiments were performed with GST fused to the SH2 domain of Ship-1 or a mutant SH2 domain in which the conserved arginine of the FLVRES motif was mutated to glutamine (Figure 2B). Tyrosine-phosphorylated proteins associated with a GST–Ship-1 SH2 domain fusion (Figure 2B, lanes 5-8), while no association was observed with GST alone (Figure 2B, lanes 1-4). IL-3 stimulation induced tyrosine phosphorylation and binding of a number of phosphoproteins (p120, p110, p97, p66, p56, and p52) to the SH2 domain of Ship-1 (Figure 2B, lane 6). More importantly, TEL-JAK2(5-19) was observed in mixes with Ba/F3-TEL-JAK2(5-19) cell lysate (Figure 2B, lanes 7 and 8). The interaction between TEL-JAK2(5-19) and Ship-1 was SH2 dependent, because a mutation in the SH2 domain abrogated TEL-JAK2(5-19) association (Figure 2B, lanes 11 and 12). Thus, TEL-JAK2 expression in Ba/F3 cells resulted in the SH2-dependent recruitment of Ship-1 to the fusion protein and constitutive tyrosine phosphorylation of Ship-1.
TEL-JAK2 fusions constitutively tyrosine phosphorylate Shc and associate with the SH2 domain of Shc
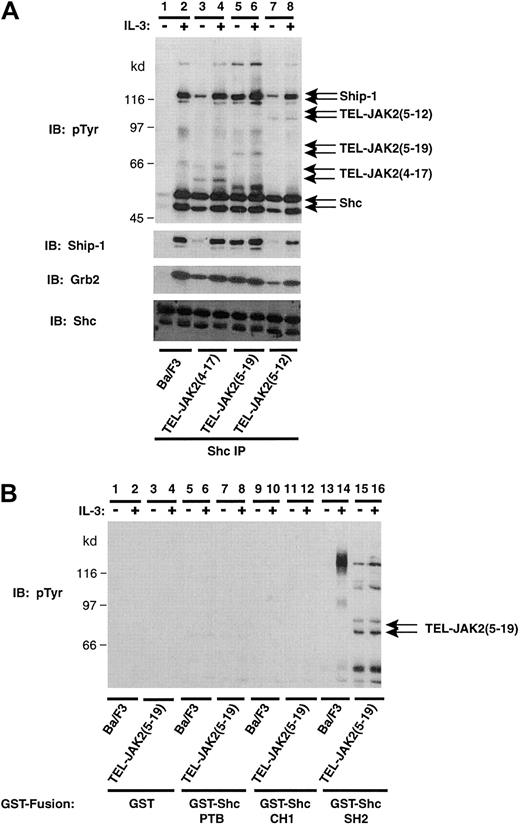
The phosphorylation of Shc following TEL-JAK2 expression was examined further by performing Shc IP and phosphotyrosine analysis (Figure 3A). As described above, IL-3 stimulation of Ba/F3 cells induced tyrosine phosphorylation of Shc (52 and 46 kd) and subsequent co-IP of Ship-1 (Figure 3A, lane 2). All 3 TEL-JAK2 isoforms stimulated constitutive Shc phosphorylation and association of Ship-1 (Figure 3A, lanes 3, 5, and 7). Longer exposure of the phosphotyrosine immunoblot revealed a clear association of TEL-JAK2(4-17) (Figure 3A, lanes 3 and 4), TEL-JAK2(5-19) (Figure 3A, lanes 5 and 6), and TEL-JAK2(5-12) (Figure 3A, lanes 7 and 8) with Shc (data not shown). Importantly, it has been previously shown that Grb2 binds to Tyr239 48,49 and Tyr317 50 of Shc in an SH2-dependent manner, and receptor localization of Grb2 leads to Ras activation. Grb2 was constitutively associated with tyrosine-phosphorylated Shc in all TEL-JAK2–expressing Ba/F3 cells (Grb2 reprobe). Equal amounts of Shc were immunoprecipitated in this experiment (Shc reprobe). These results demonstrated that TEL-JAK2 expression led to constitutive tyrosine phosphorylation and association of Shc and the possible formation of a complex that was composed of TEL-JAK2, Ship-1, Shc, and Grb2.
TEL-JAK2 expression results in constitutive tyrosine phosphorylation of Shc and association with the SH2 domain of Shc.
(A) Ba/F3 and Ba/F3-TEL-JAK2 cell lines were depleted of cytokine and then stimulated in the presence (+) or absence (−) of IL-3. IPs were performed using a Shc antibody, and tyrosine-phosphorylated proteins were detected by pTyr IB (upper panel). Total Ship-1, Grb2, and Shc proteins were detected upon reprobing with Ship-1, Grb2, and Shc antibodies, respectively (lower panels). (B) Ba/F3 and Ba/F3-TEL-JAK2(5-19) cells were depleted of cytokine and then stimulated in the presence (+) or absence (−) of IL-3. GST in vitro mixes were performed using 5 μg GST (lanes 1-4), GST-Shc PTB domain (lanes 5-8), GST-Shc CH1 domain (lanes 9-12), or GST-Shc SH2 domain (lanes 13-16). Tyrosine-phosphorylated proteins were detected by pTyr IB.
TEL-JAK2 expression results in constitutive tyrosine phosphorylation of Shc and association with the SH2 domain of Shc.
(A) Ba/F3 and Ba/F3-TEL-JAK2 cell lines were depleted of cytokine and then stimulated in the presence (+) or absence (−) of IL-3. IPs were performed using a Shc antibody, and tyrosine-phosphorylated proteins were detected by pTyr IB (upper panel). Total Ship-1, Grb2, and Shc proteins were detected upon reprobing with Ship-1, Grb2, and Shc antibodies, respectively (lower panels). (B) Ba/F3 and Ba/F3-TEL-JAK2(5-19) cells were depleted of cytokine and then stimulated in the presence (+) or absence (−) of IL-3. GST in vitro mixes were performed using 5 μg GST (lanes 1-4), GST-Shc PTB domain (lanes 5-8), GST-Shc CH1 domain (lanes 9-12), or GST-Shc SH2 domain (lanes 13-16). Tyrosine-phosphorylated proteins were detected by pTyr IB.
Shc contains a PTB domain and a SH2 domain, both of which interact with tyrosine-phosphorylated proteins. GST in vitro mixes were performed to determine which domain of Shc mediated TEL-JAK2 binding (Figure 3B). Experiments were performed with GST fusions of the PTB domain, collagen homology 1 (CH1) domain, or SH2 domain of Shc. TEL-JAK2(5-19) was found to interact with the SH2 domain of Shc (Figure 3B, lanes 15 and 16) and not with the PTB domain (Figure 3B, lanes 7,8), CH1 domain (Figure 3B, lanes 11 to 12), or GST (Figure 3B, lanes 3,4). The SH2 domain of Shc bound a prominent phosphoprotein of 140 kd in Ba/F3 cells upon IL-3 stimulation (Figure 3B, lane 14) and migrated at the expected molecular weight of the IL-3 βc chain. The expression of TEL-JAK2 in Ba/F3 cells not only induced an interaction between the SH2 domain of Shc and TEL-JAK2 but resulted in constitutive tyrosine phosphorylation of the adaptor molecule.
TEL-JAK2 fusion proteins recruit the Grb2-Sos complex through the SH2 domain of Grb2
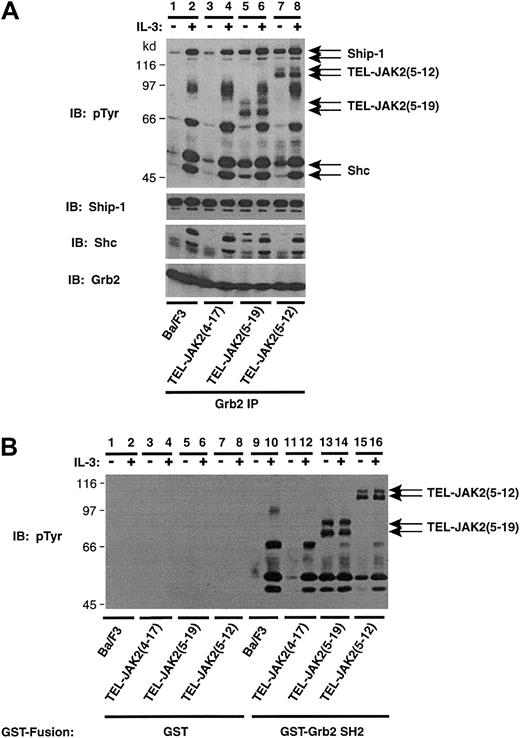
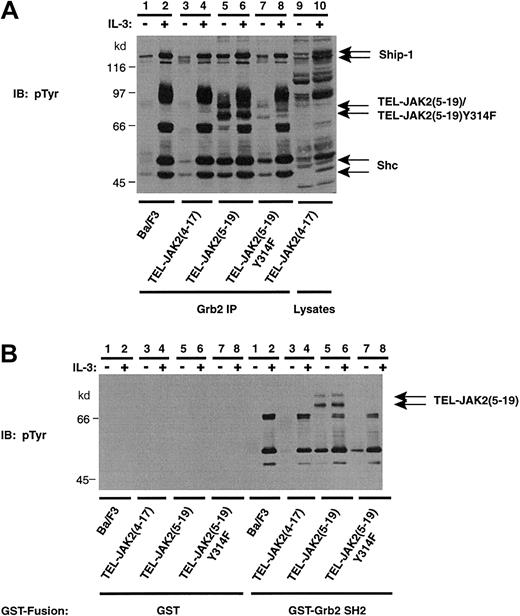
Recruitment of Grb2 to activated growth factor receptors and BCR-ABL occurs through direct and/or indirect mechanisms.24,25,51 Grb2 can bind directly to PTB motifs (pYXN) on receptor cytoplasmic tails and BCR-ABL. Indirect mechanisms involve SH2-dependent binding to the adaptor molecule, Shc,52-55 and the tyrosine phosphatase, Shp2.56-58 An additional mode of indirect recruitment involves SH3-dependent binding of Grb2 to Ship-1.19,59 To identify the protein complexes that associate with Grb2 in TEL-JAK2–expressing cells, Grb2 IPs and phosphotyrosine IB were performed (Figure 4A). IL-3 stimulation resulted in the association of 4 distinct phosphoproteins in Ba/F3 cells, 2 of which were confirmed as Ship-1 and Shc upon reprobing (Figure 4A, upper panel, lanes 1 and 2). The remaining 2 phosphoproteins, p97 and p68, are likely Gab2 (97 kd) and Shp-2 (68 kd), respectively, because IL-3 stimulates the tyrosine phosphorylation of these signaling molecules in Ba/F3 cells.57 60Constitutive Grb2 association with Ship-1 (145 kd) and Shc (52 and 46 kd) was demonstrated in Ba/F3 cells expressing TEL-JAK2(5-19) and TEL-JAK2(5-12) isoforms (Figure 4A, lanes 5 and 7). However, the constitutive association between Grb2, Shc, and Ship-1 in TEL-JAK2(4-17) cells, which was observed upon Shc IP, was not evident in Grb2 immunoprecipitates (Figure 4A, lane 3). Furthermore, co-IP of TEL-JAK2(5-19) (Figure 4A, lanes 5 and 6) and TEL-JAK2(5-12) (Figure4A, lanes 7 and 8) with Grb2 was observed, while co-IP of TEL-JAK2(4-17) was not clearly demonstrated, even following a long exposure (Figure 4A, lanes 3 and 4). A reprobe of the membrane with a Ship-1 antibody revealed constitutive association of Ship-1 in all lysates (Ship-1 reprobe). Equivalent amounts of Grb2 were present in each IP (Grb2 reprobe).
Grb2 constitutively associates with TEL-JAK2 fusions through the SH2 domain of Grb2.
(A) Ba/F3 and Ba/F3-TEL-JAK2 cell lines were depleted of cytokine and then stimulated in the presence (+) or absence (−) of IL-3. IPs were performed using a Grb2 antibody, and tyrosine-phosphorylated proteins were detected by pTyr IB (upper panel). Total Ship-1, Shc, and Grb2 proteins were detected upon reprobing with Ship-1, Shc, and Grb2 antibodies, respectively (lower panels). (B) Ba/F3 and Ba/F3-TEL-JAK2 cell lines were depleted of cytokine and stimulated in the presence (+) or absence (−) of IL-3. GST in vitro mixes were performed using 5 μg GST (lanes 1-8) or GST-Grb2 SH2 domain (lanes 9-16). Tyrosine-phosphorylated proteins were detected by IB with a pTyr antibody.
Grb2 constitutively associates with TEL-JAK2 fusions through the SH2 domain of Grb2.
(A) Ba/F3 and Ba/F3-TEL-JAK2 cell lines were depleted of cytokine and then stimulated in the presence (+) or absence (−) of IL-3. IPs were performed using a Grb2 antibody, and tyrosine-phosphorylated proteins were detected by pTyr IB (upper panel). Total Ship-1, Shc, and Grb2 proteins were detected upon reprobing with Ship-1, Shc, and Grb2 antibodies, respectively (lower panels). (B) Ba/F3 and Ba/F3-TEL-JAK2 cell lines were depleted of cytokine and stimulated in the presence (+) or absence (−) of IL-3. GST in vitro mixes were performed using 5 μg GST (lanes 1-8) or GST-Grb2 SH2 domain (lanes 9-16). Tyrosine-phosphorylated proteins were detected by IB with a pTyr antibody.
GST in vitro mixing experiments performed with the SH2 domain of Grb2 also determined that the interaction between Grb2 and TEL-JAK2 containing TEL exon 5 was SH2 dependent (Figure 4B). No binding of tyrosine-phosphorylated proteins were observed in mixes with GST (Figure 4B, lanes 1-8). TEL-JAK2(5-19) (Figure 4B, lanes 13 and 14) and TEL-JAK2(5-12) (Figure 4B, lanes 15 and 16) associated with the Grb2 SH2 domain; however, TEL-JAK2(4-17) binding was not observed (Figure4B, lanes 11 and 12). Thus, the association between TEL-JAK2 and Grb2 in TEL-JAK2(5-12) and TEL-JAK2(5-19) is mediated by SH2-phosphotyrosine interactions. In TEL-JAK2(4-17), Grb2-Sos appears to associate with the fusion protein indirectly via Ship-1 and Shc, as illustrated in Figures2 and 3.
ERK2 is preferentially phosphorylated by TEL-JAK2 and is required for factor-independent proliferation of TEL-JAK2(5-19)–expressing Ba/F3 cells
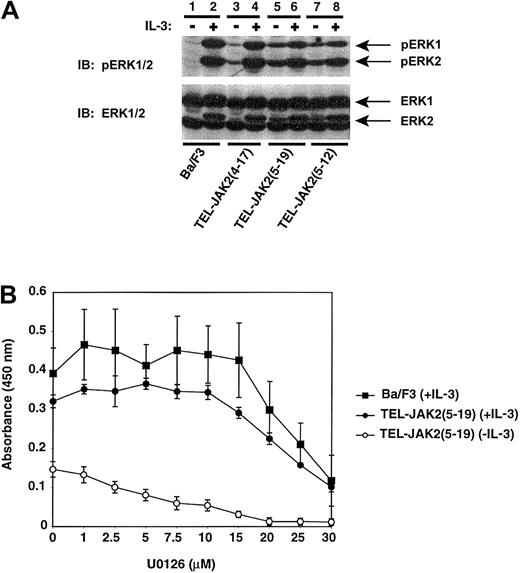
The direct or indirect recruitment of Grb2-Sos to growth factor receptors and activated tyrosine kinases activates the Ras signaling pathway. The activation of Ras then leads to the induction of a number of other pathways, most significantly, the ERK signaling cascade. We examined whether ERK was activated in Ba/F3 cells upon TEL-JAK2 expression. An activation-specific ERK antibody was used to examine phosphorylation at threonine 202 and tyrosine 204 (Thr202/Tyr204) (Figure 5A). ERK1 (44 kd) and ERK2 (42 kd) were both phosphorylated in all cell lines following IL-3 stimulation (Figure 5A, even lanes). However, unstimulated cells expressing TEL-JAK2(5-19) or TEL-JAK2(5-12) demonstrated preferential phosphorylation of ERK2 (Figure 5A, upper panel, lanes 5 and 7). The level of ERK2 phosphorylation by TEL-JAK2(4-17) was significantly lower in the absence of IL-3 stimulation (Figure 5A, lane 3) when compared with TEL-JAK2(5-19) or TEL-JAK2(5-12) (Figure 5A, lanes 5 and 7). A reprobe for total ERK1/2 indicated equivalent protein levels in each lysate sample (Figure 5A, lower panel).
TEL-JAK2 expression results in constitutive phosphorylation of ERK1 and ERK2.
(A) Ba/F3 and Ba/F3-TEL-JAK2 cells were depleted of cytokine and then stimulated in the presence (+) or absence (−) of IL-3. Phosphorylated ERK1 and ERK2 were detected by IB lysates with an antibody specific for phosphorylated ERK1/2 (pERK1/2) (upper panel). Total ERK1/2 was detected upon reprobing with an ERK1/2 antibody (lower panel). (B) U0126 inhibits IL-3 and TEL-JAK2–mediated proliferation. Ba/F3 cells growing in IL-3 (closed squares) and Ba/F3-TEL-JAK2(5-19) cells growing in the presence of IL-3 (closed circles) or in the absence of IL-3 (open circles) were incubated in increasing concentrations of U0126. An XTT assay was then performed.
TEL-JAK2 expression results in constitutive phosphorylation of ERK1 and ERK2.
(A) Ba/F3 and Ba/F3-TEL-JAK2 cells were depleted of cytokine and then stimulated in the presence (+) or absence (−) of IL-3. Phosphorylated ERK1 and ERK2 were detected by IB lysates with an antibody specific for phosphorylated ERK1/2 (pERK1/2) (upper panel). Total ERK1/2 was detected upon reprobing with an ERK1/2 antibody (lower panel). (B) U0126 inhibits IL-3 and TEL-JAK2–mediated proliferation. Ba/F3 cells growing in IL-3 (closed squares) and Ba/F3-TEL-JAK2(5-19) cells growing in the presence of IL-3 (closed circles) or in the absence of IL-3 (open circles) were incubated in increasing concentrations of U0126. An XTT assay was then performed.
To assess the relevance of ERK phosphorylation in TEL-JAK2 transformation of Ba/F3 cells, the pharmacologic inhibitor U0126 was used to inhibit the activity of MAPK kinase 1/2 (MEK1/2) and subsequent phosphorylation of ERK1/2. The ability of TEL-JAK2(5-19) to confer factor-independent proliferation in the presence of U0126 was examined. The growth of Ba/F3 and Ba/F3-TEL-JAK2(5-19) cells was examined after 2 days of growth in media containing varying concentrations of U0126 in the presence or absence of IL-3 (Figure 5B). The addition of U0126 inhibited Ba/F3-TEL-JAK2(5-19) and Ba/F3-TEL-JAK2(4-17) (data not shown) cells growing in IL-3 or in the absence of factor. U0126 also blocked IL-3–dependent growth of Ba/F3 cells. Addition of 5 or 10 μM U0126 inhibited TEL-JAK2–mediated phosphorylation of ERK1/2 (data not shown). In summary, these results indicated that ERK was constitutively phosphorylated in Ba/F3 cells expressing TEL-JAK2 and that activation of ERK was necessary for TEL-JAK2–mediated proliferation.
TEL-JAK2 expression induces p38 phosphorylation, which is not necessary for TEL-JAK2–mediated factor independence of Ba/F3 cells
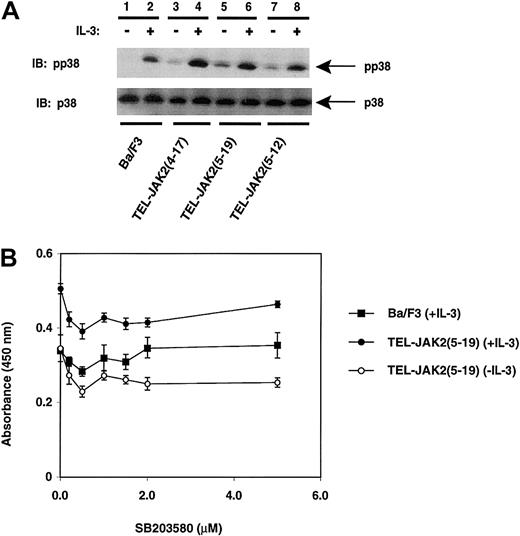
The activation of other MAPKs, such as p38 and SAPK, by TEL-JAK2 expression was also examined. Activation of p38 was detected using a phosphospecific p38 antibody that recognized p38 when phosphorylated at threonine 180 and tyrosine 182 (Thr180/Tyr182) in Western blotting experiments (Figure 6A). Phosphorylation of p38 was induced upon IL-3 stimulation of Ba/F3 cells (lane 2); however, a constitutive level of phosphorylated p38 was observed in unstimulated cells expressing TEL-JAK2 fusion proteins (Figure6A, lanes 3, 5, and 7). This phosphorylation was further increased following IL-3 stimulation (Figure 6A, lanes 4, 6, and 8).
TEL-JAK2 expression results in constitutive phosphorylation of p38.
Ba/F3 and Ba/F3-TEL-JAK2 cell lines were depleted of cytokine and then stimulated in the presence (+) or absence (−) of IL-3. Phosphorylated p38 was detected by IB lysates with a phosphorylation-specific p38 antibody (pp38) (upper panel). Total p38 was detected upon reprobing with a p38 antibody (lower panel). (B) A total of 2000 Ba/F3 cells (closed squares) and Ba/F3-TEL-JAK2(5-19) cells in the absence of IL-3 (open circles) or Ba/F3-TEL-JAK2(5-19) in the presence of IL-3 (closed circles) were seeded into 96-well plates containing media of increasing SB203580 concentration. The cells were incubated for 48 hours at 37°C prior to the addition of XTT and PMS. Absorbance was read at 450 nm following 4 hours of incubation at 37°C.
TEL-JAK2 expression results in constitutive phosphorylation of p38.
Ba/F3 and Ba/F3-TEL-JAK2 cell lines were depleted of cytokine and then stimulated in the presence (+) or absence (−) of IL-3. Phosphorylated p38 was detected by IB lysates with a phosphorylation-specific p38 antibody (pp38) (upper panel). Total p38 was detected upon reprobing with a p38 antibody (lower panel). (B) A total of 2000 Ba/F3 cells (closed squares) and Ba/F3-TEL-JAK2(5-19) cells in the absence of IL-3 (open circles) or Ba/F3-TEL-JAK2(5-19) in the presence of IL-3 (closed circles) were seeded into 96-well plates containing media of increasing SB203580 concentration. The cells were incubated for 48 hours at 37°C prior to the addition of XTT and PMS. Absorbance was read at 450 nm following 4 hours of incubation at 37°C.
Similar to the examination of ERK activation, the importance of p38 activation in TEL-JAK2–mediated proliferation was assessed by inhibiting p38 kinase activity with the pharmacologic inhibitor SB203580 (Figure 6B). Proliferation was monitored after increasing concentrations of SB203580 were added to Ba/F3 cells grown in media containing IL-3 or to Ba/F3-TEL-JAK2(5-19) cells grown in media with or without IL-3. There was no significant reduction in IL-3–mediated or TEL-JAK2–mediated proliferation with increasing amounts of inhibitor, despite inhibition of MAPKAP2 phosphorylation when monitored in a kinase reaction (data not shown). Therefore, although p38 is constitutively phosphorylated upon TEL-JAK2 expression, activation of p38 was not necessary for conferring factor-independent growth of Ba/F3 cells.
TEL-JAK2 expression induces SAPK activation, which is not necessary for TEL-JAK2–mediated factor independence of Ba/F3 cells
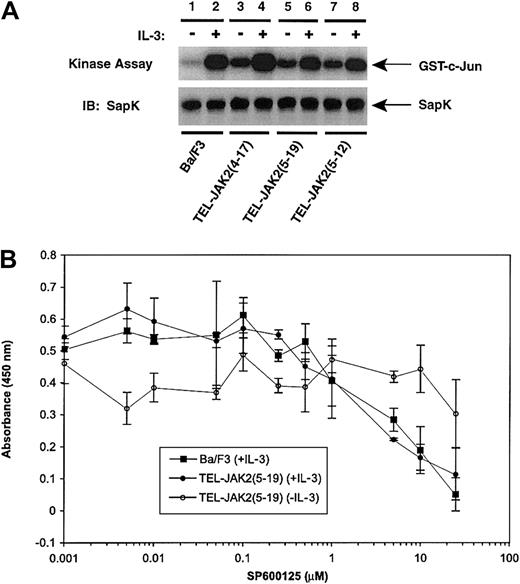
The status of SAPK activity was also of interest, because transformation by BCR-ABL activates SAPK and requires c-Jun for transformation.7 SAPK activation was examined by performing an in vitro kinase assay using GST–c-Jun as a substrate (Figure 7). IL-3 stimulation of Ba/F3 cells induced the activation of SAPK (Figure 7, lane 2). Similarly, expression of all 3 TEL-JAK2 isoforms induced constitutive SAPK activity (Figure 7, lanes 3, 5, and 7). Thus, in addition to ERK and p38, TEL-JAK2 expression in Ba/F3 cells induced the activation of SAPK.
Expression of TEL-JAK2 results in constitutive activation of SAPK/JNK activity.
Ba/F3 and Ba/F3-TEL-JAK2 cell lines were depleted of cytokine and then stimulated in the presence (+) or absence (−) of IL-3. SAPK was immunoprecipitated, and an in vitro kinase assay was performed using GST–c-Jun as substrate. Reaction mixtures were resolved by SDS-PAGE, and radiolabeled proteins were detected by PhosphorImager analysis (upper panel). Total SAPK was detected by IB with a SAPK antibody (lower panel). (B) A total of 2000 Ba/F3 cells (closed squares) and Ba/F3-TEL-JAK2(5-19) cells in the absence of IL-3 (open circle) or Ba/F3-TEL-JAK2(5-19) in the presence of IL-3 (closed circles) were seeded into 96-well plates containing media of increasing SP600125 concentration. The cells were incubated for 48 hours at 37°C prior to the addition of XTT and phenazine methosulfate (PMS). Absorbance was read at 450 nm following 4 hours of incubation at 37°C.
Expression of TEL-JAK2 results in constitutive activation of SAPK/JNK activity.
Ba/F3 and Ba/F3-TEL-JAK2 cell lines were depleted of cytokine and then stimulated in the presence (+) or absence (−) of IL-3. SAPK was immunoprecipitated, and an in vitro kinase assay was performed using GST–c-Jun as substrate. Reaction mixtures were resolved by SDS-PAGE, and radiolabeled proteins were detected by PhosphorImager analysis (upper panel). Total SAPK was detected by IB with a SAPK antibody (lower panel). (B) A total of 2000 Ba/F3 cells (closed squares) and Ba/F3-TEL-JAK2(5-19) cells in the absence of IL-3 (open circle) or Ba/F3-TEL-JAK2(5-19) in the presence of IL-3 (closed circles) were seeded into 96-well plates containing media of increasing SP600125 concentration. The cells were incubated for 48 hours at 37°C prior to the addition of XTT and phenazine methosulfate (PMS). Absorbance was read at 450 nm following 4 hours of incubation at 37°C.
A novel SAPK inhibitor, SP600125, was utilized to examine the significance of SAPK in TEL-JAK2–mediated proliferation (Figure 7B). As described above, an XTT assay was performed with Ba/F3 cells cultured in IL-3 or TEL-JAK2(5-19) cells grown in media with or without IL-3 in the presence of increasing concentrations of SP600125. Interestingly, the SAPK inhibitor affected IL-3–dependent growth of both cell lines while having minimal effects on factor-independent proliferation mediated by TEL-JAK2. Addition of 20 μM SP600125 blocked SAPK-dependent phosphorylation of c-Jun in an in vitro kinase assay (data not shown). SAPK activation contributes to IL-3–dependent proliferation in Ba/F3 cells but is not required for TEL-JAK2–mediated transformation to factor-independent proliferation.
Mutation of Tyr314 to phenylalanine in TEL-JAK2(5-19) does not affect factor independence
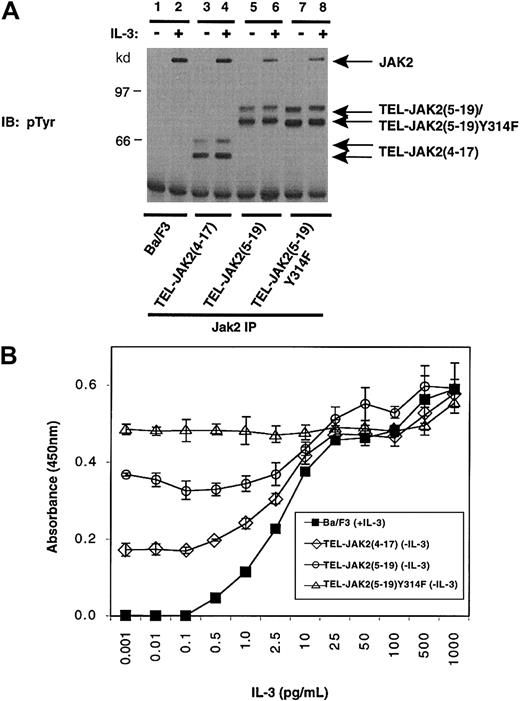
Tyr314 is a putative Grb2 binding site found within exon 5 of TEL. Therefore, this motif is found in TEL-JAK2(5-19) and TEL-JAK2(5-12), but it is not present in TEL-JAK2(4-17). Because Tyr177 in BCR-ABL has been shown to be important in BCR-ABL–mediated leukemogenesis,33-35 its role was examined by mutating Tyr314 in TEL-JAK2(5-19) to phenylalanine (Tyr314Phe). Tyrosine phosphorylation of various TEL-JAK2 isoforms including TEL-JAK2(5-19)Tyr314Phe was examined by phosphotyrosine analysis following IP of the fusion proteins with a pan-JAK antibody (Figure8A). TEL-JAK2(5-19)Tyr314Phe, like TEL-JAK2(4-17) and TEL-JAK2(5-19), was constitutively tyrosine phosphorylated in the absence of IL-3 stimulation (Figure 8A, lanes 3, 5, and 7). However, the migration of the mutant TEL-JAK2 fusion protein appeared to be slightly faster than the wild-type fusion (Figure 8A, lane 7).
Expression of TEL-JAK2(5-19)Tyr314Phe in Ba/F3 cells does not affect factor-independent growth.
(A) Ba/F3 cells expressing the indicated TEL-JAK2 constructs were depleted of cytokine and then stimulated in the presence (+) or absence (−) of 10 ng/mL IL-3. IPs were performed with a pan-Jak antibody, and tyrosine-phosphorylated proteins were detected by IB with pTyr antibody. (B) A total of 2000 Ba/F3 cells (closed squares) and Ba/F3 cells expressing TEL-JAK2(4-17) (open diamonds), TEL-JAK2(5-19) (open circles), and TEL-JAK2(5-19)Tyr314Phe (open triangles) constructs were seeded into 96-well plates containing media of increasing IL-3 concentration. The cells were incubated for 48 hours at 37°C prior to the addition of XTT and PMS. Absorbance was read at 450 nm following 4 hours of incubation at 37°C.
Expression of TEL-JAK2(5-19)Tyr314Phe in Ba/F3 cells does not affect factor-independent growth.
(A) Ba/F3 cells expressing the indicated TEL-JAK2 constructs were depleted of cytokine and then stimulated in the presence (+) or absence (−) of 10 ng/mL IL-3. IPs were performed with a pan-Jak antibody, and tyrosine-phosphorylated proteins were detected by IB with pTyr antibody. (B) A total of 2000 Ba/F3 cells (closed squares) and Ba/F3 cells expressing TEL-JAK2(4-17) (open diamonds), TEL-JAK2(5-19) (open circles), and TEL-JAK2(5-19)Tyr314Phe (open triangles) constructs were seeded into 96-well plates containing media of increasing IL-3 concentration. The cells were incubated for 48 hours at 37°C prior to the addition of XTT and PMS. Absorbance was read at 450 nm following 4 hours of incubation at 37°C.
The effect on factor-independent proliferation of the Tyr314Phe mutation in TEL-JAK2(5-19) was examined (Figure 8B). Expression of TEL-JAK2(4-17) or TEL-JAK2(5-19), as demonstrated earlier, induced factor-independent proliferation of Ba/F3 cells. Factor-independent proliferation of TEL-JAK2(4-17) was reduced when compared with TEL-JAK2(5-19). Similar observations were noted using 6 independently isolated subclones expressing TEL-JAK2(4-17) (data not shown). However, mutation of Tyr314Phe did not affect the factor-independent proliferation of Ba/F3 cells expressing TEL-JAK2(5-19).
Tyr314 is the major mediator of Grb2-Sos recruitment to TEL-JAK2
The significance of Tyr314 in the interaction between TEL-JAK2 and Grb2 was tested. Grb2 IPs were performed and analyzed by phosphotyrosine Western blotting (Figure9A). As demonstrated earlier, TEL-JAK2(5-19) (Figure 9A, lanes 5 and 6) constitutively associates with Grb2, while TEL-JAK2(4-17) binding to Grb2 was not observed (Figure 9A, lanes 3 and 4). Mutation of Tyr314 to phenylalanine in TEL-JAK2(5-19) (Figure 9A, lanes 7 and 8) significantly reduced the binding of TEL-JAK2(5-19)Tyr314Phe to the Grb2 adaptor protein. Ship-1 and Shc, however, remained constitutively tyrosine phosphorylated in unstimulated cells expressing TEL-JAK2(5-19)Tyr314Phe (Figure 9A, lane 7). GST in vitro mixing experiments with the SH2 domain of Grb2 were performed, and phosphotyrosine analysis (Figure 9B) again demonstrated the strong association of TEL-JAK2(5-19) (Figure 9B, lanes 13 and 14) with the SH2 domain of Grb2, while no association with TEL-JAK2(4-17) (Figure 9B, lanes 11 and 12) was observed. Examination of the TEL-JAK2(5-19)Tyr314Phe mutant in in vitro mixing experiments also demonstrated a significant reduction in the association of the fusion protein with the SH2 domain of Grb2 (Figure 9B, lanes 15 and 16). A long exposure of the membrane revealed that some TEL-JAK2(5-19)Tyr314Phe associated with the SH2 domain; however, no association of TEL-JAK2(4-17) could be detected (data not shown). Because TEL-JAK2(4-17) lacks the Grb2 consensus binding sequence, these experiments revealed that the major mechanism of Grb2 recruitment to TEL-JAK2 occurred directly through Tyr314 found in TEL exon 5.
TEL-JAK2(5-19)Tyr314 represents a major binding site for Grb2.
(A) Ba/F3 cells, Ba/F3-TEL-JAK2(4-17), TEL-JAK2(5-19), and TEL-JAK2(5-19)Tyr314Phe cells were depleted of cytokine and then stimulated in the presence (+) or absence (−) of IL-3. IPs were performed using a Grb2 antibody, and tyrosine-phosphorylated proteins were detected by pTyr IB (upper panel). (B) Ba/F3, TEL-JAK2(4-17), TEL-JAK2(5-19), and TEL-JAK2(5-19)Tyr314Phe cells were depleted of cytokine and stimulated with (+) or without (−) IL-3. GST in vitro mixes were performed with 5 μg GST (lanes 1-8) or GST fused to the SH2 domain of Grb2 (lanes 9-16). Tyrosine phosphorylation was assayed by IB with pTyr antibody.
TEL-JAK2(5-19)Tyr314 represents a major binding site for Grb2.
(A) Ba/F3 cells, Ba/F3-TEL-JAK2(4-17), TEL-JAK2(5-19), and TEL-JAK2(5-19)Tyr314Phe cells were depleted of cytokine and then stimulated in the presence (+) or absence (−) of IL-3. IPs were performed using a Grb2 antibody, and tyrosine-phosphorylated proteins were detected by pTyr IB (upper panel). (B) Ba/F3, TEL-JAK2(4-17), TEL-JAK2(5-19), and TEL-JAK2(5-19)Tyr314Phe cells were depleted of cytokine and stimulated with (+) or without (−) IL-3. GST in vitro mixes were performed with 5 μg GST (lanes 1-8) or GST fused to the SH2 domain of Grb2 (lanes 9-16). Tyrosine phosphorylation was assayed by IB with pTyr antibody.
Mutation of TEL Tyr314 is required for full Ras activation in TEL-JAK2(5-19)
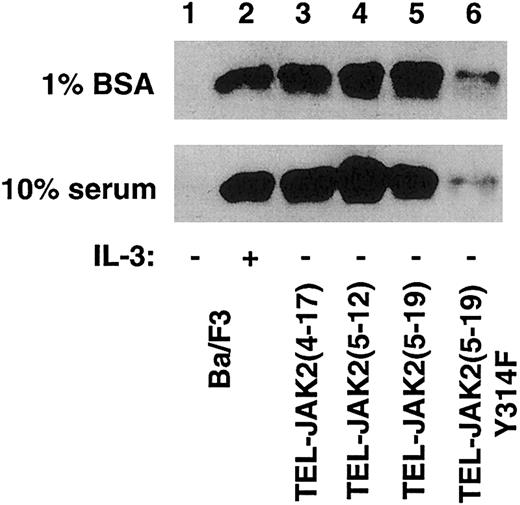
Because Grb2 binding is reduced upon mutation of TEL Tyr314 in the context of TEL-JAK2(5-19), Ras activation was examined in all 3 TEL-JAK2 isoforms as well as TEL-JAK2Tyr314Phe. In vitro binding assays were performed utilizing a GST-Raf1 fusion protein, followed by Western blotting with a monoclonal anti-Ras antibody (Figure10). Cells were cultured in RPMI medium containing 1% bovine serum albumin (Figure 10, upper panel) or 10% serum (Figure 10, lower panel). In both conditions, IL-3 stimulation activated Ras (Figure 10, lane 2) in vector-transfected cells. TEL-JAK2(4-17), TEL-JAK2(5-12), and TEL-JAK2(5-19) all activated Ras in both serum-depleted and serum-containing conditions (Figure 10, lanes 3-5). Mutation of Tyr314 resulted in dramatically lower Ras activation in TEL-JAK2(5-19)Tyr314Phe (Figure 10, lane 6) in both growth conditions. IL-3 resulted in stimulation of Ras in all Ba/F3-TEL-JAK2 subclones (data not shown).
TEL-JAK2(5-19)Tyr314Phe displays impaired Ras activation.
Ba/F3 and the indicated Ba/F3-TEL-JAK2 cell lines were cultured in RPMI containing 1% bovine serum albumin (upper panel) or RMPI supplemented with 10% fetal bovine serum (lower panel). Ba/F3 cells were depleted of cytokine and incubated in the presence or absence of IL-3 prior to lysis. Lysates were mixed with a GST-Raf fusion protein, and an anti-Ras Western blot was performed.
TEL-JAK2(5-19)Tyr314Phe displays impaired Ras activation.
Ba/F3 and the indicated Ba/F3-TEL-JAK2 cell lines were cultured in RPMI containing 1% bovine serum albumin (upper panel) or RMPI supplemented with 10% fetal bovine serum (lower panel). Ba/F3 cells were depleted of cytokine and incubated in the presence or absence of IL-3 prior to lysis. Lysates were mixed with a GST-Raf fusion protein, and an anti-Ras Western blot was performed.
Mutation of TEL Tyr314 does not affect activation of ERK1/2, p38, or SAPK
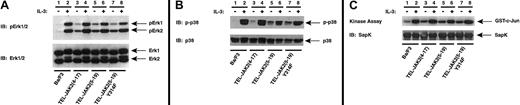
Because Grb2 recruitment leads to Ras and ERK activation, the effect of the Tyr314Phe mutation on ERK, SAPK, and p38 activation was analyzed (Figure 11A-C). The MAPK activation profiles of TEL-JAK2(4-17) and wild-type TEL-JAK2(5-19) were similar, as described earlier. Mutation of Tyr314 to phenylalanine, moreover, had no effect on ERK (Figure 11A, lane 7), p38 phosphorylation (Figure 11B, lane 7), or SAPK activation (Figure 11C, lane 7). Despite the dramatic reduction of Grb2 recruitment to TEL-JAK2(5-19)Tyr314Phe and lower activation of Ras, mutation of the putative Grb2 binding site did not affect TEL-JAK2(5-19)–mediated factor independence or MAPK activation.
TEL-JAK2(5-19)Tyr314Phe does not alter constitutive activation of ERK1/2, p38, or SAPK/JNK.
(A) Ba/F3 and the indicated Ba/F3-TEL-JAK2 cell lines were depleted of cytokine and then stimulated in the presence (+) or absence (−) of IL-3. Phosphorylated ERK1 and ERK2 were detected by IB lysates with an antibody specific for phosphorylated ERK1/2 (pERK1/2) (upper panel). Total ERK1/2 was detected upon reprobing (lower panel). (B) The indicated cell lines were depleted of cytokine and then stimulated in the presence (+) or absence (−) of IL-3. Phosphorylated p38 was detected by IB lysates with a phosphorylation-specific p38 antibody (pp38) (upper panel). Total p38 was detected upon reprobing (lower panel). (C) The indicated cell lines were depleted of cytokine and then stimulated in the presence (+) or absence (−) of IL-3. SAPK was immunoprecipitated, and an in vitro kinase assay was performed using GST–c-Jun as substrate. Reaction mixtures were resolved by SDS-PAGE, and radiolabeled proteins were detected by PhosphorImager analysis (upper panel). Total SAPK was detected by IB with a SAPK antibody (lower panel).
TEL-JAK2(5-19)Tyr314Phe does not alter constitutive activation of ERK1/2, p38, or SAPK/JNK.
(A) Ba/F3 and the indicated Ba/F3-TEL-JAK2 cell lines were depleted of cytokine and then stimulated in the presence (+) or absence (−) of IL-3. Phosphorylated ERK1 and ERK2 were detected by IB lysates with an antibody specific for phosphorylated ERK1/2 (pERK1/2) (upper panel). Total ERK1/2 was detected upon reprobing (lower panel). (B) The indicated cell lines were depleted of cytokine and then stimulated in the presence (+) or absence (−) of IL-3. Phosphorylated p38 was detected by IB lysates with a phosphorylation-specific p38 antibody (pp38) (upper panel). Total p38 was detected upon reprobing (lower panel). (C) The indicated cell lines were depleted of cytokine and then stimulated in the presence (+) or absence (−) of IL-3. SAPK was immunoprecipitated, and an in vitro kinase assay was performed using GST–c-Jun as substrate. Reaction mixtures were resolved by SDS-PAGE, and radiolabeled proteins were detected by PhosphorImager analysis (upper panel). Total SAPK was detected by IB with a SAPK antibody (lower panel).
Discussion
The recruitment of the Grb2-Sos complex to BCR-ABL is an important event in the mediation of BCR-ABL transformation. Recruitment of the complex is affected by direct binding to Tyr177 on BCR-ABL or indirectly through an interaction with Shc,24,25,61Ship-1,26 Shp2,27 and Cbl.28 30-32 Similar to BCR-ABL, TEL-JAK2 fusions contain putative PTB motifs for Shc, Ship-1, and Shp2; however, only the recruitment and tyrosine phosphorylation of Shc and Ship-1 were examined in this study. We have demonstrated that TEL-JAK2 expression, like BCR-ABL expression, results in the constitutive association and constitutive tyrosine phosphorylation of Shc and Ship-1 proteins. Direct binding of Grb2-Sos is a possibility for TEL-JAK2(5-12) and TEL-JAK2(5-19), because a putative consensus binding sequence (pYXN) for Grb2 is found in TEL exon 5 at Tyr314. Because TEL-JAK2(4-17) does not contain this motif, an alternative explanation for the association of Grb2 likely involves an indirect interaction through Shc and/or Ship-1.
The SH2 domain of Ship-1 recognizes a consensus binding motif with the peptide sequence pY-(Y/D)-X-(L/I/V),62 while the SH2 domain of Shc binds to a sequence pY-(I/E/Y/L)-X-(I/L/M).63 Analysis of the 3 TEL-JAK2 isoforms revealed the presence of 6 putative binding sites for Shc and Ship-1 on TEL-JAK2(4-17) and TEL-JAK2(5-19), with 1 additional site identified on TEL-JAK2(5-12). Expression of all 3 TEL-JAK2 isoforms resulted in the association of Shc and Ship-1 with TEL-JAK2 as well as constitutive tyrosine phosphorylation of the 2 signaling molecules. TEL-JAK2 fusion proteins would then be capable of recruiting Grb2-Sos because Grb2 associates constitutively with Ship-1 and inducibly with Shc, following tyrosine phosphorylation. Grb2-Sos recruitment can then affect the activation of various downstream signaling pathways.
Direct binding of the Grb2-Sos complex is another possible mechanism of recruitment in 2 isoforms, TEL-JAK2(5-19) and TEL-JAK2(5-12), as stated above. Grb2 IPs revealed association of TEL-JAK2(5-12) and TEL-JAK2(5-19) with Grb2; however, binding of Grb2 to TEL-JAK2(4-17) was not evident. Moreover, GST in vitro mixing experiments revealed SH2-dependent binding to TEL fusions containing exon 5 but not with TEL-JAK2(4-17). These differences may be accounted for by Tyr314 in TEL exon 5, which was examined as discussed below.
All 3 TEL-JAK2 isoforms confer robust activation of Ras as determined by an activated Ras binding assay.76Interestingly, TEL-JAK2(5-19)Tyr314Phe showed markedly reduced Ras activation, indicating that in the context of TEL-JAK2(5-19), Tyr314 is responsible for a large proportion of Ras activation through binding of Grb2. TEL-JAK2(4-17) (which also lacks Tyr314) was capable of activating Ras. This suggests that additional tyrosine residues contained within exons 17 to 19 of JAK2 are responsible for binding of Shc and/or Ship-1, allowing for indirect recruitment of Grb2 or alternative mechanisms that may be SH2 independent.
Ras activates the ERK1 and ERK2 kinase cascade involving Raf1, MEK1, and MEK2 (MEK1/2). A major consequence of ERK1/2 activation is the promotion of cellular proliferation; thus, the involvement of this pathway in TEL-JAK2–mediated leukemogenesis was examined. IL-3 stimulates the activation of both ERK isoforms in untransfected and transfected Ba/F3 cells expressing TEL-JAK2. However, in the absence of IL-3 stimulation, expression of TEL-JAK2(5-19) or TEL-JAK2(5-12) induces preferential phosphorylation of ERK2. In several experiments, lower levels of ERK2 phosphorylation were reproducibly observed in TEL-JAK2(4-17)–expressing cells.
The requirement for ERK1/2 activation in IL-3–stimulated and IL-3–unstimulated proliferation of Ba/F3 cell lines was examined using the pharmacologic inhibitor U0126, which specifically targets the upstream activators of ERK1/2, MEK1/2. In the absence of IL-3, 10 μM U0126 inhibits the proliferative signals supplied by all TEL-JAK2 isoforms. In summary, TEL-JAK2 expression induces the preferential phosphorylation of ERK2. Furthermore, the activation of ERK1/2 by TEL-JAK2 is necessary for TEL-JAK2–mediated factor independence of Ba/F3 cells.
Another MAPK family member, p38, is involved in stress responses. Initially, the role assigned to p38 involved modulation of cellular responses to stresses, such as hyperosmolarity, heat shock, as well as inflammatory cytokines. However, recent investigations implicate p38 in modulating cytokine signals, including IL-3,64,65erythropoietin (Epo),65-68 and granulocyte colony-stimulating factor,69 in differentiation and proliferation of hematopoietic cells. The exact function of p38 activation is not clear and appears to be dependent on the stimuli as well as cell type. Thus, the relevance of constitutive p38 activation in TEL-JAK2–mediated transformation is not obvious. Because p38 activation has been implicated in inducing mitogenic responses in hematopoietic cells, the inhibition of p38 in Ba/F3 cells expressing TEL-JAK2 was of interest. Inhibition of p38 activation by TEL-JAK2 was achieved by using the pharmacologic inhibitor SB203580. However, activation of p38 was not required for TEL-JAK2–mediated proliferation of Ba/F3 cells.
SAPK is commonly activated in response to toxins, physical stresses, and inflammatory cytokines.70 Early investigations on SAPK function were focused on its role in modulating apoptosis; however, recent findings have implicated SAPK activation in oncogenic transformation. In addition, several cytokines, including IL-3 71-74 and Epo,66,68,71 activate SAPK. There is increasing evidence that supports the requisite role of SAPK activation in cellular transformation. As described earlier, BCR-ABL expression in fibroblasts or hematopoietic cells activates SAPK. Moreover, BCR-ABL–mediated transformation requires SAPK activation because expression of a dominant negative c-Jun, a target of SAPK, inhibits the transformation phenotype.7 Consequently, the status of SAPK activation by TEL-JAK2 expression was examined. Our results illustrate that SAPK activity is induced upon the introduction of TEL-JAK2(4-17), TEL-JAK2(5-19), and TEL-JAK2(5-12) into a hematopoietic cell line, thus suggesting a role for SAPK in TEL-JAK2 transformation. However, we have shown using the recently described SAPK inhibitor, SP600125, that SAPK activation is dispensable for TEL-JAK2–mediated proliferation.
As discussed above, it appears that Tyr314 in TEL exon 5 may permit direct recruitment of Grb2 to TEL-JAK2. Mutation of Tyr314 in TEL-JAK2(5-19) resulted in significant reduction in TEL-JAK2(5-19) association with endogenous Grb2 in IP experiments as well as the SH2 domain of Grb2 in vitro. These data suggested that Tyr314 in TEL exon 5 may account for increased phosphorylation of ERK1/2 observed in TEL-JAK2(5-12) and TEL-JAK2(5-19). However, analysis of several subclones of TEL-JAK2(5-19)Tyr314Phe revealed comparable activation of ERK2 as compared with TEL-JAK2(5-19). In summary, our experiments indicated that TEL Tyr314 is an important motif in direct recruitment of Grb2 and activation of Ras. However, mutation of this residue does not affect downstream activation of ERK1/2, SAPK, and p38. Alternative pathways leading to MAPK family activation may account for their activation. Similar redundancy has been observed downstream of Epo receptor activation.19 21
The importance of direct Grb2 binding to BCR-ABL has been examined in numerous studies.23-25,51 Pendergast et al23demonstrated that mutation of the Tyr177 Grb2 binding site to phenylalanine abolished direct binding of Grb2 with the mutant protein. The mutation also impaired/reduced the transformation potential of BCR-ABL in bone marrow and rat fibroblast transformation assays.23 Using an identical BCR-ABL mutant, Goga et al51 later recapitulated the impaired transformation of rat fibroblast expressing BCR-ABL Tyr177Phe; however, they observed no effect on hematopoietic transformation of bone marrow cells. Despite the initial report that mutation of Tyr177 to phenylalanine impairs hematopoietic transformation, many groups have convincingly demonstrated that BCR-ABL Tyr177Phe can transform hematopoietic cell lines as well as primary bone marrow cells.
However, a more relevant method for assaying the oncogenic potential of an activated kinase fusion involves the in vivo expression of the protein in bone marrow cells of mice. The transplantation of BCR-ABL–expressing bone marrow into lethally irradiated mice induced a fatal chronic myelogenous leukemia–like disease.75Recently, several groups have demonstrated that the BCR-ABL Tyr177Phe mutant suppressed the myeloproliferative disease and induced B-cell and T-cell lymphomas.33-35 Therefore, these findings suggested that direct Grb2 recruitment is important in the development of a myeloproliferative disease, but it cannot suppress BCR-ABL–mediated transformation. In view of this these findings, it would be of interest to study the phenotype generated by TEL-JAK2(4-17), as well as TEL-JAK2(5-19)Tyr314Phe, in a bone marrow transplantation model. Transplantation of TEL-JAK2(5-19) has been shown to generate a biphenotypic myeloproliferative and lymphoproliferative disorder.41 It is plausible that TEL-JAK2(4-17) and TEL-JAK2(5-19)Tyr314Phe could generate a distinct disease phenotype or altered latency.
In summary, TEL-JAK2 isoforms are activated tyrosine kinase fusions that are capable of transforming the Ba/F3 cell line to factor independence. TEL-JAK2 fusion proteins can associate with Grb2, which likely occurs through both direct and indirect mechanisms. Recruitment of Grb2-Sos can then link TEL-JAK2 to Ras signaling. Indirect binding of Grb2 to TEL-JAK2 is mediated through interactions with tyrosine-phosphorylated Shc and/or Ship-1. Furthermore, our findings demonstrate that preferential activation of ERK2 was necessary for TEL-JAK2–mediated factor-independent transformation in Ba/F3 cells.
We thank Gary Gilliland, Peter Marynen, Brian Druker, and Dan Dumont for reagents. We thank members of the Barber laboratory for helpful discussions. This work is dedicated in memory of Douglas Hogue. This work is in partial fulfillment of an MSc degree from the University of Toronto (J.M.-Y.H.).
Supported by Cancer Research Society (D.L.B.), Canadian Institutes of Health Research (D.L.B.), National Institutes of Health award CA-73749 (M.P.C.), and the G & P Charitable Foundation for Cancer Research award (M.P.C.). D.L.B. is a National Cancer Institute of Canada Research Scientist.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Dwayne L. Barber, Ontario Cancer Institute, 610 University Ave, Toronto, Ontario, M5G 2M9, Canada; e-mail:dbarber@uhnres.utoronto.ca.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal