Rexinoids binding to both the retinoic acid receptor (RAR) and retinoid X receptor (RXR) families of rexinoid receptors have demonstrated clinical activity in hematologic malignancies and have been shown to mediate genes associated with both growth and differentiation. RXR rexinoids have demonstrated efficacy in the treatment of cutaneous T-cell lymphomas, but the mechanism of action is unclear. We explored the immunomodulatory effects of RAR and RXR rexinoids in human T- and B-cell leukemia cells and demonstrated that RXR rexinoids are capable of up-regulating high-affinity interleukin-2 receptor (IL-2R) expression. Exposure to 10−6 to 10−10 M bexarotene or Panretin for 48 hours was associated with increased expression of both the p55 and p75 subunits of the IL-2R in T-cell leukemias and p75 in B-cell leukemias. Furthermore, rexinoid exposure enhanced susceptibility of the cells to denileukin diftitox fusion toxin-targeting and -intoxicating cells expressing high-affinity IL-2R. These results suggest a rationale for combining rexinoids with IL-2R–targeted therapies in lymphoid malignancies as well as possibly in autoimmune diseases.
Introduction
Rexinoids are ligands for transcription factors of the nuclear receptor superfamily. Two subfamilies of rexinoid receptors have been identified, retinoic acid receptor (RAR)–αβγ receptors, which function in differentiation and cell growth, and retinoid X receptor (RXR)–αβγ receptors, which regulate a family of genes with RXR receptor elements and, which, in some instances, induce apoptosis in tumor cells.1,2All-trans-retinoic acid (ATRA) has been identified as an RAR-specific ligand,3 bexarotene (Targretin, LGD 1069; Ligand Pharmaceuticals, San Diego, CA) is a selective RXR ligand, and alitretinoin (Panretin, LGD 1057; Ligand Pharmaceuticals) has been demonstrated to bind to both RAR and RXR receptors.4
Rexinoids have been shown to have important immunomodulating effects on T cells, including augmenting interleukin-2 receptor (IL-2R) expression by activated T cells and inhibiting activation-induced cell death. Sidell and coworkers5 demonstrated that ATRA up-regulated the expression of IL-2Rα on human thymocytes by increasing the steady-state messenger RNA levels. Although RXR rexinoids (rexinoids) have been shown to induce apoptosis in HL60 cells and human epithelial cell lines without modulating expression of differentiation markers, it is unclear whether rexinoids (rexinoids) would similarly modulate the activation state of T cells.6-8
We examined the differential effects of both RAR and RXR rexinoids on IL-2R expression in human leukemia cells. Up-regulation of high-affinity IL-2R would enhance the susceptibility of the cells to intoxication by the IL-2 fusion toxin, denileukin diftitox (Ontak), whose mechanism of cytotoxicity is dependent on efficient binding to the high-affinity IL-2R, with subsequent receptor-mediated internalization of the toxin.9 10 We demonstrate up-regulation of the IL-2Rβ (CD122) subunit by both RAR and RXR rexinoids in T cells, but more selective effects in B cells, with up-regulation of IL-2Rβ (CD122) but not IL-2Rα (CD25) expression. In both Sezary T-leukemia cells and B cells of chronic lymphocytic leukemia (B-CLL), up-regulation of the high- affinity IL-2R was demonstrated by enhanced susceptibility to intoxication by Ontak. Our results demonstrate both a potential immunomodulatory effect of rexinoids on the activation state of T cells as well as a biomodulatory effect of these agents to sensitize leukemia cells to targeted cytotoxic therapies.
Materials and methods
Cell culture
Cutaneous T-cell lymphoma (CTCL) NCI-HUT78, HUT102, pre-B cell line NALM6, and acute lymphoblastic leukemia cell line CEM were obtained from the American Type Culture Collection (Manassas, VA). Fresh cells from patients with CTCL and B-CLL were isolated by Ficoll-Hypaque centrifugation. Cells were grown in complete medium (RPMI-1640 medium including 10% fetal bovine serum, 50 μg/mL glutathione, 50 μg/mL streptomycin, and 50 μg/mL penicillin).
Chemicals
Denileukin diftitiox (Ontak, DAB389IL2), alitretinoin (Panretin, LGD 1057; 9-cis retinoic acid; 3,7-dimethyl-9-[2,6,6-trimethyl-1-cyclohexen-1-yl]-2-trans-4-trans-6-cis-8-trans-nonatetraenoic acid), and bexarotene (Targretin, LGD 1069; 4-[1-(3,5,5,8,8,-pentamethyl-5,6,7,8-tetrahydro-2-napthalenyl) ethenyl] benzoic acid) were obtained from Ligand Pharmaceuticals.
Flow cytometry
Cells were incubated with 10−6 M and 10−8 M rexinoids (ATRA, alitretinoin, and bexarotene) in complete medium at 37°C in 5% CO2 for 48 hours and then labeled with phycoerythrin (PE)–conjugated monoclonal antihuman CD25 PE (IL-2Rα) and CD122 PE (IL-2Rβ) (Becton Dickinson, CA) antibodies, fixed, and analyzed using FACScan (Becton Dickinson, Beckman Instruments, San Jose, CA) Laser excitation was set at 488 nm.
IL-2R subunit analysis by Western immunoblot
Cells (5 × 105 cells/mL) were incubated with 10−6 M and 10−8 M rexinoids for 48 hours and lysed; proteins were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride membranes at 100 V for 1 hour. Immunoblots were performed using antibodies to human IL-2Rα, IL-2Rβ, and IL-2Rγ (Santa Cruz Biotechnology, Santa Cruz, CA). Immunoreactive bands were detected by Western blot chemiluminescence reagents (NEN Life Science, Boston, MA) and exposed on Kodak XAR film (Rochester, NY). The protein bands were stained by Coomassie blue to demonstrate the amount of protein loaded per lane. The constitutively expressed Coomassie blue–stained protein bands were used as control.
Inhibition of protein synthesis by [14C]-leucine incorporation assay
Cells were seeded in 96-well flat-bottomed tissue culture plates (Costar, Cambridge, MA), 5 × 103cells/100 μL in complete medium, and treated with10−6 to 10−10 M rexinoids for 48 hours. Cells were exposed to10−6 to 10−14 M denileukin diftitox for an additional 72 hours. The plates were centrifuged for 5 minutes at 170g, and the medium removed and replaced with 100 μL leucine-free medium containing 0.25 μCi [14C]-leucine (0.00925 MBq; New England Nuclear, Boston, MA). Cells were incubated at 37°C for 3 hours; then protein was precipitated by trichloroacetic acid (TCA) and harvested on glass fiber filters. Incorporation of [14C]-leucine was quantified by scintillation counting. All assays were performed in triplicate and results were reported ± SEM of triplicate assays.
MTT cell cytotoxicity assay
Cells were seeded in triplicate in 96-well plates in 50 μL complete media at a density of 5 × 103 cells/well. The 50-μL aliquots of rexinoids were added to each well to give a range of concentrations from 10−6 to 10−10 M in a final volume of 100 μL. After 48 hours of incubation, 20 μL denileukin diftitox was added to each well to give various concentrations from 10−6 to 10−14 M. Cells were incubated for an additional 72 hours. Then 20 μL MTT (3-(4,5-dimethylthazol-2-yl)-2,5-diphenyl tetrazolium bromide salt) from 5 mg/mL stock was added at the last day and incubated for 4 hours. The formazan crystals were solubilized by adding 100 μL 20% SDS in 50% dimethyl formamide (DMF; pH 4.7) and incubating at 37°C overnight. The absorbance of the formazan product was measured on an enzyme-linked immunosorbent assay (ELISA) plate reader at 570 nm.
Results
We and others demonstrated that the CTCL cell line HUT78, acute T-lymphoblastic leukemia cell line CEM, and the pre-B cell leukemia cell lines NALM6 and Raji express low-affinity IL-2R, consisting of the p55 (IL-2Rα) and p64 (IL-2Rγ) subunits and are thereby resistant to killing by denileukin diftitox (Ontak), whereas HUT102 T-leukemia cells expresses high-affinity IL-2R (p55, p75, p64) and are efficiently intoxicated by denileukin diftitox.11
Effects of rexinoids on T- and B-leukemia cells
To determine the effects of rexinoids on high-affinity IL-2R expression, we exposed T- and B-leukemia cell lines and fresh leukemia cells to bexarotene, an RXR ligand, and alitretinoin, which binds to both RXR and RAR receptors. After a 48-hour incubation, there was no inhibition of cell growth by MTT and, morphologically, no induction of apoptosis in the treated cells. Expression of IL-2R subunits after rexinoid exposure is shown in Figure1.
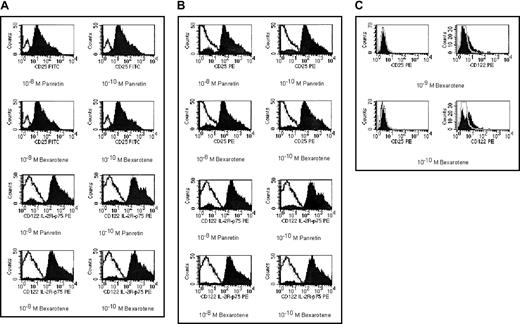
The cell surface expression of CD25 (IL-2Rα) and CD122 (IL-2Rβ).
CTCL cell line HUT78 (A), fresh Sezary cells from a patient with CTCL (B), and pre-B leukemia cell line NALM6 (C) were exposed to 10−8 M or 10−10 M alitretinoin (Panretin) or bexarotene for 48 hours. The cell surface expression of IL-2Rα (CD25) and IL-2Rβ (CD122) were detected by flow cytometry. The dot peak represents isotypic control, the linear peak represents CD25 and CD122 expression in untreated cells, and the filled peak represents the expression of CD25 or CD122 in rexinoid-treated cells.
The cell surface expression of CD25 (IL-2Rα) and CD122 (IL-2Rβ).
CTCL cell line HUT78 (A), fresh Sezary cells from a patient with CTCL (B), and pre-B leukemia cell line NALM6 (C) were exposed to 10−8 M or 10−10 M alitretinoin (Panretin) or bexarotene for 48 hours. The cell surface expression of IL-2Rα (CD25) and IL-2Rβ (CD122) were detected by flow cytometry. The dot peak represents isotypic control, the linear peak represents CD25 and CD122 expression in untreated cells, and the filled peak represents the expression of CD25 or CD122 in rexinoid-treated cells.
Both alitretinoin and bexarotene up-regulated the cell surface expression of p55 and p75 at least 4-fold in HUT78 cells and fresh CD4+CD7− Sezary cells, which lacked IL-2R expression by immunohistochemistry. Rexinoids had less effect on IL-2R expression in NALM6 and Raji cells and in human B-CLL cells, with increased the expression of p75 but not p55 (Figure 1C).
Immunoblots confirmed that both alitretinoin and bexarotene increased the expression of p55 and p75 in HUT78 (Figure2A,B) and fresh Sezary cells and only p75 in the B cells (Figure 2C).
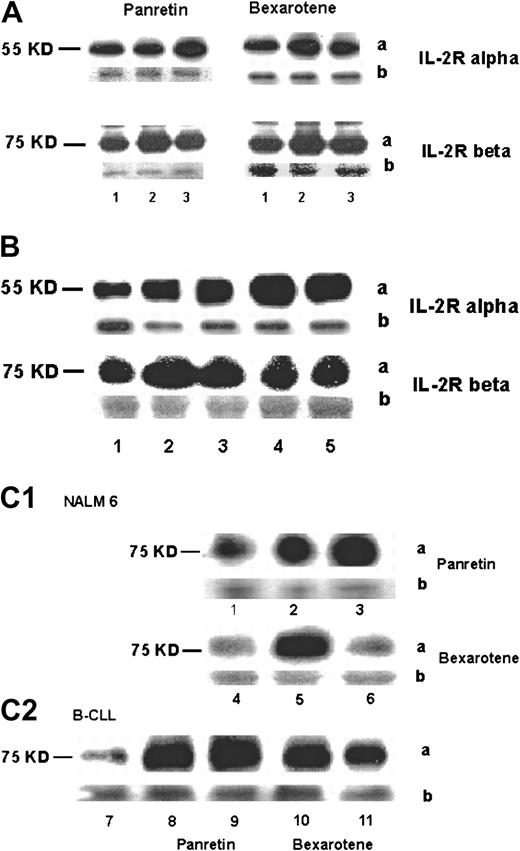
Intracellular expression of p55 (IL-2Rα) and p75 (IL-2Rβ) proteins.
Cells were treated with 10−6 M or 10−8M alitretinoin (Panretin) or bexarotene for 48 hours. The expression of p55 and p75 was analyzed by immunoblot. (A) Lane 1, untreated HUT78 cells (control); lane 2, 10−10 M rexinoid-treated cells; lane 3, 10−8 M rexinoid-treated cells. (B) Lane 1, untreated fresh Sezary cells; lanes 2-5, Sezary cells after exposure to 10−10 M Panretin (lane 2), 10−8 M Panretin (lane 3), 10−10 M bexarotene (lane 4), 10−8 M bexarotene (lane 5). (C) Expression of p75 in NALM6 (lanes 1-6) and CLL cells (lanes 7-11) after rexinoids; lanes 1, 4, and 7, untreated; lanes 2 and 8, 10−10 M Panretin; lanes 3 and 9, 10−8 M Panretin; lanes 5 and 10, 10−8 M bexarotene; lanes 6 and 11, 10−10 M bexarotene. The “a” represents protein band and the “b” represents the constitutively expressed on Coomassie blue–stained protein bands as a control for protein loading.
Intracellular expression of p55 (IL-2Rα) and p75 (IL-2Rβ) proteins.
Cells were treated with 10−6 M or 10−8M alitretinoin (Panretin) or bexarotene for 48 hours. The expression of p55 and p75 was analyzed by immunoblot. (A) Lane 1, untreated HUT78 cells (control); lane 2, 10−10 M rexinoid-treated cells; lane 3, 10−8 M rexinoid-treated cells. (B) Lane 1, untreated fresh Sezary cells; lanes 2-5, Sezary cells after exposure to 10−10 M Panretin (lane 2), 10−8 M Panretin (lane 3), 10−10 M bexarotene (lane 4), 10−8 M bexarotene (lane 5). (C) Expression of p75 in NALM6 (lanes 1-6) and CLL cells (lanes 7-11) after rexinoids; lanes 1, 4, and 7, untreated; lanes 2 and 8, 10−10 M Panretin; lanes 3 and 9, 10−8 M Panretin; lanes 5 and 10, 10−8 M bexarotene; lanes 6 and 11, 10−10 M bexarotene. The “a” represents protein band and the “b” represents the constitutively expressed on Coomassie blue–stained protein bands as a control for protein loading.
RXR-selective rexinoid bexarotene (Targretin) and RAR/RXR-specific rexinoid alitretinoin (Panretin) enhanced sensitivity of T and B cells to denileukin diftitox (Ontak)
To test modulation of expression of functional high-affinity IL-2R after rexinoid exposure, we exposed rexinoid-treated cells to denileukin diftitox and measured protein synthesis inhibition and cell growth. Because the mechanism of cytotoxicity of denileukin diftitiox is related to the diptheria toxin–mediated adenosine diphosphate ribosylation of elongation factor 2, inhibition of protein synthesis is indicative of efficient receptor-mediated internalization of the fusion toxin. As shown in Figure 3, panels A and B, a 50% to 70% inhibition of protein synthesis was demonstrated in HUT78 and fresh Sezary cells after exposure to alitretinoin or bexarotene, respectively. In the B cells, alitretinoin inhibited the protein synthesis by 35% compared to over 50% with bexarotene (Figure3C). As shown, low doses of denileukin diftitox alone were associated with increased proliferation, presumably due to cytokine-mediated signaling events from binding of denileukin diftitox to intermediate- and low-affinity receptor-expressing cells.12
Cell susceptibility to denileukin diftitox by protein synthesis inhibition.
HUT78 cells (A), Sezary cells (B), and B-CLL cells (C) were exposed to10−10 M and 10−8 M alitretinoin (Panretin) (1) or bexarotene (2) for 48 hours. The cells were incubated with denileukin diftitox (Ontak) at various concentration for 72 hours. Inhibition of protein synthesis was measured by [14C]-leucine incorporation. All the data represent percent of counts per minute ± SEM of cells pulsed with [14C]-leucine.
Cell susceptibility to denileukin diftitox by protein synthesis inhibition.
HUT78 cells (A), Sezary cells (B), and B-CLL cells (C) were exposed to10−10 M and 10−8 M alitretinoin (Panretin) (1) or bexarotene (2) for 48 hours. The cells were incubated with denileukin diftitox (Ontak) at various concentration for 72 hours. Inhibition of protein synthesis was measured by [14C]-leucine incorporation. All the data represent percent of counts per minute ± SEM of cells pulsed with [14C]-leucine.
To confirm that inhibition of protein synthesis corresponded to increased cytotoxicity, MTT assays were performed. Bexarotene had a greater effect on enhancing cytotoxicity of denileukin diftitox than alitretinoin in both T- and B-leukemia cells, concordant to the degree of protein synthesis inhibition (Figure4).
Cell cytotoxicity by the combination of rexinoids and denileukin diftitox.
HUT78 cells (A), Sezary cells (B), and B-CLL cells (C) were incubated with rexinoids (a, alitretinoin (Panretin); b, bexarotene) for 48 hours and exposed to denileukin diftitox (Ontak) for 72 hours at various concentrations. Cytotoxicity was measured by MTT assay. Assays were performed in triplicate and data represent counts ± SEM.
Cell cytotoxicity by the combination of rexinoids and denileukin diftitox.
HUT78 cells (A), Sezary cells (B), and B-CLL cells (C) were incubated with rexinoids (a, alitretinoin (Panretin); b, bexarotene) for 48 hours and exposed to denileukin diftitox (Ontak) for 72 hours at various concentrations. Cytotoxicity was measured by MTT assay. Assays were performed in triplicate and data represent counts ± SEM.
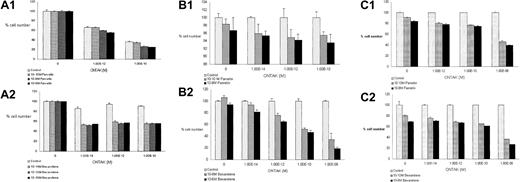
The results of the cytotoxicity assays are summarized in Table1, which demonstrates that bexarotene alone had no effect on cell number.
Cytotoxic effects of bexarotene and denileukin diftitox (Ontak) alone and in combination
| Cell lines and cells . | Expression of IL-2 receptor subunits (baseline) . | Cytotoxicity (% survival) bexarotene . | Cytotoxicity (% survival) bexarotene + Ontak (10−12 M) . | |||||
|---|---|---|---|---|---|---|---|---|
| p55α . | p75β . | p64γ . | 10−10M bexarotene . | 10−8 M bexarotene . | 10−12 M Ontak . | 10−12 M Ontak + 10−10 M bexarotene . | 10−12 M Ontak + 10−8 M bexarotene . | |
| NCI-HUT 102 | +++ | +++ | +++ | 116 | 112 | 56 | 36 | 29 |
| CEM | ± | − | +++ | 106 | 109 | 85 | 53 | 44 |
| Raji | + | − | +++ | 103 | 105 | 104 | 84 | 80 |
| CTCL patient | ± | − | +++ | 102 | 106 | 83 | 68 | 61 |
| CTCL patient | − | − | +++ | 119 | 106 | 80 | 64 | 58 |
| B-CLL patient | ± | − | +++ | 103 | 110 | 86 | 71 | 64 |
| Cell lines and cells . | Expression of IL-2 receptor subunits (baseline) . | Cytotoxicity (% survival) bexarotene . | Cytotoxicity (% survival) bexarotene + Ontak (10−12 M) . | |||||
|---|---|---|---|---|---|---|---|---|
| p55α . | p75β . | p64γ . | 10−10M bexarotene . | 10−8 M bexarotene . | 10−12 M Ontak . | 10−12 M Ontak + 10−10 M bexarotene . | 10−12 M Ontak + 10−8 M bexarotene . | |
| NCI-HUT 102 | +++ | +++ | +++ | 116 | 112 | 56 | 36 | 29 |
| CEM | ± | − | +++ | 106 | 109 | 85 | 53 | 44 |
| Raji | + | − | +++ | 103 | 105 | 104 | 84 | 80 |
| CTCL patient | ± | − | +++ | 102 | 106 | 83 | 68 | 61 |
| CTCL patient | − | − | +++ | 119 | 106 | 80 | 64 | 58 |
| B-CLL patient | ± | − | +++ | 103 | 110 | 86 | 71 | 64 |
All the data represent cell count ± SEM by MTT assay.
Discussion
Vitamin A and its analogues influence differentiation and proliferation and may also alter immune responses. Rexinoids exert their effects by binding to the rexinoid A receptor (RARαβγ) or the rexinoid X receptor (RXRαβγ) in the nuclei of cells, either directly or indirectly via nuclear transcription factors. As a result, rexinoids regulate the expression of a wide range of target genes.13
The effects of RAR and RXR rexinoids on IL-2R expression were determined in T and B lymphocytes, which do not express high-affinity IL-2R.11 14 IL-2Rα and IL-2Rβ expression is increased after exposure of the T cells to alitretinoin or bexarotene for 48 hours, whereas only IL-2Rβ expression was increased in B-CLL cells. These results demonstrate lineage-specific effects of rexinoids and suggest that modulation of IL-2Rα or IL-2Rβ may be in part related to the state of differentiation of the cell.
A number of factors have been shown to modulate expression of IL-2R subunits. The p55 subunit is induced by T-cell activation, viral gene products, protein kinase A (PKA), IL-2, IL-1, and tumor necrosis factor α (TNF-α).15 Likewise, the p75 subunit, which is expressed on resting T lymphocytes, is up-regulated by activation, IL-2, and IL-4. The p64, or common γ subunit, is constitutively expressed but up-regulated by IL-2 and interferon γ.
Up-regulation of IL-2R subunits by rexinoids is most likely mediated by modulation of the binding of transcriptional elements. In the case of the RAR rexinoid, ATRA, direct binding to thePML transcription factor in acute promyelocytic leukemia cells has been shown to be the mechanism of action.16 RXR rexinoids have been shown to bind directly to RXR transcriptional elements either as homodimers or heterodimers with RAR. RXR elements have been identified in a number of genes, including the thyroid-stimulating hormone promoter and the peroxisome proliferator-activated receptor promoter.16
The differential effect of rexinoids on IL-2R expression in T and B cells may be related to interaction with other transcriptional regulators. Both RAR and RXR rexinoids inhibit activation and prevent apoptosis in B lymphocytes while inducing cell differentiation and proliferation in T lymphocytes.17 IL-2Rα is not expressed on resting lymphocytes but is potently induced after activation.18 IL-2Rα is mainly regulated at the level of transcription by induction of nuclear factor-κB (NF-κB) and serum response element.19 Rexinoids may induce IL-2Rα expression by enhancing expression of IL-2Rβ in T lymphocytes.15
IL-2Rβ is linked to at least 2 intracellular signaling pathways mediating nuclear proto-oncogene induction. One pathway involves tyrosine phosphorylation by janus kinase 1 (JAK1) and src-family phosphotyrosine kinases (PTKs) and leads to the induction of c-fos and c-jun through the activation of p21ras. Another pathway involves induction of c-myc and bcl-2 genes.20Up-regulation of IL-2Rβ, therefore, favors proliferation. Because IL-2Rγ is constitutively expressed in lymphocytes and, via janus kinase signal transducer and activation of transcription (JAK-STAT) phosphorylation, serves as a messenger to rapidly regulate expression of target genes,21 up-regulation of IL2-Rβ by the rexinoids enhances activation and proliferation signals even in the absence of up-regulation of IL2-Rα in the B cells. This may explain the effects of the RXR rexinoids alone to induce the proliferation we observed in both T- and B-leukemia cells.
Up-regulation of functional high-affinity receptor by rexinoids could potentially confer susceptibility of the cells to IL-2R–targeted therapies. We demonstrate that rexinoid treatment enhanced the cytotoxicity of the IL-2R–directed fusion toxin, denileukin diftitox, in resistant T- and B-cell leukemia cells. The inhibition of protein synthesis by denileukin diftitox requires receptor-mediated internalization of the fusion toxin and intracellular processing to cleave the enzymatically active moiety of diphtheria toxin into the cytosol.22,23 The susceptibility of both normal and neoplastic cells to denileukin diftitox–induced cytotoxicity is dependent on the expression of the high-affinity IL-2R, consisting of the p55 (α), p75 (β), and p64 (γ) subunits.24-26 The inhibitory concentration of 50% (IC50) of high-affinity IL-2R–expressing cells is 10−12 M, whereas cells expressing intermediate-affinity receptors (p75, p64) are intoxicated at an IC50 of 10−10 M.27 Cells expressing only p55 are relatively resistant (IC50 of 10−8 M). Expression of high-affinity IL-2R is restricted to activated T lymphocytes, activated B lymphocytes, and activated macrophages, whereas intermediate-affinity receptors are found on natural killer cells and resting T cells.28 29
In addition to up-regulation of high-affinity receptor, rexinoid exposure might have other effects on multiple signaling pathways to enhance apoptosis in the presence of the fusion toxin. Denileukin diftitox has been shown to initiate signal transduction with ligand engagement, even if receptor-mediated internalization of the fusion toxin does not occur.12 Signaling events mediated by rexinoids and IL-2R–ligand engagement may contribute to the markedly enhanced cytotoxicity observed in this study.
Cell viability studies show that neither alitretinoin or bexarotene induces cell death at concentrations ranging from 10−6 to 10−8 M. Other studies have demonstrated that RXR rexinoids induce apoptosis in epithelial cell lines and in HL60 cells. RXRα has been shown to be down-regulated during the transition from G0/G1 to S phase of the cell cycle, whereas RARγ has been shown to facilitate apoptosis of T lymphocytes, suggesting a role for rexinoid homodimers and heterodimers in cell cycle control in lymphocytes.7,30 31
Our results demonstrate that RXR rexinoids up-regulate high-affinity IL-2R expression on human B- and T-leukemia cells. These results suggest that the clinical efficacy of denileukin diftitox might be enhanced by the addition of an RXR rexinoid, and phase I trials exploring this concept are under way. Further, bexarotene is widely used as a therapy for patients with CTCL, but its mechanism of action in this disease is unclear, because our studies demonstrate no direct cytotoxic effects on Sezary leukemia cells. The demonstration of an immunomodulatory effect of bexarotene to up-regulate high-affinity IL-2R may have consequences in the context of the generation of antitumor immunity in patients, and, likewise, it is unclear whether up-regulation of IL-2R might render the tumor cells more susceptible to fas-mediated cytotoxicity in vivo. Our results suggest that further studies of the in vivo immunomodulatory effects of bexarotene are warranted.
Prepublished online as Blood First Edition Paper, June 21, 2002; DOI 10.1182/blood-2002-01-0300.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Francine Foss, Hematology Oncology and Experimental Therapeutics, Tufts New England Medical Center, 750 Washington St, Boston, MA 02111; e-mail: ffoss@lifespan.org.

![Fig. 3. Cell susceptibility to denileukin diftitox by protein synthesis inhibition. / HUT78 cells (A), Sezary cells (B), and B-CLL cells (C) were exposed to10−10 M and 10−8 M alitretinoin (Panretin) (1) or bexarotene (2) for 48 hours. The cells were incubated with denileukin diftitox (Ontak) at various concentration for 72 hours. Inhibition of protein synthesis was measured by [14C]-leucine incorporation. All the data represent percent of counts per minute ± SEM of cells pulsed with [14C]-leucine.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/4/10.1182_blood-2002-01-0300/6/m_h81622990003.jpeg?Expires=1769105377&Signature=dWvhK1meZMPPU3qd8RVtK19EamujtdPmtim2Ad4GmO8KSf-J304ddpNp7iCwNoPW1-jpc3JbHffxHadiAcba~LTJRTriJl~jvDSUvauOHQuLI6DHLJMvwVNa3FEKa9jQnBNNw7Y0eg39m5h~nDhLkLibUxZITz482fOIySMGY7kbhqLRRcf6EXMe7XcLjOcu07LyZvLE~hvWuvJJYafnjRkrNABHfkFTySHYz2jWk4e88X3uEuXyAlk8A3slCT2b427h0cZidWHt60XZGdxQIrq~gN~7GamBRQQ49L~bjojCsdm3eCcyVI75wU14IYGQ5OkeMga8yhj43WaPGGXklA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal