Unlike β particle–emitting isotopes, α emitters can selectively kill individual cancer cells with a single atomic decay. HuM195, a humanized anti-CD33 monoclonal antibody, specifically targets myeloid leukemia cells and has activity against minimal disease. When labeled with the β-emitters 131I and 90Y, HuM195 can eliminate large leukemic burdens in patients, but it produces prolonged myelosuppression requiring hematopoietic stem cell transplantation at high doses. To enhance the potency of native HuM195 yet avoid the nonspecific cytotoxicity of β-emitting constructs, the α-emitting isotope 213Bi was conjugated to HuM195. Eighteen patients with relapsed and refractory acute myelogenous leukemia or chronic myelomonocytic leukemia were treated with 10.36 to 37.0 MBq/kg 213Bi-HuM195. No significant extramedullary toxicity was seen. All 17 evaluable patients developed myelosuppression, with a median time to recovery of 22 days. Nearly all the 213Bi-HuM195 rapidly localized to and was retained in areas of leukemic involvement, including the bone marrow, liver, and spleen. Absorbed dose ratios between these sites and the whole body were 1000-fold greater than those seen with β-emitting constructs in this antigen system and patient population. Fourteen (93%) of 15 evaluable patients had reductions in circulating blasts, and 14 (78%) of 18 patients had reductions in the percentage of bone marrow blasts. This study demonstrates the safety, feasibility, and antileukemic effects of 213Bi-HuM195, and it is the first proof-of-concept for systemic targeted α particle immunotherapy in humans.
Introduction
In the past decade, various approaches using native monoclonal antibodies, immunotoxins, and radioimmunoconjugates have emerged as promising strategies for the treatment of cancer, particularly for hematologic malignancies.1-8 To date, all clinical radioimmunotherapy studies have used β-emitting isotopes. Because of their long range (0.8-5 mm), β particles can crossfire to create a “field effect,” leading to the destruction of targeted cells and surrounding tumor cells. This effect, however, can also result in the killing of normal bystander cells and produce significant toxicity. In contrast, α particles are high-energy helium nuclei with path lengths of only 50 to 80 μm. The linear energy transfer of α particles is up to 500 times greater than that of β particles (100 keV/μm vs 0.2 keV/μm).9 Injured cells have limited capacity to repair DNA damage induced by α particles, and cell death may result from a single atomic decay traversing the nucleus.10 Therefore, radioimmunotherapy with α particle–emitting isotopes may produce more efficient killing of individual tumor cells with little damage to surrounding normal tissues. The specificity and efficacy of targeted α particle immunotherapy with 212Bi, 213Bi, and211At have been reported in several experimental models.11-16
HuM195 is a humanized anti-CD33 monoclonal antibody constructed by grafting complementarity-determining regions of murine M195 into a human IgG1 framework and backbone.17 The CD33 antigen is a 67-kd glycoprotein expressed on most myeloid leukemias and clonogenic leukemia progenitors. It is also found on committed myelomonocytic and erythroid progenitor cells, but not on mature granulocytes or nonhematopoietic tissues.18-20 HuM195 displayed rapid targeting of leukemia cells in patients and a pharmacology similar to that of murine M195 but without significant immunogenicity.21 Treatment with native HuM195 showed activity against minimal residual disease in patients with acute promyelocytic leukemia22 and produced rare complete remissions in patients with relapsed or refractory myeloid leukemia.23,24 When labeled with therapeutic doses of the β-particle emitters 131I and 90Y, M195 and HuM195 induced profound myelosuppression and eliminated large leukemic burdens.25,26131I-M195 and131I-HuM195 were safely combined with busulfan and cyclophosphamide as conditioning for allogeneic bone marrow transplantation (BMT).27
213Bi has a half-life of 45.6 minutes and emits an α particle of 8 MeV. Additionally, a 440-keV photon emission accompanies 26.5% of 213Bi decays, allowing detailed biodistribution and dosimetry studies to be performed. 213Bi is produced from an 225Ac/213Bi generator and is conjugated to HuM195 using the bifunctional chelating agent 2-(4-isothiocyanatobenzyl) diethylenetriamine pentaacetic acid (SCN-CHX-A-DTPA).28 Intravenous injections up to 370 MBq/kg 213Bi-HuM195 were safe in mice. The application of bismuth-labeled HuM195 in vitro resulted in dose- and specific activity–dependent killing of CD33+ HL60 cells. Approximately 50% of target cells were killed when only 2 bismuth atoms were bound to the cell surface.29 To demonstrate the proof-of-concept that targeted α particles could enhance the potency of native antibodies yet avoid the nonspecific cytotoxicity of β-emitting radioimmunoconjugates, we conducted a phase 1 dose-escalation study of 213Bi-HuM195 in patients with advanced myeloid leukemia.
Patients, materials, and methods
HuM195-CHX-A-DTPA
HuM195, supplied by Protein Design Labs (Fremont, CA), is a recombinant IgG1 monoclonal antibody to CD33, which was constructed by grafting the complementarity-determining regions of murine M195 into human framework and constant regions.17,30-32 The bifunctional chelating agent SCN-CHX-A-DTPA, a backbone-substituted derivative of DTPA obtained from Brechbiel and Gansow,33was conjugated to HuM195 by TSI Washington (Bethesda, MD) using buffer exchange and dialysis.34,35 The ligand-to- protein ratio was 4.55, as determined by the yttrium Arsenazo III spectrophotometric method.36
Isotope preparation and radiolabeling
225Ac was obtained from the Institute for Transuranium Elements (Karlsruhe, Germany) or from Oak Ridge National Laboratory (Oak Ridge, TN). 225Ac/213Bi generators were constructed, and 213Bi was eluted every 3 to 4 hours using previously described methods.28,29,34,37,38 The radiolabeling efficiency was 81%, as determined by instant thin-layer chromatography, and the immunoreactivity was 89%, as measured using described methods.28 Additional antibody was added to adjust the specific activity to range from 329 to 766 MBq/mg. The final product was diluted in normal saline with 1% human serum albumin to a total volume of 10 mL for injection.
Study design
Patients with relapsed or refractory acute myelogenous leukemia (AML), accelerated phase or myeloid blast crisis of chronic myelogenous leukemia, or chronic myelomonocytic leukemia (CMMOL) were eligible if more than 25% of their bone marrow blasts expressed CD33. Patients did not receive any antileukemic therapy for 3 weeks before entering the study except for hydroxyurea, which was permitted to control peripheral blood leukocyte counts. Concurrent use of either oral or intravenous antibiotics was allowed. Entry criteria included serum creatinine level less than 1.5 times normal, serum bilirubin of 1.5 mg/dL or less, and hepatic transaminases and alkaline phosphatase 2.5 times normal or less. Patients could not have detectable antibodies to HuM195 or active central nervous system involvement by leukemia.
213Bi-HuM195 was given as a 5-minute infusion, 2 to 4 times daily in 148 to 925 MBq fractions over 2 to 4 days. Because213Bi yields were limited by the activity of each225Ac/213Bi generator and because of constraints on the specific activity that could be achieved for any one injection, we escalated radioactivity doses by increasing the number of injections. Patients received a total of 3 to 7 injections. Five dose levels of 213Bi-HuM195 were administered—10.36, 15.54, 20.72, 25.9, and 37 MBq/kg. Three to 6 patients were treated at each dose level. Total administered activities ranged from 602 to 3515 MBq, and total antibody doses ranged from 1.6 to 6.4 mg. Complete blood counts and biochemical profiles were performed at least once weekly. Toxicity was assessed according to the common criteria established by the National Cancer Institute, version 2.0. We evaluated the biodistribution, pharmacokinetics, and dosimetry of213Bi-HuM195 using methods described below and compared differences between the first and last injections. To measure the antileukemic effects of 213Bi-HuM195, we performed bone marrow aspirations 7 to 10 days and 4 weeks following treatment.
Biodistribution
The γ emissions of 213Bi allowed biodistribution, pharmacokinetic, and dosimetry studies to be performed as previously described.39 Patient images were obtained after at least the first or last dose of 213Bi-HuM195 on a dual-head Vertex gamma camera (ADAC Laboratories, Milpitas, CA). Imaging began at the start of injection. Using a 20% photopeak window centered at 440 keV, the γ energy emitted in the β− decay of213Bi, we collected 30 1-minute images followed by 10 3-minute images in dynamic mode. Contours around the liver, spleen, and vertebrae were used to determine the number of counts in these regions at each time point. We calculated the activities in the liver and spleen as the geometric mean of the counts per minute in the anterior and posterior images. Activity in the spine was estimated from the posterior view only. Kinetic curves were generated, corrected for decay, and converted to percentage injected dose (%ID) for each region. The %ID for the spine was converted to marrow %ID by scaling a nominal estimate of the red marrow mass in the vertebrae according to body weight.39
Pharmacokinetics
Blood samples were collected at 5, 10, 15, 30, 45, 60, 90, 120, and 180 minutes following the first or last injection, or both, of213Bi-HuM195 in conjunction with gamma camera imaging. Aliquots of whole blood and plasma were analyzed for 1 minute in a gamma counter (Compugamma model 1282; LKB Wallac, Gaithersburg, MD). The data were decay corrected to the time of injection and expressed as percentage of injected activity per liter.39
Dosimetry
We used a conventional medical internal radiation dose approach to estimate the average radiation doses delivered to specific organs.40 Time-activity data were fitted to a sum or difference of 2 exponential expressions, and these expressions were integrated to determine the cumulated activity within each region or organ. Organ volumes were estimated using previously described methods39 and were converted to masses assuming unit density. The cumulated activity for each volume, Ã, was divided by the mass of each organ (MORG) to give cumulated activity concentration. Mean absorbed doses to liver, spleen, and red marrow were obtained by multiplying the cumulated activity concentration in each organ by the mean energy emitted per nuclear transition, Δ, for the electron and α particle emissions of213Bi and its daughters 213Po,209Tl, and 209Pb. Relative biologic effectiveness of 5 for cellular inactivation was assumed for α particles.41 The absorbed dose over an organ volume, DORG, is given by the equation:
We calculated the absorbed dose to the blood by first integrating the fitted expression from the pharmacokinetic analysis of serial blood samples to obtain the cumulated activity concentration in the blood, [Ã]BL. Assuming a blood density of 1 g/mL, the average absorbed dose to blood, DBL, was then determined by the equation: DBL = [Ã]BL (Δe + 5Δα). In most patients, pharmacokinetic data were collected only after the first and last injections. If no data were collected during injections, we calculated absorbed doses or dose equivalents by weighted averaging of the image-derived values. The weight assigned to known values depended on the number of injections elapsed between 2 estimates. Known values that were closer in injection number to the unknown value were given greater weight.
Results
Patient characteristics
We treated 18 patients in the study. Fourteen patients had relapsed AML, and 3 had primary refractory AML and did not achieve complete remission after 2 or more induction courses. One patient had relapsed CMMOL (Table 1). The median age was 56 years (range, 17 to 74 years), and the median number of prior treatments was 3 (range, 2 to 9). Four patients previously underwent allogeneic (n = 2) or autologous (n = 2) bone marrow or peripheral blood progenitor cell transplantation.
Patient characteristics
| Patient . | Age/sex . | Diagnosis . | Cytogenetics . | % CD33 expression . | Previous therapy . |
|---|---|---|---|---|---|
| 1 | 74/F | AML, M2, rel | + 8 | 86 | IDR/Ara-C × 2, HiDAC |
| 2 | 54/F | AML, M0, rel | inv (3) | 37 | IDR/Ara-C × 2, AlloBMT, Mito/VP-16 |
| 3 | 64/M | AML, M2, rel | NE | 68 | IDR/Ara-C, IL-2, HiDAC |
| 4 | 67/F | AML, M2, rel | Normal | 83 | IDR/Ara-C, MEC |
| 5 | 70/F | AML, M2, ref | − 5, − 7, + 8, + 11, − 18, − 20, del (20) | 48 | IDR/Ara-C × 2 |
| 6 | 66/M | AML, M6, rel | del (9) | 75 | IDR/Ara-C, Mito/VP-16 |
| 7 | 17/M | AML, M2, ref | − 7 | 57 | IDR/Ara-C, HiDAC |
| 8 | 68/F | AML, M2, rel | − 7, t(2;3) | 84 | Mito/Ara-C, HiDAC, Mito/VP-16, 2-CDA |
| 9 | 30/M | AML, M2, rel | Normal | 75 | IDR/Ara-C, Mito/VP-16, Ara-C, AuPBPCT |
| 10 | 50/F | AML, M2, rel | Normal | 40 | Mito/Ara-C, HiDAC, AuPBPCT |
| 11 | 62/M | AML, M2, rel | + 21 | 48 | IDR/Ara-C, HiDAC × 2, Mito/VP-16, HiDAC × 4 |
| 12 | 57/F | AML, M2, rel | Normal | 63 | HiDAC/IDR, HiDAC/VP-16, HuM195 |
| 13 | 45/M | AML, M2, rel | + 8 | 65 | HiDAC/IDR, HiDAC/VP-16, AlloBMT |
| 14 | 42/F | AML, M2, ref | − 4, del(5), del(7), add (17), − 20 | 92 | HiDAC/IDR, VP-16/Cy |
| 15 | 49/F | AML, M2, rel | Normal | 99 | DNR/Ara-C, HiDAC/Mito, HiDAC × 2 |
| 16 | 19/F | AML, M1, rel | t(9;11) | 97 | IDR/Ara-C, HiDAC/DNR, HiDAC × 2, Mito/VP-16, Gemcitabine |
| 17 | 63/F | CMMOL, rel | Normal | 96 | IDR/Ara-C, MEC |
| 18 | 49/M | AML, M3, rel | dup(1), t(15;17) | 73 | ATRA, Ara-C/VP-16, HiDAC × 2, ATRA/Dox, ATO × 4 |
| Patient . | Age/sex . | Diagnosis . | Cytogenetics . | % CD33 expression . | Previous therapy . |
|---|---|---|---|---|---|
| 1 | 74/F | AML, M2, rel | + 8 | 86 | IDR/Ara-C × 2, HiDAC |
| 2 | 54/F | AML, M0, rel | inv (3) | 37 | IDR/Ara-C × 2, AlloBMT, Mito/VP-16 |
| 3 | 64/M | AML, M2, rel | NE | 68 | IDR/Ara-C, IL-2, HiDAC |
| 4 | 67/F | AML, M2, rel | Normal | 83 | IDR/Ara-C, MEC |
| 5 | 70/F | AML, M2, ref | − 5, − 7, + 8, + 11, − 18, − 20, del (20) | 48 | IDR/Ara-C × 2 |
| 6 | 66/M | AML, M6, rel | del (9) | 75 | IDR/Ara-C, Mito/VP-16 |
| 7 | 17/M | AML, M2, ref | − 7 | 57 | IDR/Ara-C, HiDAC |
| 8 | 68/F | AML, M2, rel | − 7, t(2;3) | 84 | Mito/Ara-C, HiDAC, Mito/VP-16, 2-CDA |
| 9 | 30/M | AML, M2, rel | Normal | 75 | IDR/Ara-C, Mito/VP-16, Ara-C, AuPBPCT |
| 10 | 50/F | AML, M2, rel | Normal | 40 | Mito/Ara-C, HiDAC, AuPBPCT |
| 11 | 62/M | AML, M2, rel | + 21 | 48 | IDR/Ara-C, HiDAC × 2, Mito/VP-16, HiDAC × 4 |
| 12 | 57/F | AML, M2, rel | Normal | 63 | HiDAC/IDR, HiDAC/VP-16, HuM195 |
| 13 | 45/M | AML, M2, rel | + 8 | 65 | HiDAC/IDR, HiDAC/VP-16, AlloBMT |
| 14 | 42/F | AML, M2, ref | − 4, del(5), del(7), add (17), − 20 | 92 | HiDAC/IDR, VP-16/Cy |
| 15 | 49/F | AML, M2, rel | Normal | 99 | DNR/Ara-C, HiDAC/Mito, HiDAC × 2 |
| 16 | 19/F | AML, M1, rel | t(9;11) | 97 | IDR/Ara-C, HiDAC/DNR, HiDAC × 2, Mito/VP-16, Gemcitabine |
| 17 | 63/F | CMMOL, rel | Normal | 96 | IDR/Ara-C, MEC |
| 18 | 49/M | AML, M3, rel | dup(1), t(15;17) | 73 | ATRA, Ara-C/VP-16, HiDAC × 2, ATRA/Dox, ATO × 4 |
Rel indicates relapse; ref, refractory; Ara-C, cytarabine; HiDAC, high-dose cytarabine; AlloBMT, allogeneic bone marrow transplantation; Mito, mitoxantrone; VP-16, etoposide; IL-2, interleukin-2; MEC, mitoxantrone, etoposide, and cytarabine; 2-CDA, cladribine; AuPBPCT, autologous peripheral blood progenitor cell transplantation; Cy, cyclophosphamide; DNR, daunorubicin; ATRA, all-transretinoic acid; Dox, doxorubicin; ATO, arsenic trioxide; NE, not evaluable.
Adverse effects
Treatment with 213Bi-HuM195 was well tolerated. Maximum tolerated dose in this study was not reached because escalation beyond 37 MBq/kg was restricted by the availability and cost of225Ac. Because only 0.3 to 1 mg HuM195 was administered in each dose fraction, no infusion-related toxicity was seen. Grade 1 1iver function abnormalities were seen in 4 patients (22%). Two of these patients had elevations in alkaline phosphatase levels; one had hyperbilirubinemia, and one had an elevated transaminase level. Two patients (11%) had grade 2 hyperbilirubinemia. There was no correlation between administered activity and the occurrence of these abnormalities (r = 0.051; P = .841). The onset was typically 5 to 14 days following treatment, and these abnormalities resolved within 3 to 14 days.
Myelosuppression, demonstrated by a decline in the number of normal peripheral white blood cells or blasts, was seen in all 17 evaluable patients. Thirteen (76%) of the 17 patients developed grade 3 (n = 2) or 4 (n = 11) leukopenia; however, substantial clearing of circulating blasts (more than 95%) accounted for this finding in 11 patients (85%). Decreases in normal leukocyte counts could be explained by the destruction of myeloid progenitors that express CD33 and by the nonspecific irradiation of normal blood elements. Median time from initiation of treatment to resolution of grade 3 or 4 leukopenia was 22 days (range, 12-41 days). Dose-limiting toxicity, defined as grade 4 leukopenia for more than 35 days from the start of therapy, was seen in one patient treated at the 37 MBq/kg dose level following relapse after allogeneic BMT. The duration of myelosuppression was unrelated to the level of CD33 expression (r = 0.119; P = .648), the number of previous treatments (r = 0.207; P = .426), or the administered activity (r = 0.390; P = .121).
Because pancytopenia is a prominent clinical feature of leukemia, we could not evaluate other hematologic toxicities in most patients. Among the 6 patients who had an absolute neutrophil count (ANC) of 1.5 × 109/L or more before receiving213Bi-HuM195, 4 (66%) developed grade 4 neutropenia (ANC less than 0.5 × 109/L). Eight (44%) of the 18 patients were hospitalized for neutropenic fever. Of the 2 patients who had platelet counts greater than 50 × 109/L before treatment, one required transfusions for a platelet count of less than 10 × 109/L following therapy.
Biodistribution
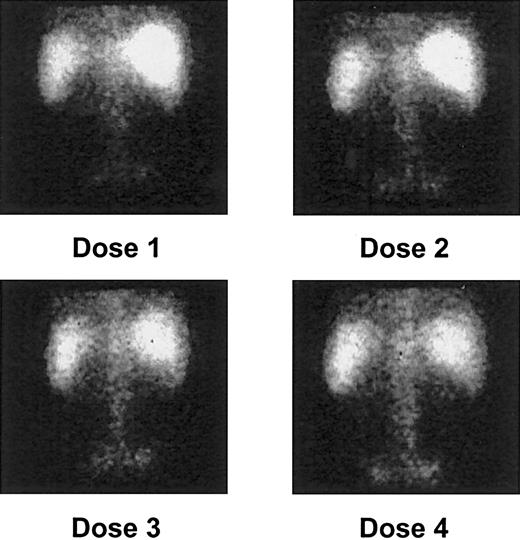
Gamma camera imaging showed localization of 213Bi to expected areas of leukemic involvement, including the bone marrow of the vertebrae and pelvis, the liver, and the spleen in all patients (Figure 1). Despite avidity for free bismuth, the kidneys were not visualized. This suggested that no significant catabolism or clearance of the drug occurred, thereby confirming the stability of the construct in vivo. Uptake by the marrow, liver, and spleen, accounting for 70% to 100% of the administered activity, occurred within 5 to 10 minutes after injection and was maintained throughout the 1-hour period of image collection (Figure 2).
Posterior gamma camera imaging of patient 2 after each of 4 213Bi-HuM195 injections.
Quantitative targeting of isotope to areas of leukemic involvement, including the bone marrow of the vertebrae and pelvis, the liver, and the spleen, is demonstrated. As noted in most patients, there is a progressive increase in bone marrow uptake of the isotope after each dose.
Posterior gamma camera imaging of patient 2 after each of 4 213Bi-HuM195 injections.
Quantitative targeting of isotope to areas of leukemic involvement, including the bone marrow of the vertebrae and pelvis, the liver, and the spleen, is demonstrated. As noted in most patients, there is a progressive increase in bone marrow uptake of the isotope after each dose.
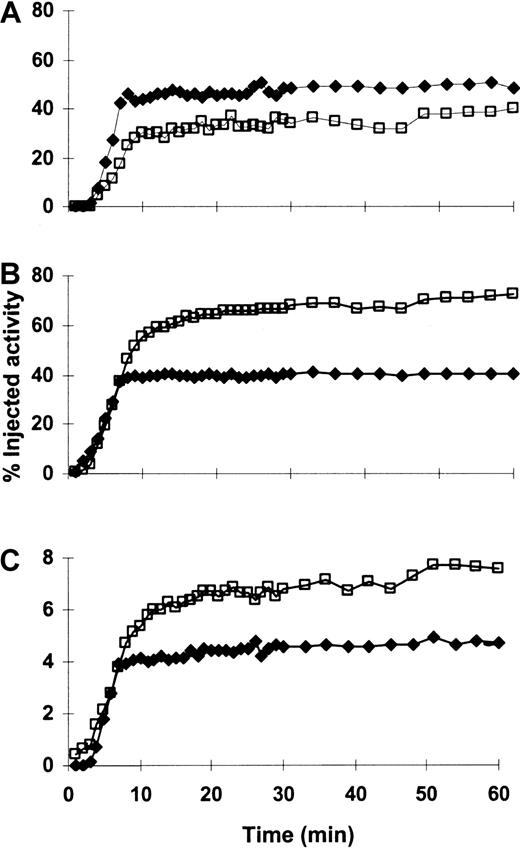
Time-activity curves for a representative patient (patient 15).
213Bi rapidly localized to and was retained in the marrow (A), liver (B), and spleen (C) throughout the 1-hour imaging period. Percentage injected activity increased in the marrow and decreased in the liver and spleen after multiple injections of213Bi-HuM195. This pattern was the most commonly observed in the patients who were studied (see “Results”). The red marrow region sampled on gamma camera imaging is scaled to represent total marrow. Plotted activity is adjusted for decay. (—■—) indicates the first injection; (—♦—) indicates the fourth (last) injection.
Time-activity curves for a representative patient (patient 15).
213Bi rapidly localized to and was retained in the marrow (A), liver (B), and spleen (C) throughout the 1-hour imaging period. Percentage injected activity increased in the marrow and decreased in the liver and spleen after multiple injections of213Bi-HuM195. This pattern was the most commonly observed in the patients who were studied (see “Results”). The red marrow region sampled on gamma camera imaging is scaled to represent total marrow. Plotted activity is adjusted for decay. (—■—) indicates the first injection; (—♦—) indicates the fourth (last) injection.
Sixteen patients underwent gamma camera imaging after at least 2 injections of 213Bi-HuM195 during the treatment course. The pattern of uptake within the bone marrow was variable. Six patients (38%) demonstrated an increase in marrow activity with greater numbers of injections. Five patients (31%) showed a decrease, and activity remained constant in 5 patients (31%). The effect of multiple doses on biodistribution within the liver and spleen was more consistent. Twelve (75%) of the 16 patients had a decrease in liver activity with increasing numbers of injections. Two patients (12.5%) had an increase in liver activity, and the activity was constant in the remaining 2 patients (12.5%). Similarly, 9 patients (56%) had a decrease in activity within the spleen after multiple injections; 1 patient (6%) had an increase, and 6 patients (38%) had no change. This tendency toward decreased liver and spleen uptake after multiple injections of213Bi-HuM195 suggested a CD33 antigen saturation effect at these sites after the administration of several milligrams of antibody.
Pharmacokinetics
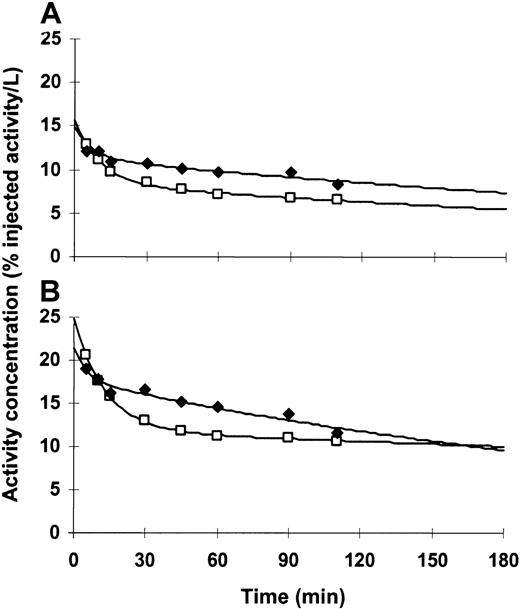
Blood and plasma antibody concentrations displayed typical α distributions over the first 20 to 40 minutes, followed by slower β clearance over the remaining 3 hours of sample collection (Figure3). Additionally, we found that clearance rates were independent of the number of injections. Estimated initial distribution volumes in blood and plasma were similar for all patients, indicating that a significant fraction of activity in the blood was associated with cellular elements. Among the 15 patients for whom pharmacokinetic studies were performed after at least 2 injections, 13 (87%) had higher activity concentrations in blood or plasma following later injections. This suggested that increasing levels of antigen saturation at sites within the bone marrow, liver, and spleen occurred after multiple injections of 213Bi-HuM195.
Time-activity curves for a representative patient (patient 15).
Blood (A) and plasma (B) antibody levels increased after multiple injections, consistent with a CD33 antigen saturation effect. This pattern was seen in 13 of 15 (87%) patients who were studied. Plotted activity is adjusted for decay. (—■—) indicates the first injection; (—♦—) indicates the fourth (last) injection.
Time-activity curves for a representative patient (patient 15).
Blood (A) and plasma (B) antibody levels increased after multiple injections, consistent with a CD33 antigen saturation effect. This pattern was seen in 13 of 15 (87%) patients who were studied. Plotted activity is adjusted for decay. (—■—) indicates the first injection; (—♦—) indicates the fourth (last) injection.
Dosimetry
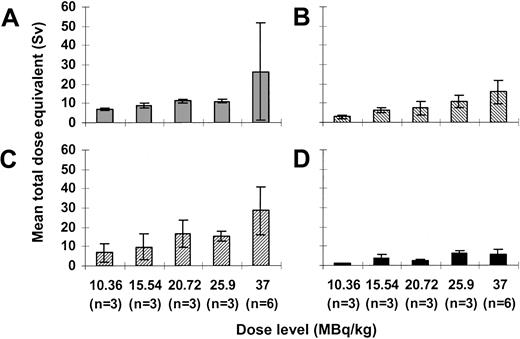
Mean ± standard deviation absorbed dose per amount of injected activity to the marrow was 9.8 ± 6.5 mSv/MBq (range, 2.6-29.4 mSv/MBq). Mean absorbed doses per injected activity for the liver, spleen, and blood were 5.8 ± 1.6 mSv/MBq (range, 3.9-9.7 mSv/MBq), 10.8 ± 5.4 mSv/MBq (range, 3.8-24.2 mSv/MBq), and 2.6 ± 1.2 mSv/MBq (range, 1-5.1 mSv/MBq), respectively. Estimated total dose equivalents to the marrow, and therefore to CD33+ target cells, ranged from 6.6 to 73 Sv. Total dose equivalents to the liver, spleen, and blood ranged from 2.4 to 23.5 Sv, 2.9 to 36.8 Sv, and 1.1 to 11 Sv, respectively (Figure4). As expected, we found linear correlations between the total injected activity and total dose equivalents to the liver (r = 0.764;P < .001), spleen (r = 0.754;P < .001), and blood (r = 0.608;P = .007). Additionally, there was a strong trend toward a positive correlation between injected activity and dose equivalents delivered to the marrow (r = 0.456;P = .057).
Dosimetry for 213Bi-HuM195.
Mean total dose equivalents ± SD delivered to the red marrow (A), liver (B), spleen (C), and blood (D) with administered activities ranging from 10.36 to 37 MBq/kg are shown.
Dosimetry for 213Bi-HuM195.
Mean total dose equivalents ± SD delivered to the red marrow (A), liver (B), spleen (C), and blood (D) with administered activities ranging from 10.36 to 37 MBq/kg are shown.
Because of the short range of the α emissions, the whole-body radiation dose was composed only of photon emissions. Because of the short half-life of 213Bi, we assumed identical residence times for each patient, making the whole-body dose per amount of injected activity 3.6 × 10−4 mSv/MBq. Absorbed doses to organs not targeted by 213Bi-HuM195 were calculated by adding the contributions from the photon dose and the electron dose from both 213Bi and its daughter, 209Pb. Estimated absorbed doses to the heart, kidney, and lungs ranged from 4.1 × 10−3 to 3.9 × 10−2 mSv/MBq.
Antileukemic effects
Fifteen of 18 patients had leukemic blasts in the peripheral blood before treatment with 213Bi-HuM195. Fourteen (93%) of these 15 patients had reductions in circulating blasts following therapy (Figure 5A). Mean ± standard deviation percentage reduction in circulating blasts for these 14 patients was 90% ± 21%, (median, 98.5%; range, 34%-100%). Even at the lowest dose level, 2 of the 3 patients had elimination of more than 99% of peripheral blasts. Up to 3 logs of circulating leukemia cells were killed, and 4 patients (27%) had complete eradication of peripheral leukemia cells. Circulating blast counts decreased rapidly, with nadirs occurring after a median of 10 days (range, 4-17 days). Suppression of peripheral blast counts lasted a median of 19 days (range, 8-42 days).
Antileukemic effects of 213Bi-HuM195.
(A) Numbers of circulating blasts before and after treatment. Fourteen (93%) of 15 evaluable patients had reductions in the number of peripheral blood blasts. (B) Percentages of bone marrow blasts before and after treatment are shown for each dose level from 10.36 to 37 MBq/kg. Fourteen (78%) of the 18 patients had reductions in the percentages of bone marrow leukemia cells after 7 to 10 days.
Antileukemic effects of 213Bi-HuM195.
(A) Numbers of circulating blasts before and after treatment. Fourteen (93%) of 15 evaluable patients had reductions in the number of peripheral blood blasts. (B) Percentages of bone marrow blasts before and after treatment are shown for each dose level from 10.36 to 37 MBq/kg. Fourteen (78%) of the 18 patients had reductions in the percentages of bone marrow leukemia cells after 7 to 10 days.
Fourteen (78%) of the 18 patients had reductions in the percentages of bone marrow leukemia cells 7 to 10 days after treatment (Figure 5B). We did not find a clear correlation between the degree of elimination of blasts in the peripheral blood and bone marrow (r = 0.454;P = .089), likely because 213Bi-HuM195 rapidly targeted and cleared circulating blasts before reaching leukemia cells within the marrow. Among the 4 patients with complete elimination of peripheral blood blasts, 3 had reductions in bone marrow blasts. The percentage reduction of marrow blasts and the level of CD33 expression were also unrelated (r = 0.074; P = .769). Reductions in bone marrow blasts, however, occurred more consistently with higher injected activities. Only 4 (44%) of the first 9 patients had partial responses with at least a 25% decrease in the percentage of bone marrow blasts, whereas 8 (89%) of the last 9 patients had partial responses (P = .046). Across all dose levels, the mean percentage reduction in bone marrow blasts for the 14 responders was 41% ± 22% (median, 41%; range, 8%-79%). Although213Bi-HuM195 produced significant antileukemic effects accounting for hundreds of billions of selectively killed cells, no complete remissions were observed.
Discussion
This is the first study to show the proof-of-concept for α particle immunotherapy in humans. Radioimmunotherapy with long-range β emitters can potentially overcome antigen heterogeneity within the tumor, and it offers advantages when treating bulky disease. For example, β particle–emitting anti-CD33 and anti-CD45 constructs have shown activity in the treatment of advanced acute leukemias.8,25-27 Significant toxicities, however, may result from the nonspecific irradiation of normal tissues, and therapy at high doses is associated with prolonged myelosuppression that requires hematopoietic stem cell transplantation.6,8 25-27Conversely, targeted therapy with high-energy, short-range α particles can potentially provide more efficient and selective delivery of radiation to individual tumor cells. 213Bi-HuM195 represents the first in this new class of drugs to be administered systemically to patients.
Although myelosuppression was seen in all evaluable patients, treatment with 213Bi-HuM195 produced no significant extramedullary toxicity. The drug rapidly cleared from the blood to expected areas of leukemic involvement, including the bone marrow, liver, and spleen, within 5 to 10 minutes of injection. No uptake by any other organ was seen. Of the 16 patients who were studied, 12 (75%) showed decreased activity in the liver after multiple injections of213Bi-HuM195, and 9 (56%) had decreased activity in the spleen. This is likely because of first-pass binding to leukemia cells and CD33+ monocytes at these sites. The pattern of biodistribution in the marrow after multiple injections was variable, with 6 patients (38%) showing an increase in activity, 5 (31%) showing a decrease, and 5 (31%) showing no change. This variability, likely because of differences in tumor burden and first-pass binding, explains the lack of correlation between the duration of myelosuppression and administered activity.
The dosimetry of α particle–emitting radionuclides is distinguished from that of conventional β emitters by a number of characteristics. Few α emitters, including 213Bi, decay to stable or short-lived daughter products. The shorter range and higher linear energy transfer of α particles result in a relative biologic effectiveness for cell sterilization of 3 to 7.41Although a single α particle track traversing the nucleus can result in cell death, several thousands are required for β particles. In this study, we used conventional medical internal radiation dose methodology to determine average radiation doses to tumor cells and normal organs delivered by α and β emissions of 213Bi and its daughters, given the high concentration of α emissions in the regions of interest. We reported radiation dose equivalents (in sieverts) rather than the more commonly used absorbed dose (in grays) to account for the relative biologic effectiveness of the α particle component of the total radiation dose. We chose this more conservative approach because in vitro experimental data suggest that dose equivalents provide a value consistent with the expected biologic response for β emitters.
Although leukemia is characterized predominantly by the unregulated growth of blasts within the bone marrow, extramedullary involvement of the liver and spleen is common. Therefore, most CD33+target cells reside in these 3 sites. Dose equivalents up to 73 Sv, 23.5 Sv, and 36.8 Sv were delivered to the marrow, liver, and spleen, respectively. Calculated absorbed doses to each of these organ volumes, however, may not reflect the actual dose to individual target cells or to normal organ parenchymal cells. Because of the short range of α emissions and their stochastic nature, the cells immediately adjacent to a targeted cell could receive no α particle radiation at all. As a result, 213Bi-HuM195 can deliver extremely large absorbed radiation doses to leukemia and other CD33+ cells within the bone marrow, liver, and spleen while sparing normal tissues, as suggested by the lack of significant extramedullary toxicity in this study. Microdosimetric and stochastic analyses that account for the spatial distribution of various cell types and the distribution of α decays within the organ will be necessary to estimate the absorbed dose to leukemia cells and normal tissues more accurately.42 43
Our previous studies with131I-M195,25,44131I-HuM195,21 and90Y-HuM19526 allowed us to compare the dosimetry of α- and β-emitting radioimmunoconjugates within the same antigen system and patient population. Absorbed dose ratios between the bone marrow, liver, spleen, and the whole body were approximately 1000 times higher for 213Bi-HuM195 than those for the β-emitting constructs (Table 2) because of the markedly decreased whole-body doses and the greater target organ doses for 213Bi compared with 131I and 90Y.
Dosimetry for α and β particle–emitting M195 and HuM195 conjugates
| Isotope . | Absorbed dose (mSv/MBq) . | Red marrow/liver absorbed dose ratio . | Red marrow/whole-body absorbed dose ratio . | ||
|---|---|---|---|---|---|
| Red marrow . | Liver . | Whole body . | |||
| 131I | 2.7 ± 3.1 | 0.8 ± 0.3 | 0.16 ± 0.06 | 3.4 ± 3.3 | 14.4 ± 13.6 |
| 90Y | 6.8 ± 4.8 | 4.0 ± 2.5 | 0.49 ± 0.04 | 1.9 ± 1.0 | 13.9 ± 9.0 |
| 213Bi | 9.8 ± 6.4 | 5.8 ± 2 | 0.0004 ± 0 | 1.7 ± 1.0 | 27 300 ± 17 700 |
| Isotope . | Absorbed dose (mSv/MBq) . | Red marrow/liver absorbed dose ratio . | Red marrow/whole-body absorbed dose ratio . | ||
|---|---|---|---|---|---|
| Red marrow . | Liver . | Whole body . | |||
| 131I | 2.7 ± 3.1 | 0.8 ± 0.3 | 0.16 ± 0.06 | 3.4 ± 3.3 | 14.4 ± 13.6 |
| 90Y | 6.8 ± 4.8 | 4.0 ± 2.5 | 0.49 ± 0.04 | 1.9 ± 1.0 | 13.9 ± 9.0 |
| 213Bi | 9.8 ± 6.4 | 5.8 ± 2 | 0.0004 ± 0 | 1.7 ± 1.0 | 27 300 ± 17 700 |
Values are expressed as mean ± SD.
213Bi-HuM195 displayed clear antileukemic activity. Fourteen (93%) of 15 evaluable patients had reductions in peripheral blood leukemia cells, and 14 (78%) of the 18 patients had reductions in the percentages of bone marrow blasts. There was a predictable increase in the absorbed dose to target organs when higher activities of 213Bi-HuM195 were administered. Accordingly, reductions in bone marrow blasts occurred more consistently at higher dose levels. Observation of a clear dose-response effect over all dose levels, however, was confounded by the small number of patients who displayed a wide variability in tumor burden, target antigen expression, and tumor cell radiation resistance, in part because of previous treatment.
Although 213Bi-HuM195 killed large leukemic volumes in many patients, none achieved complete remission. Because of the nature of α particle radiation, complete remission at 30 days after treatment would have required the individual targeting and killing of 99.9% of the leukemia cells. Given that these patients have tumor burdens of up to 1012 cells, each with an average CD33 density of 10 000/cell, roughly 1016 leukemic binding sites are available to HuM195. Because approximately 1 in 2700 molecules of HuM195 carry the radiolabel at the specific activities injected, it remains difficult to deliver 1 to 2 213Bi atoms to every leukemia cell, even if we assume optimal antibody targeting. Moreover, because of the selectivity of α particle irradiation, CD33− leukemic progenitors may escape its cytotoxic effects. Treatment of overt leukemia with 213Bi-HuM195 as a single agent would require extraordinarily high injected activities. On the other hand, because of the short range and the high linear energy transfer, α particle immunotherapy is ideally suited to the treatment of residual disease. This observation led to a trial in which213Bi-HuM195 is given for the elimination of minimal disease after partial cytoreduction with cytarabine in patients with myeloid leukemia.
The current study provides proof-of-concept for the use of α particle immunotherapy for a variety of malignancies, particularly those in which small-volume, minimal residual, or micrometastatic disease is present. Antitumor effects of α particle immunotherapy may be further enhanced by the use of alternative radioisotopes. We recently reported that tumor-specific 225Ac radioimmunoconjugates could kill a variety of tumor cell lines in vitro at doses 1000 times lower than213Bi-containing constructs. Moreover, in xenograft models of disseminated human lymphoma and solid prostate carcinoma, single doses at kBq (nanocurie) levels prolonged survival and cured a substantial fraction of animals without toxicity.45 The increased potency of these 225Ac constructs compared to213Bi analogs can be explained by the longer (10-day) half-life of 225Ac and by the ability of 225Ac conjugates to act as atomic nanogenerators, emitting 4 α particles within an individual tumor cell as it decays. Based on these data, we plan to conduct a phase 1 trial of 225Ac-HuM195 in advanced myeloid leukemia.
We thank Peter G. Maslak and Ellin Berman for clinical care; Bipin M. Mehta, Michael Curcio, Yan Ma, Jing Qiao, and Lawrence Lai for laboratory assistance; Jenny Jimenez for assistance with data management; Lucy Dantis and her staff for expert research nursing (Memorial Sloan-Kettering Cancer Center, New York, NY); Chuanchu Wu for assistance in preparing the HuM195 immunoconjugate (Radioimmune and Inorganic Chemistry Section, National Cancer Institute, Bethesda, MD); Sead Mirzadeh (Oak Ridge National Laboratory, TN), Christos Apostolidis (Institute for Transuranium Elements, Karlsruhe, Germany), Maurits Geerlings, Sr (Pharmactinium, Alexandria, VA) for supplying225Ac, and Daniel Levitt (Protein Design Labs, Fremont, CA) for supplying HuM195. We dedicate this work to the memory of Otto Gansow, who was instrumental in the development of chelating agents for α particle–emitting radiometals.
Supported by National Institutes of Health grants PO1 CA33049 and RO1 CA55349. J.G.J. is a recipient of a Clinical Oncology Career Development Award from the American Cancer Society. D.A.S. is a Translational Investigator of the Leukemia Society of America and a Doris Duke Distinguished Clinical Scientist.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Joseph G. Jurcic, Memorial Sloan-Kettering Cancer Center, Box 458, 1275 York Ave, New York, NY 10021; e-mail:jurcicj@mskcc.org.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal