Abstract
Activation of intracellular signaling pathways is important for cellular transformation and tumorigenesis. The nonreceptor tyrosine kinases Jak1 and Jak3, which bind to the v-Abl oncoprotein, are constitutively activated in cells transformed with the Abelson murine leukemia virus. A mutant of p160 v-Abl lacking the Jak1-binding region (v-Abl Δ858-1080) has a significant defect in Jak/STAT (signal transducers and activators of transcription) activation, cytokine-independent cell growth/survival, and tumorigenesis. To identify the pathways downstream of Jak kinases in v-Abl–mediated signaling, we examined the activation of several signaling molecules by p160 v-Abl or the v-Abl Δ858-1080 mutant. We demonstrate that, in addition to the decreased Ras activation, signaling through phosphatidylinositol-3 kinase and Akt are impaired in cells expressing mutant v-Abl. The proliferative defect of v-Abl Δ858-1080 was rescued by activated v-Akt and was also moderately rescued by activated v-H-Ras. However, constitutive active phosphatidylinositol-3 kinase (p110CAAX) did not complement this effect. Cells expressing v-Abl Δ858-1080 demonstrated reduced tumor formation in nude mice. In contrast, cells coexpressing v-Akt with v-Abl Δ858-1080 demonstrated reduced latency and increased frequency of tumor formation in nude nice compared with cells expressing v-Abl Δ858-1080 alone, whereas v-H-Ras or p110CAAX had minimum effects on tumor formation. These results suggest that Jak1–dependent Akt activation is important in v-Abl–mediated transformation.
Introduction
Aberrant activation of the Abl nonreceptor tyrosine kinase has been shown to cause lymphoid malignancies in mice and humans.1 Three oncoproteins have been identified among the Abl family of tyrosine kinases; v-Abl is a gag-Abl fusion protein of the Abelson murine leukemia virus, whereas Bcr-Abl and Tel-Abl are fusion proteins produced by chromosomal translocations and are found expressed in human leukemias. All of these proteins have deregulated tyrosine kinase activities that are essential for transformation, as evidenced by the inability of kinase-deficient mutants to transform cells. The molecular mechanisms responsible for Abl-induced transformation are complex. Most Abl-interacting proteins or substrates identified to date are involved in signaling leading to gene transcription, cellular proliferation, and/or survival. However, multiple signaling pathways, such as Ras, phosphatidylinositol-3 kinase (PI 3-K), and Myc, are affected by Abl expression. Because many proteins have been reported to interact with Abl, it has been difficult to define the precise role each of these proteins play in Abl-induced transformation.2
Recent work from our laboratory has demonstrated that certain Jak kinases and STAT (signal transducers and activators of transcription) proteins, including Jak1, Jak2, Jak3, STAT1, STAT5, and STAT6, are constitutively activated in Abelson murine leukemia virus–transformed pre–B-cell lines.3 The full activation of Jak1 in the murine pro B-cell line Ba/F3 requires direct interaction between Jak kinases and v-Abl, which results in activation of STATs and cellular proliferation.4 Several groups have also reported constitutive STAT activation in Bcr-Abl transformed cells,5-9 although consistent activation of Jak kinases has not been observed in all these cells. To understand the molecular basis of v-Abl–induced Jak activation and its consequence, fine mapping of the Jak-binding domain in p160 v-Abl has revealed that amino acids 858 to 1080 within the carboxy-terminus of v-Abl are required for Jak1 binding and its activation.4 A mutant of v-Abl lacking this region exhibited a loss in Jak1 binding, inability to activate Jak and STAT proteins, and a significant defect in supporting either proliferation or survival of Ba/F3 cells in the absence of interleukin-3 (IL-3). All of these effects are likely to be Jak1 dependent, because the expression of kinase-inactive mutant of Jak1 protein prevents v-Abl–induced STAT activation and cytokine-independent growth of Ba/F3 cells.4 Furthermore, cells expressing the v-Abl Δ858-1080 mutant showed extended latency and decreased frequency in generating tumor in nude mice.
Jak kinases are essential for the activation of STATs downstream of cytokine receptors. In addition, the activation of other signaling molecules downstream of these receptors utilizes Jak kinases. Previously, we have demonstrated activation of STATs downstream of Jak kinases in a v-Abl–dependent manner. Whether the activation of Jaks by v-Abl leads to the activation of other signaling pathways is unclear. There has been accumulating evidence suggesting that the full transforming activity of v-Abl involves the activation of signaling pathways, including Ras, PI 3-K, and Akt. Interestingly, a recent report demonstrated that activation of either Ras10,11 or PI 3-K by cytokines12 requires Jak activity. Therefore, it is possible that Jak kinases participate in the activation of these signaling molecules upon v-Abl expression, leading to cytokine-independent proliferation and transformation.
Our previous work has demonstrated that Ras activation is slightly decreased in Ba/F3 cells expressing the v-Abl Δ858-1080 compared with wild-type v-Abl, whereas no significant difference in the phosphorylation of Shc and Dok-1 (p62Dok) and protein expression of Myc was observed.4 However, the functional significance of reduced Ras activation induced by v-Abl Δ858-1080 remains unclear. In this report, we demonstrate that the activation of PI 3-K and Akt are also impaired in Ba/F3 cells expressing v-Abl Δ858-1080. Coexpression of either v-H-Ras, v-Akt, or p110CAAX with v-Abl Δ858-1080 in Ba/F3 cells reveals that v-Akt complements the defect of v-Abl Δ858-1080 in cytokine-independent proliferation of Ba/F3 cells (in vitro) and tumor formation in nude mice. Taken together, these results imply that v-Abl–induced Akt activation is an important signaling pathway in v-Abl–induced cytokine-independent cell growth and that the Jak-binding domain of v-Abl is important for activation of Akt.
Materials and methods
Cell culture and stable transfections
IL-3–dependent murine pro B-cell line Ba/F3 and Ba/F3 transfectants were cultured in RPMI 1640 medium supplemented with 10% fetal calf serum, 5 μM mercaptoethanol, 1 mM sodium pyruvate, 1 mMl-glutamine, and 5% WEHI-3 supernatant. Transfections of Ba/F3 cells were carried out by electroporation as described previously.4 Ba/F3 cells stably expressing v-Abl and a construct encoding a kinase-inactive/dominant-negative Jak1 under the control of the metallothionine promoter were incubated overnight in 10−4 M final ZnSO4.
Plasmid
The p160 v-Abl expression vector and the expression vector for v-Abl lacking the entire Jak1-binding domain (Δ858-1080) were described previously.4 The expression vectors for v-Akt and p110CAAX were generously provided by Dr Tomas Franke (Columbia University), and the expression vectors for v-H-Ras13 were a generous gift from Dr Konstantina Alexandropoulos (Columbia University).
Antibodies, immunoprecipitation, and immunoblotting
The Abl antibody (Ab-2) was purchased from Calbiochem (Cambridge, MA). The anti-Akt antibody and antiphosphoserine-Akt antibody were from New England Biolab (Beverly, MA). The antibodies against Ras and p110 subunit of PI 3-K were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The antiphosphotyrosine antibodies were purchased from Upstate Biotechnology (Lake Placid, NY). The β-actin antibody was purchased from Sigma Immunochemicals (St Louis, MO). Preparation of whole-cell extracts, immunoprecipitation, and immunoblotting were done as described previously.4
PI 3-K assay
For in vitro PI 3-K assay, Ba/F3 cells were washed and resuspended in complete RPMI media in the absence of IL-3. Cells were left untreated or treated with IL-3 (10 ng/mL) for 5 minutes. Reactions were stopped with 8 vol ice-cold phosphate-buffered saline (PBS) (0.4 mM Na3VO4, 0.4 mM ethylenediaminetetraacetic acid, without Ca2+ or Mg2+), and cells were lysed in lysis buffer (20 mM Tris [pH 8], 138 mM NaCl, 10% glycerol, 1% Nonidet P-40, 1 mM phenylmethylsulfonyl fluoride, 100 μg/mL aprotinin, 100 μg/mL leupeptin, 10 μg/mL pepstatin, 1 mM Na3VO4, 2 mM ethylenediaminetetraacetic acid, and 10 mM NaF). Extracts were immunoprecipitated with anti–PI 3-K p85 antibody, and each immunoprecipitate was washed with 0.5 M LiCl in 0.1 M Tris (pH 7.5) and then with reaction buffer (20 mM HEPES [pH 7.4], 15 mM MgCl2, 1 mM Na3VO4, 0.5 mM phenylmethylsulfonyl fluoride). Immunoprecipitates were prewarmed to room temperature and resuspended in reaction buffer with 25 μM cold adenosine triphosphate, 10 μCi (.37 MBq) γ32P–adenosine triphosphate, and 20 μg/mL PI (Avanti Polar Lipids, Alavaster, AL) for 15 minutes. Reactions were stopped by adding 40 μL of 1N HCl, and lipids were extracted in 80 μL chloroform:MeOH (1:1). Lipids were resolved by thin-layer chromatography in chloroform/acetone/methanol/acetic acid/H2O (46:17:15:14:8). For in vivo PI 3-K assay, cells were starved from IL-3 overnight and were incubated in the presence of 0.06 mCi (2.22 MBq) orthophosphate for 6 hours in phosphate-free RPMI media supplemented with 0.1% bovine serum albumin. Reactions were terminated by the addition of ice-cold 1N HCl:MeOH (1:1). Lipids were extracted with chloroform and resolved by thin-layer chromatography.
Proliferation assays
Cells were washed extensively in medium without IL-3 and resuspended at a density of 5 × 104 cells in 100 μL of complete RPMI 1640 medium in 96-well plates. After 48 hours, the cells were pulsed with 1 μCi (0.037 MBq) of3H-TdR for 6 hours in culture, and 3H incorporation was quantified by using scintillation counter.
Apoptosis assay
Cells were washed extensively in medium without IL-3 and cultured for time periods described. For staining with propidium iodide, cells were washed extensively with ice-cold PBS, and the samples were resuspended in buffer containing 0.1 mg/mL propidium iodide in PBS. Then the samples were analyzed by fluorescence-activated cell sorter.
Nude-mouse injections
Cells were washed extensively, and 5 × 106 cells were resuspended in 200 μL PBS. Female nude mice (4 to 8 weeks old) were injected subcutaneously and were monitored for visible signs of cell growth during a 30-day period after injection.
Ras assays
Cells were starved of cytokines, and the assays were performed as described previously.4
Results
Activation of the PI 3-K/Akt and Ras pathways is impaired downstream of v-Abl Δ858-1080
We previously reported that the v-Abl Δ858-1080 mutant has defects in supporting cytokine-independent proliferation, cell survival upon growth factor withdrawal, tumor formation in nude mice, and bone marrow transformation.4,14 To analyze the impaired signaling pathways downstream of this mutant, we have compared the activation of various signal transduction pathways between p160 v-Abl and the Δ858-1080 mutant. As described previously, the level of tyrosine-phosphorylated Shc, Dok-1 (p62Dok), or the level of c-Myc protein in Ba/F3 cells transfected with the v-Abl Δ858-1080 mutant is unaffected when compared with cells expressing wild-type v-Abl.4 However, the level of Ras activation observed in v-Abl–expressing Ba/F3 cells is higher than that in Ba/F3 cells transduced with v-Abl Δ858-1080.4 To further examine the impact of this deletion on protein function, several other signaling pathways normally activated by v-Abl or those implicated in proliferation and cell survival were analyzed.
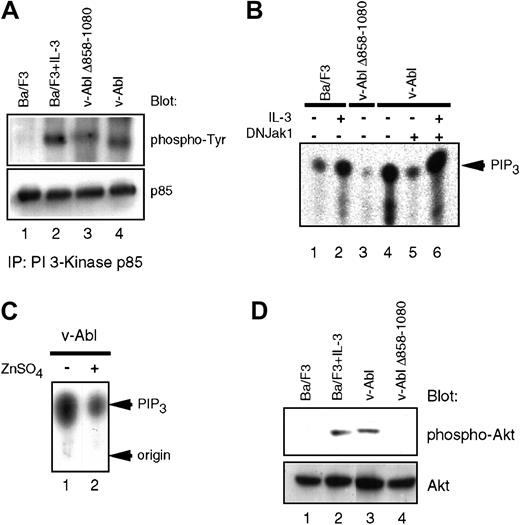
Lymphoid cells derived from Jak-1–deficient mice have specific defects in IL-7–induced proliferation,15 and Jak inhibitors have previously been shown to block IL-7–induced PI 3-K activation.16 Because we have also demonstrated that IL-7 could reconstitute several aspects of v-Abl–mediated signaling, which leads to cell cycle progression and prevention of apoptosis,17 we assessed the activation of PI 3-K in the v-Abl transfectants. Interestingly, the phosphorylation level of the regulatory subunit of PI 3-K (p85) in cells expressing v-Abl Δ858-1080 is reduced when compared with wild-type p160 v-Abl (Figure1A), suggesting that the PI 3-K enzymatic activity may be reduced when this domain in the carboxyl-terminal portion of v-Abl is deleted. Indeed, in vitro and in vivo kinase assays demonstrate that the v-Abl–induced PI 3-K activity, as assessed by the levels of phosphoinositide 3 phosphate and phosphoinositide 3,4,5 tris phosphate (PIP3), is diminished in v-Abl Δ858-1080 (Figure 1B). The kinase function of Jak1 is required for v-Abl to activate PI 3-K, because inducible expression of a kinase-deficient Jak1 blocks this effect (Figure 1B,C). IL-3 treatment of cells that express kinase-deficient Jak1 restores the PI 3-K activity, suggesting that the defect in activation of PI- 3K in the presence of the mutant Jak1 is not cell autonomous (Figure 1B).
Impaired activation of PI 3-K/Akt pathway in the v-Abl Δ858-1080 mutant.
(A) Tyrosine phosphorylation of the p85 subunit of PI 3-K is affected downstream of the v-Abl Δ858-1080. Extracts prepared from the indicated cell lines were immunoprecipitated with an anti-p85 antibody and immunoblotted with antiphosphotyrosine antisera. (B) Deletion of the Jak1-binding domain or expression of a kinase-deficient Jak1 reduces PI 3-K activation downstream of v-Abl in vitro. In vitro PI 3-K activation assays were performed by assessing lipid kinase activity in phosphotyrosine immunoprecipitates prepared from the indicated cell lines. L-α phosphatidylinositol phosphate (PIP) was used as substrate. (C) Jak1 kinase activity is required for maximum induction of PIP3 in vivo. In vivo PIP3 levels were measured by extracting lipids from the p160 v-Abl–expressing Ba/F3 cell line prior to (lane 1) or after induction of kinase-inactive Jak1 (lane 2). (D) Activation of Akt downstream of the v-Abl Δ858-1080. Eighty micrograms of whole cell extracts was fractionated on 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and immunoblotted with an antibody specific for activated Akt. For loading control, the blot was stripped and reprobed with an antibody to pan-Akt, which recognizes Akt regardless of its state of activation.
Impaired activation of PI 3-K/Akt pathway in the v-Abl Δ858-1080 mutant.
(A) Tyrosine phosphorylation of the p85 subunit of PI 3-K is affected downstream of the v-Abl Δ858-1080. Extracts prepared from the indicated cell lines were immunoprecipitated with an anti-p85 antibody and immunoblotted with antiphosphotyrosine antisera. (B) Deletion of the Jak1-binding domain or expression of a kinase-deficient Jak1 reduces PI 3-K activation downstream of v-Abl in vitro. In vitro PI 3-K activation assays were performed by assessing lipid kinase activity in phosphotyrosine immunoprecipitates prepared from the indicated cell lines. L-α phosphatidylinositol phosphate (PIP) was used as substrate. (C) Jak1 kinase activity is required for maximum induction of PIP3 in vivo. In vivo PIP3 levels were measured by extracting lipids from the p160 v-Abl–expressing Ba/F3 cell line prior to (lane 1) or after induction of kinase-inactive Jak1 (lane 2). (D) Activation of Akt downstream of the v-Abl Δ858-1080. Eighty micrograms of whole cell extracts was fractionated on 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and immunoblotted with an antibody specific for activated Akt. For loading control, the blot was stripped and reprobed with an antibody to pan-Akt, which recognizes Akt regardless of its state of activation.
Because Akt was shown to be important for transformation of hematopoietic cells by Bcr/Abl,18 the levels of Akt activation were examined in Ba/F3 cells expressing either wild-type v-Abl or v-Abl Δ858-1080. Although the activation of Akt in Ba/F3 cells expressing wild-type v-Abl is observed even after growth factor starvation, the activation of Akt in cells expressing the v-Abl Δ858-1080 mutant is significantly decreased to a level comparable to growth factor–depleted parental Ba/F3 cells (Figure 1D). Taken together, these results suggest a role for Jak1 in the functional regulation of both PI 3-K and Akt by v-Abl.
We performed similar experiments in another IL-3–dependent cell line of myeloid lineage, 32D. We found that 32D cells expressing wild-type v-Abl proliferate even in the absence of IL-3. On the contrary, 32D cells expressing v-Abl Δ858-1080 do not proliferate well in the absence of cytokine (data not shown). Furthermore, 32D cells expressing wild-type v-Abl demonstrated activation of Akt in the absence of IL-3. In contrast, the level of Akt activation in 32D cells expressing v-Abl Δ858-1080 was much weaker (data not shown). These results suggest that the phenotypic alterations found in cells expressing the v-Abl Δ858-1080 mutant are not specific to Ba/F3 cells but can also be demonstrated in other cytokine-dependent cell lines.
Expression of v-Akt or v-H-Ras restores the proliferative defect of the v-Abl Δ858-1080 mutant
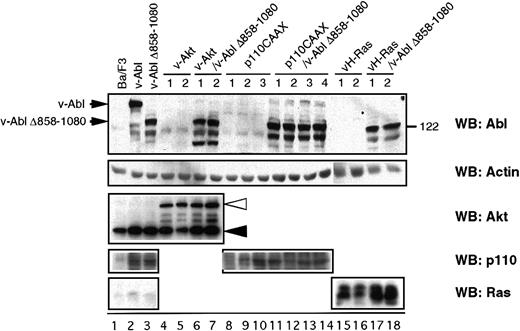
To investigate the functional relevance of the signaling molecules differentially activated by the v-Abl Δ858-1080 mutant, Ba/F3 cell lines stably expressing the v-Abl Δ858-1080 mutant were stably transfected with activated forms of either Ras (v-H-Ras), Akt (v-Akt), or p110CAAX (a membrane-targeted constitutively active form of the p110 subunit of PI 3-K). Stable transfectants expressing v-H-Ras, v-Akt, or p110CAAX in the absence of v-Abl Δ858-1080 were also generated as controls. As shown in Figure 2, the expression levels of the introduced proteins were compared by Western blot analysis. Because all control transfectants have similar or higher levels of expression than the cells that also express v-Abl Δ858-1080, these cell lines serve as controls to explore any v-Abl–independent effect of the proteins.
Characterization of Ba/F3-derived transfectants.
Cell extracts prepared from the indicated cell lines were analyzed by Western blot for the tyrosine phosphorylation status and the expression of either p160 v-Abl, Akt, p110 subunit of PI 3-K, or v-H-Ras. The closed and open triangles indicate the endogenous c-Akt and introduced v-Akt, respectively.
Characterization of Ba/F3-derived transfectants.
Cell extracts prepared from the indicated cell lines were analyzed by Western blot for the tyrosine phosphorylation status and the expression of either p160 v-Abl, Akt, p110 subunit of PI 3-K, or v-H-Ras. The closed and open triangles indicate the endogenous c-Akt and introduced v-Akt, respectively.
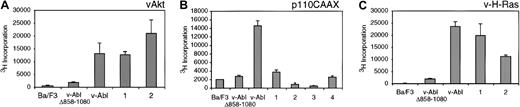
To examine the ability of the complemented cell lines to proliferate in the absence of IL-3, thymidine incorporation assays were performed (Figure 3). As previously reported, Ba/F3 cells expressing the v-Abl Δ858-1080 have reduced ability to proliferate in the absence of IL-3 when compared with cells expressing wild-type p160 v-Abl.4 When cells expressing v-Abl Δ858-1080 with v-Akt are examined, the proliferative activity is restored to levels seen with wild-type v-Abl (Figure 3A). The effect of v-Akt is more striking than that of v-H-Ras, increasing proliferation to levels even higher than observed with wild-type v-Abl (Figure 3C). However, coexpression of p110CAAX does not result in increased proliferation with respect to cells expressing v-Abl Δ858-1080 alone (Figure 3B), indicating that recruitment of activated PI 3-K to the plasma membrane alone is not sufficient to mimic the effects of v-Akt. Neither v-H-Ras, v-Akt, nor p110CAAX expression alone affects the IL-3 dependency of Ba/F3 cells (data not shown), indicating that activation of any of these proteins in the absence of v-Abl is insufficient to confer cytokine independence in Ba/F3 cells. Together, these results suggest that the activated forms of either Ras or Akt can complement the v-Abl Δ858-1080 mutation when cytokine-independent growth is examined.
Analysis of cytokine-independent cell growth of Ba/F3-derived transfectants.
Parental Ba/F3 cells and other transfectants were washed extensively and resuspended in the media without cytokine at a density of 5 × 104/100 μL complete RPMI and plated in 96-well plates. The cells were cultured for another 48 hours and pulsed with 1 μCi (0.037 MBq) of 3H-TdR for 8 hours, and then3H incorporation was quantified. The number underneath each column represents independent Ba/F3 clone coexpressing v-Abl Δ858-1080 and either v-Akt (A), p110CAAX (B), or v-H-Ras (C), respectively.
Analysis of cytokine-independent cell growth of Ba/F3-derived transfectants.
Parental Ba/F3 cells and other transfectants were washed extensively and resuspended in the media without cytokine at a density of 5 × 104/100 μL complete RPMI and plated in 96-well plates. The cells were cultured for another 48 hours and pulsed with 1 μCi (0.037 MBq) of 3H-TdR for 8 hours, and then3H incorporation was quantified. The number underneath each column represents independent Ba/F3 clone coexpressing v-Abl Δ858-1080 and either v-Akt (A), p110CAAX (B), or v-H-Ras (C), respectively.
The PI 3-K/Akt pathway is not sufficient for v-Abl–mediated protection from apoptosis
The Ras,19,20 PI 3-K, and Akt pathways21,22 have been reported to regulate induction of apoptosis through regulation of antiapoptotic or proapoptotic genes. Ba/F3 cells expressing wild-type v-Abl are resistant to apoptosis after cytokine withdrawal, whereas Ba/F3 cells expressing v-Abl Δ858-1080 are not.4 To determine whether this defect in protection from apoptosis is due to the defective activation of either Ras, Akt, or PI 3-K by v-Abl Δ858-1080, the complemented cell lines were incubated in the absence of IL-3 and stained with propidium iodide at different time points after cytokine withdrawal. Surprisingly, coexpression of either v-Akt or p110CAAX with v-Abl Δ858-1080 does not increase cell survival upon cytokine starvation when compared with cells expressing v-Abl Δ858-1080 alone (Figure4A,B). This suggests that activation of either the PI 3-K or the Akt pathways is not sufficient to complement the loss of the Jak-dependent protection from apoptosis. Expression of v-H-Ras with v-Abl Δ858-1080 results in slightly increased cell survival with respect to cells expressing v-Abl Δ858-1080 alone, but this slight increase in cell survival is also observed when v-H-Ras is expressed alone. Therefore, we cannot exclude the possibility that v-H-Ras can increase cell survival of these cells independently of Jak activation by v-Abl. These results also indicate that the hyperproliferation of Ba/F3 cells coexpressing v-Abl Δ858-1080 and v-Akt is mainly due to enhanced cell cycle progression rather than suppression of apoptosis.
Apoptosis analysis of Ba/F3-derived transfectants in the absence of IL-3.
Parental Ba/F3 cells and other transfectants were washed extensively and resuspended at a density of 2 × 105 to 3 × 105 cells per milliliter in the media without cytokine. The aliquots of the cells were harvested after every 24 hours, stained with propidium iodide, and analyzed by fluorescence-activated cell sorter. Each column represents the independent Ba/F3 clones of each category.
Apoptosis analysis of Ba/F3-derived transfectants in the absence of IL-3.
Parental Ba/F3 cells and other transfectants were washed extensively and resuspended at a density of 2 × 105 to 3 × 105 cells per milliliter in the media without cytokine. The aliquots of the cells were harvested after every 24 hours, stained with propidium iodide, and analyzed by fluorescence-activated cell sorter. Each column represents the independent Ba/F3 clones of each category.
Akt activation restores the efficiency and latency of tumorigenesis of Ba/F3 cells expressing v-Abl Δ858-1080
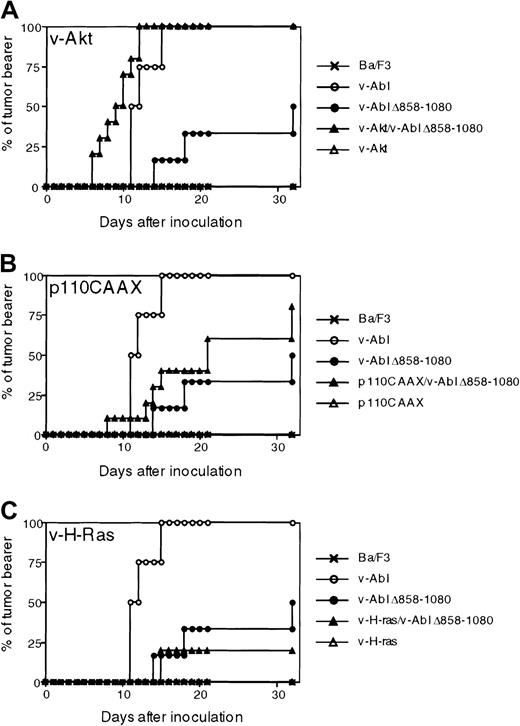
We have previously reported that, upon subcutaneous injection in mice, cells expressing v-Abl Δ858-1080 form tumors less efficiently and with extended latency than those expressing wild-type v-Abl.4 Therefore, we examined the ability of v-H-Ras, v-Akt, and PI 3-K to restore the tumorigenicity of cell lines expressing v-Abl Δ858-1080. Nude mice were inoculated subcutaneously with Ba/F3 cells coexpressing v-Abl Δ858-1080 with either v-H-Ras, v-Akt, or p110CAAX. Subcutaneous injections with cells expressing wild-type v-Abl alone or v-Abl Δ858-1080 alone were also included as controls, as well as injections with cells expressing either v-H-Ras, v-Akt, or p110CAAX in the absence of v-Abl Δ858-1080. Mice were examined over a period of 30 days for signs of visible tumor growth. Tumors were detected within 2 weeks after inoculation in most of the nude mice challenged with wild-type v-Abl–expressing Ba/F3 transformants (Figure 5). In contrast, only 10% of the mice inoculated with cells expressing the Jak1-binding mutant of v-Abl showed visible growth during this period. Tumors were eventually visible more than 30 days after inoculation in half of the mice that received cells expressing the mutant v-Abl. Parental Ba/F3 cells did not give rise to any tumors during the course of these experiments.
Akt, but not p110CAAX or v-H-Ras, complements the efficiency and latency of the v-Abl Δ858-1080 mutant in tumorigenesis.
Nude mice were injected with either parental IL-3–dependent Ba/F3 cells; Ba/F3 transfectants expressing wild type; v-Abl Δ858-1080 mutant; single transfectants that express either v-Akt, p110CAAX, or v-H-Ras; or double transfectants that express v-Abl Δ858-1080 mutant and either v-Akt, p110CAAX, or v-H-Ras. The mice were monitored for visible signs of growth over the 30-day period of after injection. Data from 3 experiments are pooled. The total numbers of mice injected for each category of transfectants were as follows: 2 for parental Ba/F3 cells, 4 for p160 v-Abl–Ba/F3, 4 for Jak1-binding mutant Ba/F3, 6 for Akt-Ba/F3, 4 for Ras-Ba/F3, 4 for p110CAAX-Ba/F3, 10 for Akt/J1BM-Abl-Ba/F3, 10 for Ras/J1BM-Abl-Ba/F3, and 10 for p110CAAX/J1BM-Abl-Ba/F3.
Akt, but not p110CAAX or v-H-Ras, complements the efficiency and latency of the v-Abl Δ858-1080 mutant in tumorigenesis.
Nude mice were injected with either parental IL-3–dependent Ba/F3 cells; Ba/F3 transfectants expressing wild type; v-Abl Δ858-1080 mutant; single transfectants that express either v-Akt, p110CAAX, or v-H-Ras; or double transfectants that express v-Abl Δ858-1080 mutant and either v-Akt, p110CAAX, or v-H-Ras. The mice were monitored for visible signs of growth over the 30-day period of after injection. Data from 3 experiments are pooled. The total numbers of mice injected for each category of transfectants were as follows: 2 for parental Ba/F3 cells, 4 for p160 v-Abl–Ba/F3, 4 for Jak1-binding mutant Ba/F3, 6 for Akt-Ba/F3, 4 for Ras-Ba/F3, 4 for p110CAAX-Ba/F3, 10 for Akt/J1BM-Abl-Ba/F3, 10 for Ras/J1BM-Abl-Ba/F3, and 10 for p110CAAX/J1BM-Abl-Ba/F3.
Of the complemented cells assayed for tumor formation, the cells coexpressing v-Akt with v-Abl Δ858-1080 showed the most drastically recovered tumor formation (Figure 5A). Tumor growth in these mice was observed 6 days after inoculation, and all the mice inoculated showed typical signs of tumor growth after 12 days, whereas at this time point tumors had not begun to appear in mice injected with cells expressing v-Abl Δ858-1080 alone. Furthermore, the cells coexpressing v-Akt and v-Abl Δ858-1080 formed tumors faster and more efficiently than cells expressing wild-type v-Abl. These observations suggest that the Jak-dependent activation of Akt is important in rendering Ba/F3 cells highly tumorigenic in nude mice. The mice inoculated with Ba/F3 cells coexpressing p110CAAX and v-Abl Δ858-1080 showed a moderate increase in the rate of tumor formation with respect to mice inoculated with cells expressing v-Abl Δ858-1080 alone (Figure 5B), although the difference in tumor incidence between v-Abl Δ858-1080 and p110 CAAX/v-Abl Δ858-1080 was not significant by statistical analysis. However, tumor formation was not as efficient as in mice inoculated with Ba/F3 cells expressing wild-type v-Abl, implying that lack of PI 3-K activation in Ba/F3 cells expressing v-Abl Δ858-1080 may contribute to the defect in tumorigenicity but is not likely the sole cause of the defect. In contrast, inoculation of cells coexpressing v-H-Ras and v-Abl Δ858-1080 showed a minimum effect for both tumor latency and rate of incidence (Figure 5C), indicating that the induction of Ras activation alone is not sufficient for rescuing the tumor formation defect of Ba/F3 cells expressing v-Abl Δ858-1080.
Analysis of the crosstalk of Akt, PI 3-K, and Ras in the transfectants expressing v-Abl Δ858-1080
Akt has been shown to be recruited to the plasma membrane and activated by interacting PIP3, one of the major products of PI 3-K.23-26 Ras is also known to induce PI 3-K activation,27-29 which results in subsequent Akt activation in various cells. Furthermore, oncogenic Ras induces the transformation of epithelial cells by maintaining cell cycle progression and by preventing apoptosis,30 31 which depends on activation of both PI 3-K and Akt. To investigate the crosstalk of these molecules in the transfectants expressing v-Abl Δ858-1080, we analyzed the activation of Akt, PI 3-K, and Ras in these cells.
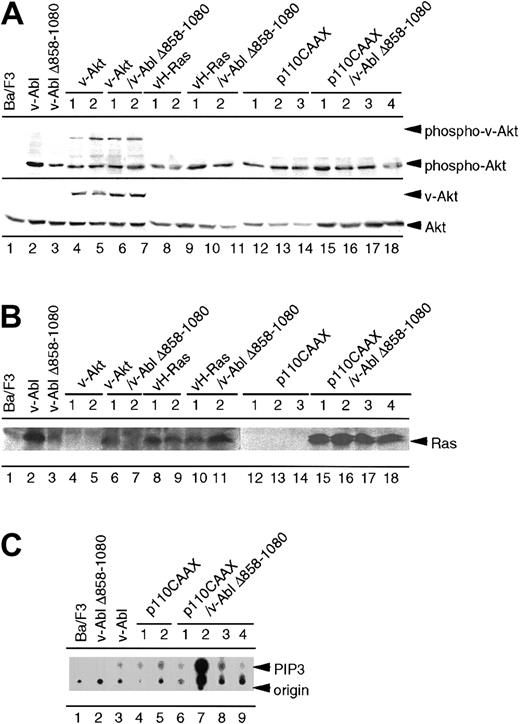
As shown in Figure 6A, activation of Akt is observed in Ba/F3 cells expressing wild-type v-Abl, while the activation of Akt in cells expressing the v-Abl Δ858-1080 mutant was significantly reduced. The residual phosphorylation of Akt detected in Ba/F3 cells expressing the v-Abl Δ858-1080 was greater compared with the result shown in Figure 1D, in which almost complete inhibition of Akt was observed in Ba/F3 cells expressing the v-Abl Δ858-1080. We have found increased residual activation of Akt in cell lines that have been passaged for several generations in culture. The serine phosphorylation of introduced v-Akt was observed in cells transfected with v-Akt. The activation of Akt was not induced to the level of that of Ba/F3 cells expressing wild-type v-Abl in the cells that express v-Abl Δ858-1080 with either v-H-Ras or p110CAAX. This indicates that neither v-H-Ras nor p110CAAX could fully activate Akt to complement the defect of the Ba/F3 cells expressing v-Abl Δ858-1080 mutant.
Analysis of the mutual activation of Ras, PI 3-K, and Akt in the parental Ba/F3 cells and the transfectants.
(A) Parental Ba/F3 cells and other transfectants were cultured in the media without IL-3 overnight. Cell extracts prepared from the indicated cell lines were analyzed by Western blotting using antiphosphoserine-Akt antibody. The membrane was reprobed with pan-Akt antibody. (B) Parental Ba/F3 cells and other transfectants were cultured in the media without IL-3 for 4 hours. Cell extracts prepared from the indicated cell lines were subjected to pull-down analysis using GST-Raf fusion protein. Precipitates were separated on sodium dodecyl sulfate–polyacrylamide gel electrophoresis and blotted with anti-Ras antibody. (C) Parental Ba/F3 cells and transfectants expressing p110CAAX alone or p110CAAX/v-Abl Δ858-1080 were subjected to PIP3 assay as described in “Materials and methods.”
Analysis of the mutual activation of Ras, PI 3-K, and Akt in the parental Ba/F3 cells and the transfectants.
(A) Parental Ba/F3 cells and other transfectants were cultured in the media without IL-3 overnight. Cell extracts prepared from the indicated cell lines were analyzed by Western blotting using antiphosphoserine-Akt antibody. The membrane was reprobed with pan-Akt antibody. (B) Parental Ba/F3 cells and other transfectants were cultured in the media without IL-3 for 4 hours. Cell extracts prepared from the indicated cell lines were subjected to pull-down analysis using GST-Raf fusion protein. Precipitates were separated on sodium dodecyl sulfate–polyacrylamide gel electrophoresis and blotted with anti-Ras antibody. (C) Parental Ba/F3 cells and transfectants expressing p110CAAX alone or p110CAAX/v-Abl Δ858-1080 were subjected to PIP3 assay as described in “Materials and methods.”
Using the pull-down assay with GST-Raf fusion protein, we also investigated the Ras activation in these cells. As previously shown, Ba/F3 cells expressing the v-Abl Δ858-1080 mutant have less Ras activation than cells expressing p160 v-Abl.4 As shown in Figure 6B, strong Ras activation was observed in Ba/F3 cells overexpressing either constitutive active Ras or v-Abl Δ858-1080 along with v-H-Ras. No Ras activation could be observed in the clones expressing either v-Akt alone or p110CAAX alone. Introduction of v-Akt into Ba/F3 cells expressing v-Abl Δ858-1080 resulted in no change of Ras activation. Moderate activation of Ras was observed in each of the Ba/F3 clones expressing both v-Abl Δ858-1080 and p110CAAX.
To exclude the possibility that the inability of p110CAAX to rescue the defect of Ba/F3 clones expressing both v-Abl Δ858-1080 and p110CAAX is due to the lack of enzymatic activity of introduced p110 proteins, we examined PI 3-K activity in these cells. All Ba/F3 cells expressing p110CAAX with or without v-Abl Δ858-1080 have higher activity to catalyze the formation of PIP3 (Figure 6C), indicating that the introduction of active p110 alone is not sufficient for v-Abl Δ858-1080 clones to confer the proliferative activity or tumorigenic ability.
Discussion
The studies described here have focused on understanding the involvement of signaling molecules downstream of Jak kinases (ie, Akt, PI 3-K, and Ras) in v-Abl–induced cellular proliferation, apoptosis, and tumorigenesis. We have demonstrated that, in Ba/F3 cells expressing either a mutant of p160 v-Abl lacking Jak-binding region (v-Abl Δ858-1080) or a p160 v-Abl along with a kinase-deficient mutant of Jak1, there are significant defects in activation of the PI 3-K, Ras, and Akt pathways. By using the IL-3–dependent Ba/F3 cells coexpressing v-Abl Δ858-1080 and constitutively active forms of either Akt, PI 3-K, or Ras, we have also shown in several independent assays that activated Akt can rescue several defects of Ba/F3 cells expressing the v-Abl Δ858-1080 mutant, while expression of either active PI 3-K or active Ras showed moderate or minimum effect.
There are several pathways that could lead to v-Abl–induced Ras activation. It is still not known, however, which of those pathways is the dominant or essential one. First, the direct binding of Shc to the SH2 domain of v-Abl and subsequent tyrosine phosphorylation leads to the assembly of a signaling complex with Grb-2/SOS.32Secondly, a link between the carboxy-terminus of v-Abl and the Ras pathway has also been suggested,33 and a variety of SH3-containing proteins, including Crk, CrkL, Nck, and Grb-2, may be involved in Ras activation by binding to the proline-rich region in the C-terminus of v-Abl.34 Furthermore, Dok family proteins, which could recruit p120 RasGAP to its binding partner, have been reported to interact with v-Abl and Bcr-Abl.35,36 The Abi family proteins,37,38 which interact with C-terminal proline-rich domain of Abl kinase through their SH3 domain, could also potentially modulate Ras. Recently, Fan et al39 have demonstrated that Abi-1 could interact with SOS GEF and down-regulate v-Abl–induced activation of extracellular signal-regulated kinases, implying that Abi-1 modulates v-Abl–induced Ras activation directly by unknown mechanisms or indirectly by precluding the interaction of SH3-containing adapter proteins. We have previously shown that the level of activated Ras is partially reduced downstream of the v-Abl Δ858-1080 mutant.4 This result indicates that there is a novel Ras-activating pathway that is dependent on the Jak-binding domain of v-Abl and that the other pathway(s) leading to Ras activation may be intact in Ba/F3 cells expressing v-Abl Δ858-1080. Considering the moderate effect of constitutive active Ras on reconstituting cytokine-independent cell growth and the minimum effect on rescuing tumor formation, the reduction of Ras activation downstream of v-Abl Δ858-1080 may not be functionally important. This also suggests that the levels of activated Ras in cells expressing v-Abl Δ858-1080 may be enough to support the Ras-mediated functions of v-Abl during transformation. Notably, the introduction of Ras into Ba/F3 cells has some protective effect against apoptosis irrespective of the expression of v-Abl. Recently, cooperative and redundant effects of STAT5 and Ras signaling have been shown in Bcr/Abl transformed hematopoietic cells, where expression of v-H-Ras alone in Ba/F3 cells provided nearly complete protection from apoptosis.40 The reason our results differ from those published is not clear. One possibility is the expression level of Ras in these cells. Ras was introduced differently in our work and the work of Hoover et al40(electroporation vs retroviral infection). Retroviral infection may result in higher protein expression in the cells. In addition, the concentration of G418 used for selection (1 mg/mL) in our study is half of the concentration used in the previous work. This could have allowed the growth of clones expressing less Ras.
The SH2 and the kinase domains of v-Abl have been shown to regulate the recruitment of p85 and the activation of PI 3-K in fibroblasts.41 Our results suggest that other mechanisms could be involved in the regulation of this pathway, mediated by the Jak-binding domain of v-Abl. One possible mechanism by which Jak kinase may directly affect PI 3-K is through recruitment and phosphorylation of the p85 regulatory subunit of PI 3-K.42 Another potential mechanism may involve an indirect effect via Ras. It has previously been suggested that the p110 catalytic subunit of PI 3-K is a bona fide Ras effector molecule, because it binds GTP Ras and is positively regulated by this exchange factor.27-29 The involvement of Ras, however, is unlikely because concomitant expression of v-Ras with v-Abl Δ858-1080 in Ba/F3 cells showed at most a moderate effect on cytokine-independent proliferation, prevention of apoptosis, and tumor formation. On the other hand, the observed reduction of the in vivo levels of PIP3 downstream of the v-Abl Δ858-1080 mutant or the kinase-inactive Jak1 protein may reflect a more complex regulation of lipid turnover involving phosphoinositide phosphatases, such as PTEN and SHIP. A recent report has implicated a role for both PTEN and the PH domain–containing protein Akt in transformation by Bcr-Abl.18 Furthermore, SHIP is phosphorylated downstream of Bcr-Abl and may play a functional role in transformation by this oncogenic form of Abl.43 It is therefore possible that Jak kinase activation leads to an increase in the level of PIP3 by regulating PTEN44-46 or SHIP47 48 in the context of v-Abl. These results have interesting implications for Jak1 function both in the context of cytokine signal transduction and in the context of signals regulated by the carboxy-terminus of v-Abl and their potential roles in transformation of lymphoid cells.
Studies have shown that inhibition of the PI 3-K/Akt pathway using wortmannin or a dominant-negative form of Akt results in reduced Bcr-Abl–dependent colony formation of murine bone marrow cells and reduced development of leukemia in severe combined immunodeficient mice,18 underscoring the important role of this pathway in Bcr-Abl–mediated transformation. Furthermore, PI 3-K/Akt activation is required for the Bcr-Abl–dependent induction of Bcl-2 and c-Myc18 and for inhibition of expression of the cell cycle inhibitor p27.49 Recently, Tel-Jak2, another fusion protein detected in lymphoid and myeloid leukemia with constitutive tyrosine kinase activity,50 was shown to mediate constitutive activation of the PI 3-K/Akt signaling pathway.51 Our results demonstrated that activation of Akt is essential for v-Abl–induced cellular proliferation and tumorigenesis of Ba/F3 cells. Introduction of viral Akt protein (v-Akt) rescues the defects of the v-Abl Δ858-1080 mutant in both tumor formation in nude mice and cytokine-independent growth of Ba/F3 cells. Furthermore, much more aggressive tumor growth is observed in the mice inoculated with Ba/F3 cells coexpressing the v-Abl Δ858-1080 and v-Akt than in the mice inoculated with cells expressing wild-type v-Abl. Because v-Akt, the oncogene of acute retrovirus AKT8 isolated from AKR mice, is capable (by itself) of rendering a nontumorigenic T-cell lymphoma line highly tumorigenic, introduction of v-Akt into Ba/F3 cells with the v-Abl Δ858-1080 may cause the activation of additional signaling pathways, resulting in the hypertumorigenic activities of those transfectants. However, v-Akt expression alone does not render Ba/F3 cells tumorigenic.
Introduction of the membrane-targeted active form of the catalytic subunit of PI 3-K (p110CAAX) partially complements the defect of the v-Abl Δ858-1080 mutant in tumor formation in nude mice. This partial effect of p110CAAX may be a consequence of insufficient PI 3-K activation for tumorigenesis in vivo. Alternatively, it is possible that other signaling pathways, still impaired in the p110CAAX/v-Abl Δ858-1080 double transfectants, are required for full compensation. One possible candidate is STATs. Supporting evidence for this hypothesis is that STAT5 activation, which is impaired in Ba/F3 cells expressing v-Abl Δ858-1080 and has been shown to induce antiapoptotic bcl-xL downstream of Bcr-Abl, is still impaired in cells coexpressing p110CAAX and v-Abl Δ858-1080 (S.O. et al, unpublished observation, December 2001). Interestingly, p110CAAX showed no effect in supporting cytokine-independent cell proliferation, indicating that the requirement of signaling pathways for tumor formation in vivo and cytokine-independent cell proliferation in vitro is slightly different in the context of Ba/F3 cells.
Analysis of the crosstalk of Akt, PI 3-K, and Ras revealed that the strong activation of Akt was achieved only by introducing wild-type v-Abl or v-Akt into Ba/F3 cells, and all the other clones, including Ba/F3 cells expressing p110CAAX, could not fully activate Akt comparable to Ba/F3 cells expressing wild-type v-Abl. The introduced p110CAAX, however, was functional because Ba/F3 clones expressing p110CAAX showed significantly higher PI 3-K activity than all the other clones. Considering that the cellular responses of Ba/F3 cells expressing v-Abl Δ858-1080 seem to be differentially regulated after introduction of either p110CAAX or v-Akt in proliferation, apoptosis, and tumor formation in vivo, there are 2 possibilities that could explain this phenomenon. The first possibility is that PI 3-K and Akt are independently regulated in the context of Ba/F3 cells expressing v-Abl. The second possibility is that activation of PI 3-K is necessary but not sufficient for full activation of Akt induced by v-Abl in Ba/F3 cells. Taken together, our results indicate that Akt activation, which is impaired in cells expressing v-Abl Δ858-1080, has a critical role on v-Abl–induced transformation.
We thank Tomas Franke for expression vectors for v-Akt and p110CAAX and thank Konstantina Alexandropoulos for expression vectors for v-H-Ras.
Supported by National Institutes of Health grants RO1 CA77862 (to P.B.R.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Paul B. Rothman, Dept of Medicine/Microbiology, Columbia University, 630 W 168th St, New York, NY 10032-3702; e-mail:pbr3@columbia.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal