Abstract
Primary (AL) amyloidosis is a plasma cell dyscrasia characterized by extracellular deposition of monoclonal light-chain variable region (V) fragments in the form of amyloid fibrils. Light-chain amyloid is rare, and it is not fully understood why it occurs in only a fraction of patients with a circulating monoclonal component and why it typically associates with λ isotype and λVI family light-chain proteins. To provide insights into these issues, we obtained complete nucleotide sequences of monoclonal Vλ regions from 55 consecutive unselected cases of primary amyloidosis and the results were compared with the light-chain expression profile of polyclonal marrow plasma cells from 3 healthy donors (a total of 264 sequences). We demonstrated that: (1) the λIII family is the most frequently used both in amyloidosis (47%) and in polyclonality (43%); (2) both conditions are characterized by gene restriction; (3) a very skewed repertoire is a feature of amyloidosis, because just 2 germline genes belonging to the λIII and λVI families, namely 3r (22% of cases, λIII) and 6a (20%, λVI), contributed equally to encode 42% of amyloid Vλ regions; (4) these same 2 gene segments have a strong association with amyloidosis if their prevalences are compared with those in polyclonal conditions (3r, 8.3%,P = .024; 6a, 2.3%, P = .0008, χ2 test); (5) the Jλ2/3 segment, encoding the fourth framework region, appears to be slightly overrepresented in AL (83% versus 67%, P = .03), and this might be related to preferential Jλ2/3 rearrangement in amyloid (11 of 12 cases) versus polyclonal 3r light chains (13 of 22 cases). These findings demonstrate that Vλ-Jλ expression is more restricted in plasma cells from amyloidosis than from polyclonal bone marrow and identify 3r as a new disease-associated gene segment. Overusage of just 2 gene segments,3r and 6a, can thus account for the λ light-chain overrepresentation typical of this disorder.
Introduction
Immunoglobulin light chain–related (AL) amyloidosis is the most common form of amyloidosis in Western countries and the only one caused by a tumor. A marrow plasma cell clone1 synthesizes structurally unstable monoclonal light chains whose variable (V) domains form systemic amyloid deposits.2 In most cases AL amyloidosis leads to organ failure and finally death.3 4
Only a few Bence Jones proteins form amyloid in vivo and λ light chains are observed approximately 2 times more frequently than κ ones.2,5 These observations suggest the existence of Vλ genes with a propensity to form amyloid (amyloidogenic). Indeed, light-chain protein sequencing6 and enzyme-linked immunosorbent assay (ELISA) typing7 showed a very high prevalence of λVI family light chains in amyloidosis and a strong association with this disorder: with rare exceptions, λVI monoclonal light chains are found only in patients with AL. Since this seminal observation, no other amyloid-associated genes have been identified.
Virtually any organ excluding the brain parenchyma can be the target of amyloid systemic deposition and in almost any combination. The reasons for the diversity in organ distribution of AL amyloidosis are unknown. Very recently, Comenzo et al8 documented that λVI light chains were more likely to be found in patients with dominant renal involvement at diagnosis, raising the possibility that, at least in some instances, the diverse organ tropism of amyloid light chains may be influenced by VL gene usage.
To provide insights into the above issues, we report here the first comparative Vλ germline gene usage analysis in plasma cells from amyloidosis and normal polyclonal bone marrow and analyze the relationship between amyloid Vλ expression and specific organ damage.
Patients, materials, and methods
Patient population, clinical data, and materials
The patient population consisted of 55 consecutive λ amyloid cases enrolled at the coordinating center of the Italian Amyloid Program (Internal Medicine and Medical Oncology, University of Pavia, Pavia, Italy). All patients had biopsy-proven amyloidosis (apple-green birefringence of Congo red–stained specimens) in one or more sites. Abdominal fat aspiration was performed in all patients (85% positive), and a biopsy was taken from the clinically dominant organ in 25 cases (45%, all positive, often performed in other institutions), and from other typical sites (ie, rectum, gingiva, labial salivary glands) in 6 cases (11%, all positive). Patients were categorized for the dominant syndrome at presentation according to the major clinical manifestations and laboratory data, including ultrasound studies.9 10 In the case of multiple organ involvement, patients were assigned according to the most prominent characteristic. For instance, a patient with nephrotic syndrome and congestive heart failure, with characteristic echocardiographic changes, was assigned to the heart group, whereas a patient with clinically overt nephrotic syndrome and asymptomatic heart involvement (with minor echocardiographic changes only, ie, interventricular septum thickness of 12 mm) was assigned to the kidney group. Renal involvement was defined as 24-hour proteinuria more than 0.5 g or serum creatinine more than 1.2 mg/dL, or renal failure and dialysis. The following echocardiographic features defined heart involvement: mean interventricular wall thickness 13 mm or more (or ≥ 12 mm in case of typical granular sparkling or diastolic dysfunction, ≥ 14 mm in the presence of arterial hypertension). Patients were stratified according to the New York Heart Association classification and those defined as having dominant cardiac involvement were in class II or higher. Liver involvement was defined as hepatomegaly (at ultrasound) and an increase of serum alkaline phosphatase level more than 279 U/L (institutional upper reference limit), with alanine transaminase and aspartate transaminase below double the upper reference limit. Neuropathic involvement was defined based on clinical history, paresthesias, orthostatic hypotension, delayed emptying at gastric scans, diarrhea or persistent constipation, abnormal electromyography, impotence, absence of intraday variations of heart frequency at Holter electrocardiography. Patients with unobvious involvement, such as gastrointestinal or urinary tract, lung, soft tissue, or lymph node involvement, underwent organ biopsies to confirm amyloid.
Association with clinically overt multiple myeloma was excluded by the absence of osteolytic lesions or generalized osteoporosis, hypercalcemia, anemia, and bone marrow plasma cells (BMPCs) 20% or higher.
A bone marrow aspirate was taken at diagnosis after obtaining the patients' informed consent. BMPCs from 3 donors were used as controls for Vλ gene usage in the normal population. The donors had no history of chronic infection and were apparently in good health.
Cloning and sequencing of immunoglobulin light-chain variable regions
The Vλ regions were isolated by an inverse polymerase chain reaction (PCR)–based procedure previously described in detail.11 Briefly, double-stranded complementary DNA (cDNA) from Ficoll-separated bone marrow mononuclear cells was blunt-ended, ligated on itself to form a circle using T4 DNA ligase (Gibco BRL, Grand Island, NY), PCR-amplified with primers specific for the 5′ and 3′ ends of the λ light-chain constant region and oriented toward the V region (5′ primer) or toward the 3′ end of the constant region (3′ primer). The PCR products were gel purified and plasmid cloned. Several plasmid inserts were then sequenced on either strand using an automated DNA sequencer and compared to each other. Because primers (both placed on the constant region) cannot generate amplification bias, the presence of the same V region sequence in multiple clones indicates its monoclonal origin. That the monoclonal V region did indeed correspond to the amyloidogenic light chain was demonstrated by partial amino acid sequencing from the deposited light chain.12 Conversely, in the case of normal bone marrow, cloned Vλ sequences differed from each other, as expected for a polyclonal condition.
In the case of amyloid light chains, multiple inserts (median, 5; range, 3-12) from each cloning procedure were sequenced for a total of 284 plasmid clones. In every case (100% efficiency) it was possible to identify a single, identical, repeated Vλ region that was considered monoclonal. In 29 patients (53%), all sequenced inserts contained just the same monoclonal Vλ region fragment. In other cases, different Vλ sequences were observed together with the predominant monoclonal ones, an occurrence that had been previously noted and attributed to residual polyclonal plasma cells.12 Monoclonal amyloid sequences were derived from analysis of an average of 4 (range, 3-9) repeated plasmid clones. Even when several repeated clones were analyzed from the same patient (7 cases, 6 inserts; 1 case, 7 inserts; 1 case, 8 inserts; 1 case, 9 inserts), no significant nucleotide substitution was observed. Nucleotide sequences of the Vλ regions were submitted to the GenBank database (accession numbers:AF462643-AF462689, AF026919-AF026926).
Because there is no information on the expression repertoire of Vλ genes in polyclonal marrow plasma cells, inverse PCR was used to characterize Vλ regions from 3 healthy donors. A total of 264 diverse sequences were analyzed and were all potentially functional. In healthy donors, the inverse PCR was run using total RNA extracted from plasma cells isolated via CD138 (syndecan-1) indirect immunomagnetic selection (Dynal, Oslo, Norway). RNA from unselected bone marrow mononuclear cells was used in the case of amyloid patients.
Identification of Vλ and Jλ germline segments
To identify the presumed germline genes of Vλ regions, alignment was made with the current releases of EMBL-GenBank and V-BASE (V BASE Sequence Directory, Tomlinson et al, MRC Centre for Protein Engineering, Cambridge, England) sequence directories using the BLAST13 and DNAPLOT (Althaus H-H, University of Cologne, Germany) search tools, respectively. Sequences were all potentially functional (no stop codons, frameshifts, or pseudogenes).
Statistical analysis
Means and SDs were used to describe continuous variables and absolute and relative frequencies to describe categorical variables. CIs (at 95% level) were calculated to assess comparability of Vλ family and germline use frequencies in the 3 individual healthy donors. The frequency distributions of families or germline genes in monoclonal and polyclonal light chains were compared by means of χ2or Fisher exact test; the mean mutation rates were compared with an unpaired t test (accounting for unequal variances). The distributions of the dominant organ syndromes in the 3r and6a light-chain cases were compared by means of Fisher exact test. The Mann-Whitney U test was used to compare the number of organs involved in the 2 groups. Observed and expected gene frequencies were compared by means of a χ2 test. Significance was lessened according to Bonferroni in all post hoc tests. Median survival was computed based on Kaplan and Meier estimates of cumulative survival. Stata 7 (Statacorp, College Station, TX) was used for all computations.
Results
Patient population
The patients'characteristics are listed in Table1. No history or clinical features of secondary or hereditary amyloidosis were recorded. A monoclonal component was found either in serum or urine in all cases and there was concordance between the monoclonal component isotype and the bone marrow plasma cell isotype ratio at immmunofluorescence.14The most frequently observed dominant syndromes at diagnosis involved the kidney and heart (together they accounted for 71% of all organs).
Clinical and laboratory findings for the 55 AL amyloidosis patients studied
| Characteristic . | No. . |
|---|---|
| Median age, y (range) | 57 (39-80) |
| Men/women | 37/18 |
| Median survival from diagnosis, mo (range)* | 38 (1-131+) |
| Bone marrow aspirate, % | |
| Plasma cell infiltration | 7 (median, range 2-20) |
| Serum monoclonal component, no. of patients (%) | |
| Present | 50 (91) |
| IgGλ | 17 (31) |
| IgAλ | 7 (13) |
| IgDλ | 1 (2) |
| λ light chains only | 25 (45) |
| Urine monoclonal component, no. of patients (%) | |
| Present | 47 (85) |
| IgGλ | 5 (9) |
| IgAλ | 1 (2) |
| IgGλ + free λ light chains | 1 (2) |
| IgAλ + free λ light chains | 1 (2) |
| λ light chains only | 39 (71) |
| No. of organs involved†, no. of patients (%) | |
| 1 | 21 (38) |
| 2 | 23 (42) |
| 3 | 9 (16) |
| 4 | 2 (4) |
| Therapy, no. of patients (%) | |
| Treated | 53 (96) |
| MP | 27 (49) |
| M-HDex | 5 (9) |
| HDex | 8 (15) |
| HDCT+ASCT | 8 (15) |
| Iodo-DOX | 5 (9) |
| Characteristic . | No. . |
|---|---|
| Median age, y (range) | 57 (39-80) |
| Men/women | 37/18 |
| Median survival from diagnosis, mo (range)* | 38 (1-131+) |
| Bone marrow aspirate, % | |
| Plasma cell infiltration | 7 (median, range 2-20) |
| Serum monoclonal component, no. of patients (%) | |
| Present | 50 (91) |
| IgGλ | 17 (31) |
| IgAλ | 7 (13) |
| IgDλ | 1 (2) |
| λ light chains only | 25 (45) |
| Urine monoclonal component, no. of patients (%) | |
| Present | 47 (85) |
| IgGλ | 5 (9) |
| IgAλ | 1 (2) |
| IgGλ + free λ light chains | 1 (2) |
| IgAλ + free λ light chains | 1 (2) |
| λ light chains only | 39 (71) |
| No. of organs involved†, no. of patients (%) | |
| 1 | 21 (38) |
| 2 | 23 (42) |
| 3 | 9 (16) |
| 4 | 2 (4) |
| Therapy, no. of patients (%) | |
| Treated | 53 (96) |
| MP | 27 (49) |
| M-HDex | 5 (9) |
| HDex | 8 (15) |
| HDCT+ASCT | 8 (15) |
| Iodo-DOX | 5 (9) |
MP indicates mephalan and prednisone; M-HDex, melphalan plus high-dose dexamethasone; HDex, high-dose dexamethasone; HDCT, high-dose chemotherapy; ASCT, autologous stem cell transplant; Iodo-DOX, 4”-iodo-4”-deoxydoxorubicin.
According to Kaplan and Meier.
Major organs involved are reported in Figure 6.
Family and germline gene usage in amyloid and polyclonal λ light chains
Amyloid (no. 55) and polyclonal (no. 264) sequences were tested in databases to identify the best matching Vλ and Jλ germline segments. Unequivocal assignment was possible in all cases. Vλ family (Figure 1) and germline gene uses (data not shown) were found to be quite similar in the 3 healthy donors (widely overlapping CIs), and results were pooled.
Vλ family expression in BMPCs from 3 healthy donors.
The frequencies of expression are quite similar in the different individuals. Significant representation is limited to the first 3 families (λI, λII, and λIII), with λIII being the most prevalent.
Vλ family expression in BMPCs from 3 healthy donors.
The frequencies of expression are quite similar in the different individuals. Significant representation is limited to the first 3 families (λI, λII, and λIII), with λIII being the most prevalent.
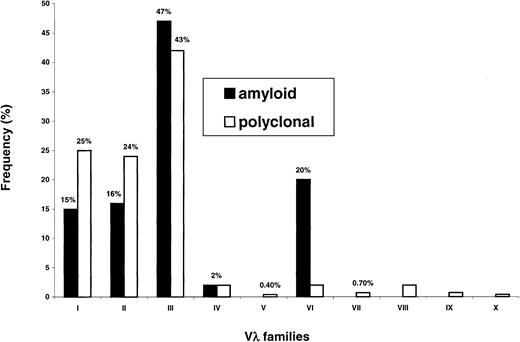
Figure 2 illustrates Vλ family uses in amyloidosis and in polyclonal conditions. In both instances, the λIII family was by a large amount the most represented. This family is the most complex (9 germline gene members) and almost half of amyloid and polyclonal λ light chains (47% and 43%, respectively) belonged to this group. Despite this fact, the Vλ family expression patterns markedly differed in the 2 conditions (P < 1 × 10−4), mainly because of increased usage of λVI in amyloidosis (20% versus 2%,P < 1 × 10−4), a well-established feature of this disorder.
Vλ family expression in plasma cells from amyloidosis and normal bone marrow.
In both conditions, the most frequently observed Vλ family is the λIII. Overrepresentation of the λVI family, a well-known feature of amyloidosis, is very significant (P < 1 × 10−4).
Vλ family expression in plasma cells from amyloidosis and normal bone marrow.
In both conditions, the most frequently observed Vλ family is the λIII. Overrepresentation of the λVI family, a well-known feature of amyloidosis, is very significant (P < 1 × 10−4).
Figure 3 reports the use of Vλ germline genes. Only a fraction of the 31 functional segments contributed significantly to the repertoires, indicating restriction. Again, the germline gene expression patterns differed in the 2 conditions (P = .002).
Vλ germline gene repertoires in plasma cells from primary amyloidosis and polyclonal bone marrow differ markedly (P = .002).
Restriction in gene usage is observed in both conditions, but is particularly evident in amyloid because of 2 gene segments, namely 3r and 6a, which are very significantly overrepresented and, therefore, associated with this disorder.
Vλ germline gene repertoires in plasma cells from primary amyloidosis and polyclonal bone marrow differ markedly (P = .002).
Restriction in gene usage is observed in both conditions, but is particularly evident in amyloid because of 2 gene segments, namely 3r and 6a, which are very significantly overrepresented and, therefore, associated with this disorder.
Just 5 germline segments accounted for approximately 50% of polyclonal λ light chains (Figure 3, blank bars). A member of the λIII family,3h, was the most frequently observed (15%), followed by2a2 (λII, 12%), 1e (λI), 3m(λIII), and 3r (λIII), each found in 8% of sequences. The rearrangement frequencies of the above germline genes were significantly higher than predicted from their presence in the genome (P < .001). Some segments were rarely observed, whereas others (2d, 3e, 4a, 4c,5e, 5c, 7b) were never found.
Biased expression was even more evident in amyloidosis because only 3 gene segments were sufficient to encode 53% of Vλ regions (in decreasing order of magnitude, 3r, 6a,3h). The rearrangement frequencies of 3r (22%) and 6a (20%) were much higher than in polyclonality, revealing a preferential association of these genes with amyloidosis (3r, P = .024; 6a,P = .0008, significance lessened according to Bonferroni). Germline segment 3h, the one most frequently involved in polyclonal light chains (15%), was rearranged to a similar degree in AL (11%).
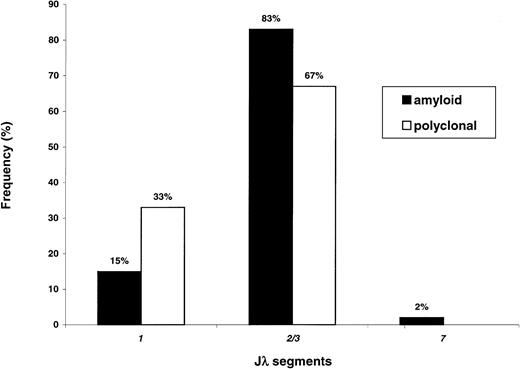
Figure 4 reports the Jλ rearrangement frequencies in the 2 populations. Overexpression of Jλ2/3was found in both cases. However, the frequency of this segment appears to be significantly higher in AL (83% versus 67% of cases,P = .030 with Bonferroni correction), with relative underutilization of Jλ1 (15% versus 33%,P = .016). This finding could be partly explained by the virtually exclusive association of amyloid 3r–derived sequences with Jλ2/3 (11 of 12 cases, 92%). In contrast, polyclonal 3r light chains presented more even rearrangement frequencies with Jλ2/3 (13 of 22, 59%) andJλ1 (9 of 22, 41%). The difference in theJλ2/3 rearrangement frequencies of amyloid and polyclonal3r light chains approached statistical significance (P = .061).
Jλ segment expression in amyloidosis and normal BMPCs.
The Jλ2/3 segment is the most frequently used in both conditions; however, usage of this segment appears to be higher in amyloidosis as compared to in polyclonality (P = .030); conversely, the Jλ1 segment is less frequently involved in AL (P = .016).
Jλ segment expression in amyloidosis and normal BMPCs.
The Jλ2/3 segment is the most frequently used in both conditions; however, usage of this segment appears to be higher in amyloidosis as compared to in polyclonality (P = .030); conversely, the Jλ1 segment is less frequently involved in AL (P = .016).
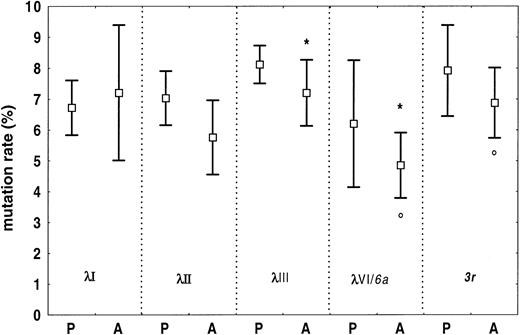
Somatic mutations were found in all sequences, and the overall frequency of nucleotide substitution was lower in amyloidosis than in polyclonality (amyloid, mean 6.4%, 95% CI, 5.7%-7.2%; polyclonality, mean 7.4%, 95% CI, 7.0%-7.8%,P = .021). Figure 5 reports the mutation rates of individual Vλ families for the 2 conditions. In amyloidosis, the λIII family, and its subset of 3r light chains, were significantly less homologous to germline than λVI/6a-light chains (P = .019 andP = .018, respectively).
Somatic mutations in amyloid and polyclonal Vλ regions from BMPCs.
Somatic mutation rates for specific subgroups are reported as means and 95% CIs. Overall, the frequency of somatic changes is significantly higher in polyclonal than in amyloid λ light chains. In amyloidosis, the λIII light chains are more mutated than the λVI (6a) light chains (*P = .019). This holds true also for3r light chains versus 6a light chains (°P = .018). Germline gene 6a is the single germline gene of the λVI family. P indicates polyclonal; A, amyloidosis.
Somatic mutations in amyloid and polyclonal Vλ regions from BMPCs.
Somatic mutation rates for specific subgroups are reported as means and 95% CIs. Overall, the frequency of somatic changes is significantly higher in polyclonal than in amyloid λ light chains. In amyloidosis, the λIII light chains are more mutated than the λVI (6a) light chains (*P = .019). This holds true also for3r light chains versus 6a light chains (°P = .018). Germline gene 6a is the single germline gene of the λVI family. P indicates polyclonal; A, amyloidosis.
Germline gene usage in λ amyloid light chains and the dominant organ syndrome
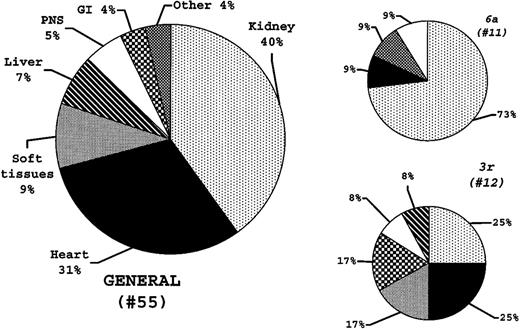
The distributions of the dominant organ syndromes at diagnosis in the 55 consecutive patients and in the 3r and 6alight-chain cases are reported in Figure6. The distribution observed in the3r patients paralleled that in the general patient population (Figure 6). By contrast, kidney involvement was overrepresented in the 6a patients (P = .019). There was no difference in the numbers of organs involved at diagnosis between patients with 3r and 6a light chains (P = .207).
Vλ germline gene usage and major amyloid organ involvement at diagnosis.
The distributions of the major organs involved are reported for the general population and for the 3r and 6a light chain patients. The 6a light chains are more frequently observed in patients with major kidney amyloid (P = .019), whereas 3r light chains appear to be able to infiltrate all amyloid organ targets.
Vλ germline gene usage and major amyloid organ involvement at diagnosis.
The distributions of the major organs involved are reported for the general population and for the 3r and 6a light chain patients. The 6a light chains are more frequently observed in patients with major kidney amyloid (P = .019), whereas 3r light chains appear to be able to infiltrate all amyloid organ targets.
Discussion
This study reports the first expression repertoire analyses of Vλ-Jλ segments in plasma cells from amyloidosis and normal bone marrow. Useful information is derived for λ light-chain genetics in both conditions. Vλ gene usage is highly restricted in both conditions, amyloid and normal, with significant overexpression of 2 germline genes, 3r and 6a, and, possibly, of the Jλ2/3 segment in amyloidosis.
Repertoire analyses in pathologic conditions must satisfy some fundamental features; these include an unbiased and efficient sequencing strategy, an unselected patient population, and information on the normal repertoire to test the significance of findings.
Sequencing data were obtained by means of an established inverse-PCR sequencing strategy11; because both primers are located on the constant region, all V regions can be efficiently amplified independently of their sequence and, after plasmid cloning, the fraction of clones with a given V region sequence is proportional to the amount of its RNA in the sample. These features make inverse PCR useful for repertoire analysis of both monoclonal and polyclonal conditions; in the former instance, an identical repeated V region is identified, whereas multiple diverse sequences are found in the case of polyclonality. The successful cloning of monoclonal light or heavy chains in a series of 72 consecutive patients12 15 (plus this report; and V.P., unpublished results, August 2001), as well as polyclonal light and heavy chains from bone marrow and peripheral blood of healthy subjects (this report; and V.P., unpublished results, August 2001) illustrates the general applicability of this approach.
The patient population of this study consisted of consecutive cases seen at our institution. The Pavia center coordinates a National Amyloid Program with collaboration spread throughout Italy and patients are referred here for diagnosis and treatment.16 Although we cannot formally exclude biases in recruitment, the patients' characteristics summarized in Table 1 indicate the generality of our population. Survival, monoclonal component characteristics, plasma cell percentage, amyloid-dominant organ syndrome, number of organs involved, and treatment are fairly representative of larger patient series.16-19
Three marrow donors with no evidence of chronic infections were used to study the expressed repertoire in polyclonal conditions. Sequencing data in polyclonal conditions were obtained by means of the same PCR approach and from isolated marrow plasma cells. This step was crucial because available information on the normal Vλ repertoire was limited to peripheral blood lymphocytes,20,21 and results from this cell population may differ. Although peripheral blood lymphocytes comprise both pregerminal and postgerminal center cells, marrow plasma cells constitute a homogenous population of typically antigen-selected, postgerminal center cells and a major source of serum immunoglobulins, particularly of IgG.22 23 Information on the expressed repertoire has implications for basic and clinical immunology.
Comparable numbers of plasmid clones were sequenced from each individual donor for a total of 264 polyclonal Vλ regions. A few germline genes dominated the repertoire and their frequency of use was rather similar in the 3 different individuals. Results were, therefore, pooled. Just 5 segments accounted for approximately half of all polyclonal light chains, and these included 3 members of the λIII family (3h, 15%; 3m and 3r, 8% each), a λII (2a2, 12%), and a λI member (1e, 8%). The most frequently used gene segment was the λIII family member 3h, which was found to be rearranged at a remarkably similar frequency (11.9%, 14.6%, 13.8%). With the exception of 1e, the other 4 dominating segments are less than 200 kb close to theJλ-Cλ cluster and belong to cluster A, which contains the λII and λIII families and one member of the λIV family.24 The polyclonal expressed repertoire is therefore restricted due to bias in the rearrangement of a few germline genes. Because restriction was similar in different individuals, it is likely that it is a general attribute of the λ light-chain repertoire. Indeed, Ignatovich et al20 and Farner et al,21 who have published the only 2 studies on the topic, documented similar restrictions in the expression repertoires of peripheral blood lymphocytes from individual donors. With few exceptions, the bias was found essentially for the pool of germline genes we identified. However, the extent of use of single Vλ segments was rather different. In both studies, there was lower expression of the λIII family (11%-20%20; 16%21), whereas the λII family (36%-49%20; 33%21) and its member2a2 (27%20; 17%21), were the most frequently observed. Diverse PCR strategies were used and technical differences may underlie divergences among studies. On the other hand, we believe that the different nature of the cell populations analyzed played a major role. In line with this hypothesis, Farner et al21 found a greater use of the VλIII family in CD5− versus CD5+ IgM+ B cells, whereas Meffre et al25 recently documented a shift in the Vλ repertoire between naive and memory B cells.
The reason why only a few of the 30 functional Vλ segments are used to a significant extent is unclear. In line with the postgerminal center origin of marrow plasma cells,26 all of the 264 sequences analyzed presented somatic mutations and, presumably, most of them were selected by antigen. For this reason it is impossible to test whether germline gene restriction results from intrinsic mechanisms (preferential rearrangement, efficient promoters, or gene-specific enhancer sequences) or from antigenic selection. Presumably, overrepresented germline gene segments possess structural features making them particularly capable of binding a variety of antigens (or the ones most commonly encountered), or accepting somatic mutations without major structural alterations.
Inverse PCR allowed unequivocal identification of monoclonal light chains from all 55 amyloid bone marrows. In line with the postgerminal center origin of amyloid plasma cells, there was no significant intraclonal nucleotide substitution, confirming our previous report.12 We found that the Vλ expression repertoire in AL was characterized by marked overrepresentation of 2 genes, namely3r and 6a. Together these genes accounted for up to 42% of amyloid light chains. The preferential association of λVI (6a) light chains with amyloidosis is well established by the pioneering studies of Solomon et al6 in the early 1980s. Subsequent work using protein sequencing unequivocally demonstrated that virtually all λVI monoclonal light chains isolated so far are from amyloidosis patients. By means of ELISA typing with monoclonal antibodies to the various λ families, Ozaki and colleagues7 established that the prevalence of λVI light chains in amyloidosis could reach 30%, whereas λVI light chains are barely found in normal serum (5%). Our report is in line with the results of these studies and confirms the rarity of polyclonal plasma cells expressing λVI light chains (2.3%).
Our previous sequencing work on somatic mutations in amyloid light chains showed that 4 of 9 λ light chains used the 3r gene, suggesting that this segment might be frequently rearranged in this condition.12 Before our present study, information on the rearrangement rate of 3r in the expression repertoire was limited to the above-cited analysis on normal peripheral blood B cells, studies that identified 3r only at low frequencies (3% and 7%).20,21 We now establish that the prevalence of3r light chains in amyloidosis (22%) is 3 times higher than in polyclonal marrow plasma cells (8.3%), thus identifying a new amyloid-associated Vλ gene (P = .024). The present sequencing data suggest that bias in 3r usage may be typical of amyloidosis, possibly related to its amyloidogenic potential, rather than a general feature of plasma cell dyscrasias. A recent exhaustive revision of nucleotide sequencing data in myeloma and related conditions found no evidence of bias in Vλ use;273r was rearranged only twice in a total of 27 myeloma Vλ regions (7.4%).28-30 It is unknown whether the 2 3r-myeloma patients had amyloid. We are now sequencing myeloma Vλ regions in patients without associated amyloidosis (biopsy negative with follow-up > 2 years) and found3r in 1 of 10 cases (V.P., unpublished observations, August 2001). Overall these data indicate that the3r-rearrangement frequency in myeloma may be similar to polyclonality (3 of 37 sequences, 8.1%), and that nonamyloidogenic3r-monoclonal light chains exist. Unlike 6a light chains, whose germline gene pathogenic potential is such that differently mutated 6a light chains virtually always form amyloid, the putative amyloidogenic propensity of 3r light chains can be counteracted by stabilizing somatic mutations. Biochemical and thermodynamic studies with pathogenic and nonpathogenic3r monoclonal light chains will help to understand the nature of the 3r association with amyloidosis.
Analysis of Jλ segment use suggested that Jλ2/3 was overrepresented in AL (83% versus 67%, P = .03), andJλ1 relatively less frequent (P = .016), in amyloid than in polyclonal light chains. However, becauseJλ2/3 was remarkably used in normal conditions too (Figure4), further studies are needed to test the association of Jλ2/3with amyloidosis. On the other hand, 3r amyloid light chains rearranged Jλ2/3 almost exclusively (11 of 12 cases), whereas polyclonal 3r light chains usedJλ1 and Jλ2/3 at similar frequencies. Thus, the preferential association of 3r amyloid light chains with the Jλ2/3 segment cannot be entirely attributed to intrinsic mechanisms, such as intrinsic preferential rearrangement of3r segment with Jλ2/3. Jλ segments encode the primary structure of framework 4; it is possible that the few amino acid differences between Jλ1 and Jλ2/3 play a role in determining the amyloidogenicity of certain 3rlight chains.
Somatic mutations were found in all sequences, and the overall frequency of mutations was lower in amyloid than in polyclonality. This phenomenon could be partly attributed to the high prevalence of λVI light chains in amyloid, the family that was more homologous to germline compared to the most common one, the λIII. Of interest, amyloid 6a light chains were less mutated than their3r counterparts too. High mutation rates indicate prolonged permanence or repeated selection circuits through the germinal center, a feature that may be typical of clones with frequently used light chains, such as those with λIII light chains.
A secondary aim of the study was to determine whether germline gene use might relate to the different organ tropism that is typical of primary amyloidosis. To this same end, Comenzo et al8 very recently reported the sequencing results from 60 patients with amyloidosis (48 of the λ type). The authors8 were able to establish an association between 6a light chains and major or exclusive amyloid kidney involvement, an association that was further substantiated by amyloid formation from λVI light chains cultured in a human mesangial cell model. In the present study, we confirmed this seminal observation8: 8 of 11 λVI light chains (P = .019) were isolated from patients with predominant kidney involvement at diagnosis. On the other hand, the present analysis does not allow identification of other germline genes with such a direct relationship to organ damage: 3r light chains seem to be capable of infiltrating various targets, with a distribution that apparently parallels that in the general amyloid population (Figure 6). Given the dispersion of data (multiple organ involvement and various gene usage), further gene usage analyses will be needed to address this matter of great biologic and clinical relevance more fully.
In conclusion, this study reports the first comparative Vλ gene usage analysis in amyloid and polyclonal marrow plasma cells. It demonstrates that a relevant proportion of amyloidogenic and polyclonal λ light chains are synthesized through recombination of just a few gene segments. Such a restriction is particularly evident in amyloidosis in which 2 gene segments, namely 3r and6a, are preferentially expressed. Because these segments are responsible for approximately 40% of total amyloid λ light chains, overrepresentation of 3r and 6a can account for the λ light chain predominance typical of AL amyloidosis. In addition, although 3r patients apparently present amyloid infiltration of various organs, this study confirms the previous observation that 6a light chains preferentially generate amyloid in the kidney.8 These findings may have relevance for a better comprehension of the mechanisms underlying amyloidogenicity of light chains. The fact that just 2 genes are highly overrepresented in amyloidosis will help in designing DNA-based vaccine approaches as well as molecules capable of interfering with the amyloid deposition process.
Prepublished online as Blood First Edition Paper, April 17, 2002; DOI 10.1182/blood-2002-01-0114.
Supported by European Biomed 2 (Programme no. BMH4-CT 98-3689), Progetto di Ateneo, MURST 1999 (no. 9906038391-007), Fondazione Ferrata-Storti, and IRCCS Policlinico S. Matteo.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Giampaolo Merlini, Biotechnology Research Laboratories, Department of Biochemistry, University of Pavia and IRCCS Policlinico S. Matteo, P.le Golgi 2, 27100 Pavia, Italy; e-mail:gmerlini@smatteo.pv.it.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal