Abstract
Hemophilia is a rare congenital bleeding disorder that is due to the deficiency of blood coagulation factor VIII or IX. Recurrent musculoskeletal bleeding is common and bleeding into joints results in a chronic inflammatory condition termed hemophilic synovitis. This destructive process is characterized by hemosiderin deposition in the superficial and deeper layers of the synovial membrane as well as a proliferation of synovial fibroblasts and vascular cells. The hyperplastic synovium and neovascular changes are reminiscent of the histopathologic appearance observed in malignant tissues. Indeed, the benign hyperplastic synovium in patients with hemophilia displays similar invasive and destructive behaviors suggesting the possibility of analogous disturbances in growth control and locally invasive mechanisms. Iron plays a role in malignant cell growth, local invasion, and tumor progression, possibly due to changes in the expression of the proto-oncogene, c-myc. We hypothesized that iron plays a similar role in hemophilic synovitis. To explore this hypothesis, we investigated the in vitro effects of iron on the proliferation of a primary, human synovial fibroblast cell (HSFC) line and the involvement of c-myc in this process. We also examined the role of ceramide, a sphingolipid capable of inducing apoptosis in this model system. HSFC proliferation was increased in a dose-dependent fashion and c-myc expression was enhanced by ferric citrate compared to sodium citrate control. Ceramide prevented both the iron-induced increases in HSFC proliferation andc-myc expression. These results indicate that iron probably plays a role in the proliferative changes observed in hemophilic joint disease and that aberrant expression of c-myc may underlie the iron effects. Furthermore, these results suggest that there may be a therapeutic role for ceramide in reversing these changes.
Introduction
Recurrent musculoskeletal bleeding is the most frequent manifestation of severe hemophilia. Bleeding into a joint results in a complex arthritis known as “hemophilic arthropathy” that in time evolves into a chronic, persistent inflammatory disorder termed “hemophilic synovitis.” It has been speculated that the persistent presence of blood in the joint space is involved in the pathogenesis of this disorder.1 Iron deposition in synovial tissues is one of the hallmarks of hemophilic arthropathy.2-4 In vitro, DNA synthesis by synovial fibroblast cells exposed to ferric citrate at concentrations up to 1 mM for 72 hours is increased compared to cells cultured with sodium citrate.5 Iron, in combination with interleukin-1β (IL-β), IL-7, tumor necrosis factor α (TNF-α), or interferon γ (IFN-γ) exerts an additive effect on DNA synthesis suggesting that iron increases cell proliferation by a mechanism distinct from inflammatory cytokines.5 In vitro, cells isolated from hemophilic synovial tissue produce more IL-1β, IL-6, and TNF-α compared to cells from normal synovial tissue.4 Monocytes and synovial fibroblasts are capable of generating metabolically active oxygen metabolites in the presence of iron, which combined with the effects of inflammatory cytokines, may play a role in the proliferative and destructive changes seen in hemophilic arthropathy. Together, these data suggest that iron in addition to inflammatory cytokines and oxygen metabolites may be important stimuli for synovial fibroblast cell proliferation.
The proto-oncogene c-myc plays a pivotal role in cell growth control, differentiation, and apoptosis.6,7 Aberrant expression of this oncogene is a feature of many human and experimental neoplasms.8,9 Hyperplastic synovium has a histopathologic appearance similar to malignant tissue and displays the same invasive and destructive behavior, thought to underlie the pathologic process observed in hemophilic joints10 as well as rheumatoid joints.11,12 In rheumatoid, reactive, and bacterial arthritis, oncogene expression, including c-myc expression has been related to both the extent of synovial hypercellularity and the intensity of the mononuclear cell infiltration.13 In hemophilic joint disease, one of the earliest pathologic changes observed following joint hemorrhage is villous hypertrophy and an increase in the vascularity of synovial tissue. Villous hypertrophy may be the result of dysregulated cell growth or abrogation of cell death or both. As in malignant cells, aberrant expression of one or more proto-oncogenes may underlie these changes. We examined the hypothesis that iron increases the proliferation of human synovial fibroblast cells by inducing aberrant expression of c-mycproto-oncogene, and that reversal of this process by ceramide leads to synovial cell apoptosis. These data are the first description of an association between iron and c-myc playing a role in human synovial cell proliferation.
Material and methods
Cells and culture
Primary cultures of normal human synovial fibroblast cells (HSFCs) were a gift from Dr Katalin Mikecz (Department of Biochemistry, Rush University, Chicago, IL). HSFCs were cultured in Dulbecco modified Eagle medium (DMEM; Biowhittaker, Walkersville, MD) with 10% fetal bovine serum, penicillin G (100 IU/mL), and streptomycin (100 μg/mL) in a humidified, 5% CO2 atmosphere at 37°C. All experiments were performed with cells between the 10th to18th passage.
Iron salts and ceramide
Ferric or sodium citrate (Sigma, St Louis, MO) was dissolved in Hanks buffered saline solution (Gibco BRL, Gaithersburg, MD) and the pH adjusted to 7.2 with 1 N HCl or 1 N NaOH as described.14C2-ceramide (Matreya, Pleasant Gap, PA) was dissolved in ethanol according to the manufacturer's instructions. The final concentration of ethanol in the culture media was 0.1%. Our preliminary observations of cell morphology and growth showed that HSFCs chronically exposed to 0.1% ethanol behaved identical to cells grown in media alone.
Cell proliferation assay
Cells (2 × 103) were placed into each well of a flat-bottom 96-well plate (Falcon 3072) and incubated for 9 days. Cell number was determined on days 1, 3, 5, 7, and 9 using the CellTiter 96 Aqueous nonradioactive cell proliferation assay kit according to the manufacturer's instructions (Promega, Madison, WI). A solution of 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium and phenazine methosulfate (MTS/PMS; 20 μL) was added to each well and incubated for 3 hours at 37°C in a humidified 5% CO2atmosphere; then absorbance at 450 nm was determined using an enzyme-linked immunosorbent assay (ELISA) plate reader (Ceres UV 900 H D; Biotek Instruments, Winooski, VT). Quadruplicate wells were used for all proliferation experiments.
Preparation of RNA, complementary DNA, and polymerase chain reaction amplification of complementary DNA
Total cellular RNA was extracted from cultured cell lines using RNAzol (Cinna/Biotecx Laboratories International, Friendswood, TX), according to the manufacturer's instructions. For polymerase chain reaction (PCR) analysis, complementary DNA (cDNA) was made by reverse transcription (RT) of each RNA sample, using the Universal RiboClone cDNA synthesis system (Promega). PCR reactions were performed with 5 μL of each cDNA, 20 μM of each sense and antisense primers, and 1.25 U Taq polymerase (Fisher Biotech, Pittsburgh, PA). Thirty-two cycles of denaturation, annealing, and extension (94°C for 50 seconds, 60°C for 45 seconds, and 72°C for 45 seconds, respectively) were performed on a GeneAmp PCR System 2400 thermal cycler (Perkin-Elmer, Branchburg, NJ). The primers used were as follows: (1) human c-myc sense: 5′-TCGCAAGACTCCAGCGCCTTCTCTC-3′, (2) human c-myc antisense 5′-TGACACTGTCCAACTTGACCCTCTT-3′, (3) human β-actin sense 5′-GGGTCAGAAGGATTCCTATC-3′, and (4) human β-actin antisense 5′-TCTCAAACATGATCTGGGTC-3′. The amplified samples were resolved on 1.5% agarose gels and photographed.
Fluorescent microscopic apoptosis assay
The ApoAlert DNA fragmentation assay kit (Clontech, Palo Alto, CA) was used to detect apoptosis. HSFCs were isolated following exposure to cell dissociation solution, washed with phosphate-buffered saline (PBS), and resuspended in PBS at 2 × 107cells/mL. The cell suspension (50 μL) was transferred onto a slide coated with poly-l-lysine and fixed with 4% paraformaldehyde in PBS. The cells were permeablized with 0.2% Triton X-100 in PBS, incubated with terminal deoxynucleotidyl transferase and stained with propidium iodide (PI). The slides were photographed using fluorescence microscopy with a dual-pass fluorescein isothiocyanate/PI filter set through which apoptotic cells appear yellow.
Results
Ferric citrate increases proliferation of synovial cells
Because iron is known to increase DNA synthesis,5 we asked if HSFC proliferation would be affected by iron in vitro. HSFCs used in these experiments proliferate slowly with a mean doubling time of approximately 5 days. The proliferation of HSFCs was significantly increased in the presence of iron compared to controls (Figure1). Exposure to 0.1 or 1 mM ferric citrate for 9 days resulted in a 2-fold increase in cell proliferation compared to control HSFCs: 0.17 ± 0.03 or 0.19 ± 0.01 versus 0.09 ± 0.01, respectively. The increase in cell number was evident after 6 days in the presence of iron citrate. Culturing HSFCs with sodium citrate had no effect on cell proliferation compared with control cells (data not shown). The lowest concentration of ferric citrate tested (0.01 mM) had no effect on cell proliferation over 9 days of observation, similar to sodium citrate and control cells. Maximal stimulation of HSFC proliferation was observed at 1 mM ferric citrate and, therefore, this concentration was used in subsequent experiments.
Effect of ferric citrate and sodium citrate on HSFC proliferation.
Approximately 2 × 103 HSFCs were added to flat-bottom 96-well plates. The cells were maintained in DMEM with 10% fetal bovine serum, penicillin G, and streptomycin in 5% CO2humidified atmosphere at 37°C. The cultures were incubated for 9 days in the presence of iron or sodium salt. Cell number was determined on days 1, 3, 5, 7, and 9 using a CellTiter 96 Aqueous nonradioactive cell proliferation assay kit according to the manufacturer's instructions. Briefly, 20 μL combined MTS/PMS solution was added into each well and incubated for 3 hours at 37°C in humidified 5% CO2atmosphere. Absorbance was measured at 450 nm using an ELISA plate reader.
Effect of ferric citrate and sodium citrate on HSFC proliferation.
Approximately 2 × 103 HSFCs were added to flat-bottom 96-well plates. The cells were maintained in DMEM with 10% fetal bovine serum, penicillin G, and streptomycin in 5% CO2humidified atmosphere at 37°C. The cultures were incubated for 9 days in the presence of iron or sodium salt. Cell number was determined on days 1, 3, 5, 7, and 9 using a CellTiter 96 Aqueous nonradioactive cell proliferation assay kit according to the manufacturer's instructions. Briefly, 20 μL combined MTS/PMS solution was added into each well and incubated for 3 hours at 37°C in humidified 5% CO2atmosphere. Absorbance was measured at 450 nm using an ELISA plate reader.
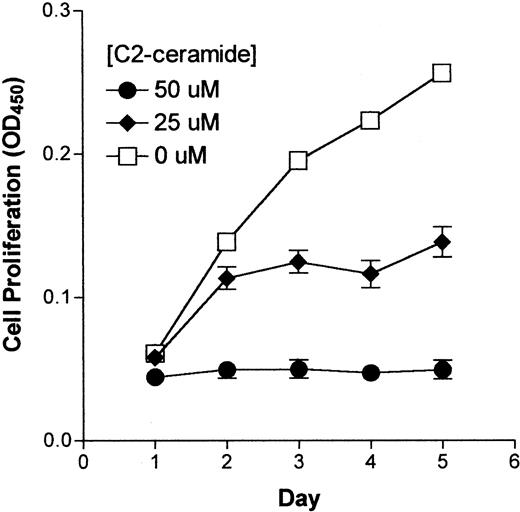
Effect of C2-ceramide on synovial cell proliferation
Ceramide affects a number of cellular processes including cell differentiation, apoptosis, and proliferation. Because ceramide inhibits the proliferation of several cell types,15 16 we asked whether synovial cell proliferation would also be inhibited. To assess the effects of ceramide on HSFC growth, cells were cultured for 9 days with a membrane-permeable ceramide analogue, C2-ceramide, at concentrations up to 50 μM. Cell proliferation was inhibited in a concentration-dependent manner (Figure2). At 50 μM, C2-ceramide completely abrogated cell proliferation and by 9 days a decrease in cell number was observed (Figure 2). Concentrations of C2-ceramide less than 25 μM did not have a significant effect on HSFC proliferation until the duration of exposure reached 1 week (OD450 0.068 versus 0.1, C2-ceramide versus control, respectively; Figure 2).
Effect of C2-ceramide on HSFC growth.
HSFCs were seeded into 96-well plates and incubated with C2-ceramide at the indicated concentration for 9 days. Details of the experiment and determination of cell number was as described for Figure 1.
Effect of C2-ceramide on HSFC growth.
HSFCs were seeded into 96-well plates and incubated with C2-ceramide at the indicated concentration for 9 days. Details of the experiment and determination of cell number was as described for Figure 1.
Effects of C2-ceramide on iron-stimulated synovial cell proliferation
To test effects of C2-ceramide on HSFC proliferation stimulated by iron, cells were precultured with 1 mM ferric citrate for 7 days then harvested and seeded in 96-well plates in the presence of 1 mM ferric citrate with or without C2-ceramide. The rate of cell proliferation was determined over an additional 9 days. C2-ceramide at a concentration of 25 or 50 μM significantly inhibited iron-induced synovial cell proliferation (Figure 3).
Effect of C2-ceramide on ferric citrate–stimulated HSFC proliferation.
HSFCs were cultured with 1 mM ferric citrate for 7 days to induce proliferation then transferred into a 96-well plate and incubated for an additional 9 days with media containing ferric citrate to which 25 or 50 μM of C2-ceramide was added and cell proliferation compared to ferric citrate alone. Cell number was determined as described for Figure 1.
Effect of C2-ceramide on ferric citrate–stimulated HSFC proliferation.
HSFCs were cultured with 1 mM ferric citrate for 7 days to induce proliferation then transferred into a 96-well plate and incubated for an additional 9 days with media containing ferric citrate to which 25 or 50 μM of C2-ceramide was added and cell proliferation compared to ferric citrate alone. Cell number was determined as described for Figure 1.
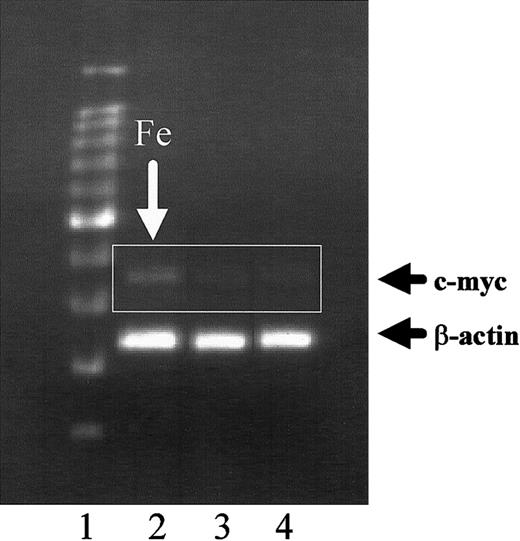
Expression of c-myc is induced by iron and abrogated by ceramide
Because iron increases HSFC proliferation (Figure 1) and c-myc has been related to synovial hypercellularity in other forms of arthritis,13 we speculated that iron may increase HSFC proliferation by inducing aberrant expression of c-myc. Using reverse transcription-PCR, cells cultured with 1 mM ferric citrate demonstrated prominent c-myc expression (Figure4, lane 2) compared to cells exposed to 1 mM sodium citrate (lane 3) or control cells (lane 4) grown in standard medium without salt supplementation. The increase in c-mycexpression induced by iron (Figure 5, lane 3) was abrogated by exposure of the cells to 25 μM C2-ceramide (lane 2).
Effect of ferric citrate on c-myc expression by human HSFC.
HSFCs were cultured in media with 1 mM ferric citrate (lane 2) or 1 mM sodium citrate (lane 3) for 7 days. The cells were harvested, total RNA was extracted, and RT-PCR was performed. The amplified samples were resolved on 1.5% agarose gels. Lane 1, DNA markers and lane 4, HSFC control cells grown in standard medium.
Effect of ferric citrate on c-myc expression by human HSFC.
HSFCs were cultured in media with 1 mM ferric citrate (lane 2) or 1 mM sodium citrate (lane 3) for 7 days. The cells were harvested, total RNA was extracted, and RT-PCR was performed. The amplified samples were resolved on 1.5% agarose gels. Lane 1, DNA markers and lane 4, HSFC control cells grown in standard medium.
Effect of C2-ceramide on iron-inducedc-myc expression by HSFCs.
HSFCs were precultured with 1 mM ferric citrate for 7 days, then transferred to 96-well plates and incubated with 25 μM C2-ceramide in the presence of 1 mM ferric citrate for another 24 hours. Then the cells were harvested and RNA was extracted and examined by RT-PCR. The remainder of the experiment was performed as described in Figure 4. Lane 1, DNA markers; lane 2, HSFCs precultured with ferric citrate then cultured with ferric citrate plus C2-ceramide; lane 3, HSFCs cultured with ferric citrate.
Effect of C2-ceramide on iron-inducedc-myc expression by HSFCs.
HSFCs were precultured with 1 mM ferric citrate for 7 days, then transferred to 96-well plates and incubated with 25 μM C2-ceramide in the presence of 1 mM ferric citrate for another 24 hours. Then the cells were harvested and RNA was extracted and examined by RT-PCR. The remainder of the experiment was performed as described in Figure 4. Lane 1, DNA markers; lane 2, HSFCs precultured with ferric citrate then cultured with ferric citrate plus C2-ceramide; lane 3, HSFCs cultured with ferric citrate.
Cell morphology and apoptosis
Cell morphology was unaffected by ferric citrate (Figure6B) or sodium citrate (data not shown) at the concentrations and exposure durations used in these experiments. Cells exposed to 25 μM C2-ceramide for 24 hours (Figure6C) retracted their processes and the cell body appeared rounded regardless of whether they were precultured with iron or sodium salt. More prolonged exposure to concentrations of C2-ceramide at or below 25 μM did not further alter cell morphology. To determine if the growth inhibition and morphologic changes observed following exposure of HSFC to C2-ceramide were due to apoptosis, we examined cells with a DNA fragmentation assay. HSFCs precultured with iron (or sodium citrate) then exposed to 25 μM (not shown) or 50 μM of C2-ceramide (Figure 7B) for 24 hours exhibited strong nuclear fluorescence indicative of apoptosis. In the absence of C2-ceramide, none of the iron-stimulated cells demonstrated DNA fragmentation (Figure7A).
Effects of iron and C2-ceramide on cell morphology.
HSFCs were cultured as described for Figure 5 then examined microscopically and photographed. Original magnification, × 40. (A) Control HSFCs; (B) iron-exposed HSFCs; and (C), HSFCs exposed to iron followed by C2-ceramide.
Effects of iron and C2-ceramide on cell morphology.
HSFCs were cultured as described for Figure 5 then examined microscopically and photographed. Original magnification, × 40. (A) Control HSFCs; (B) iron-exposed HSFCs; and (C), HSFCs exposed to iron followed by C2-ceramide.
Effects of iron and C2-ceramide on HSFC apoptosis.
HSFCs were cultured as described for Figure 5 and stained as described in “Materials and methods.” HSFCs were examined microscopically for the presence of DNA fragmentation using the ApoAlert assay after iron exposure (A) or iron followed by C2-ceramide for 24 hours (B). Original magnification, × 100.
Effects of iron and C2-ceramide on HSFC apoptosis.
HSFCs were cultured as described for Figure 5 and stained as described in “Materials and methods.” HSFCs were examined microscopically for the presence of DNA fragmentation using the ApoAlert assay after iron exposure (A) or iron followed by C2-ceramide for 24 hours (B). Original magnification, × 100.
Discussion
Bleeding into a joint produces an acute, transient inflammatory reaction known as hemophilic synovitis. With repeated episodes of bleeding, synovial fibroblasts hypertrophy and proliferate. This proliferative response is accompanied by an intense neovascularization of the synovial membrane.17 These early pathologic features have been described in dogs with spontaneous hemophilia, and changes similar to hemophilic arthritis have been produced in experimental animals following injection of autologous blood.18,19 The component of blood that initiates and perpetuates this proliferative and inflammatory reaction is not known. The hallmark of hemophilic synovitis is deposition of iron in superficial and subsynovial layers of the joint. Morris et al2 studied 5 hemophilic synovial membranes by light microscopy and found that all cases contained intracellular and extracellular granular deposits of iron in the most superficial cells. In the deeper synovial layers there were numerous pleomorphic ovate bodies. Using a computerized electron-probe and x-ray microanalytical techniques, these investigators demonstrated the granular material to be highly iron-saturated ferritin and the ovate bodies to be almost pure iron.
Iron participates in a wide range of biochemical reactions that are necessary for cell viability and proliferation. Iron is essential for DNA synthesis.20 Clinical correlations link cellular iron content to the development and progression of human cancer. Iron is necessary for proliferation of cell lines derived from solid tumors21 and is essential for the clonal expansion of the malignant phenotype in neoplastic cells of all origins.20In vitro, iron increases DNA synthesis in cultured synovial fibroblasts.5 These data led us to speculate that iron may be an important component in the synovitis that follows recurrent joint bleeding in hemophilia. In the present study, we show that iron, in the form of ferric citrate at a concentration more than 0.1 mM, significantly increases HSFC proliferation. We also show that iron increases c-myc expression and C2-ceramide abrogates the increase in cell proliferation and reduces the c-myc expression induced by iron. Furthermore, C2-ceramide induces apoptosis of HSFCs. These results indicate that iron plays a critical role in synovial fibroblast proliferation and that ceramide may represent a novel therapeutic agent to treat iron-induced synovial hypertrophy.
Similarities between hemophilic synovitis and rheumatoid arthritis are well documented. In both it has been suggested that iron mediates the inflammatory and proliferative responses as well as the tissue damage.18,22,23 In both processes, microbleeding is well described and iron deposition correlates closely with the extent of erosive disease and is associated with a poor prognosis.23,24 Aberrantly activated gene sequences, proto-oncogenes, have been suggested to play a role in the invasive behavior of rheumatoid synovial fibroblasts.12 Increased expression of oncogenes and their gene products have been detected in human synovial tissues from patients with rheumatoid arthritis.25 The proto-oncogene c-myc plays a pivotal role in growth control, differentiation, and apoptosis,6,7 and its abnormal expression has been associated with many naturally occurring neoplasms.8,9Expression of c-myc in human synovial tissue was significantly correlated both to the degree of synovial hypercellularity and the synovial lymphocytic infiltration in rheumatoid, reactive, bacterial, and osteoarthritis.13Interestingly, c-myc antisense oligonucleotides induce apoptosis and down-regulate fas expression in rheumatoid synovial cells.26 Here we show that iron increases c-myc expression in HSFCs. Kyriakou et al27showed that desferrioxamine (DFX), an iron chelating agent, decreased expression of c-myc in peripheral blood mononuclear cells from patients with thalassemia. A rapid decrease in c-mycmessenger RNA and protein levels followed by apoptosis and inhibition of DNA synthesis followed exposure of HL-60 or K562 leukemic cells to DFX.28 These effects were blocked by simultaneous addition of ferric chloride. These studies support our data suggesting that iron-stimulated synovial cell proliferation is associated with up-regulation of c-myc. Furthermore, c-myc was shown to suppress expression of H-ferritin, an iron-binding protein, and up-regulate iron regulatory protein-2 with a net effect of increasing the intracellular iron pool.29 The result of increased cellular iron is increased activity of ribonucleotide reductase, the rate-limiting enzyme in DNA synthesis.30
Ceramide is a recently identified intracellular messenger, which is generated in the sphingomyelin cycle in response to various stimuli. It regulates a variety of cellular processes such as cell differentiation, induction of apoptosis, and inhibition of cell growth.13,14 The addition of a cell-permeable ceramide to the culture medium increases the intracellular ceramide concentration of the cells and mimics the activation of cellular sphingomyelinases.31 Ceramide has been shown to induce apoptosis of rheumatoid arthritis synovial cells.32 In the present study, we show that C2-ceramide induces a dose-dependent inhibition of normal or iron-stimulated HSFC proliferation. Iron-stimulated c-myc expression by HSFCs is abrogated by ceramide, which also induces the morphologic changes characteristic of apoptosis and results in DNA fragmentation (Figure6). Development of therapeutic strategies designed to inhibit synovial fibroblast cell proliferation or induce apoptosis may be useful to treat hemophilic joint disease thereby avoiding surgical or radioactive interventions.
The authors wish to thank Samuel P. Gotoff, MD, for his support of this research project and his thoughtful review of the manuscript; Martha Cook for her scientific contributions and editorial assistance in the preparation of the manuscript. Finally, the authors wish to thank all of the patients with hemophilia who engendered enthusiasm for these investigations.
Prepublished online as Blood First Edition Paper, May 13, 2002; DOI 10.1182/blood-2002-02-0390.
Supported by the Women's Board Pediatric Oncology Research Fund. This work has been presented in abstract form at the 1999 Annual Meeting of the National Hemophilia Foundation and at the 2001 Annual Meeting of the American Society of Hematology.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Leonard A. Valentino, Rush Children's Hospital, 1653 W Congress Pkwy, Chicago, IL 60612; e-mail: lvalentino@rush.edu.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal