Abstract
Numerous pathologies may involve toxic side effects of free heme and heme-derived iron. Deficiency of the heme-catabolizing enzyme, heme oxygenase-1 (HO-1), in both a human patient and transgenic knockout mice leads to an abundance of circulating heme and damage to vascular endothelium. Although heme can be directly cytotoxic, the present investigations examine the possibility that hemoglobin-derived heme and iron might be indirectly toxic through the generation of oxidized forms of low-density lipoprotein (LDL). In support, hemoglobin in plasma, when oxidized to methemoglobin by oxidants such as leukocyte-derived reactive oxygen, causes oxidative modification of LDL. Heme, released from methemoglobin, catalyzes the oxidation of LDL, which in turn induces endothelial cytolysis primarily caused by lipid hydroperoxides. Exposure of endothelium to sublethal concentrations of this oxidized LDL leads to induction of both HO-1 and ferritin. Similar endothelial cytotoxicity was caused by LDL isolated from plasma of an HO-1–deficient child. Spectral analysis of the child's plasma revealed a substantial oxidation of plasma hemoglobin to methemoglobin. Iron accumulated in the HO-1–deficient child's LDL and several independent assays revealed oxidative modification of the LDL. We conclude that hemoglobin, when oxidized in plasma, can be indirectly cytotoxic through the generation of oxidized LDL by released heme and that, in response, the intracellular defense—HO-1 and ferritin—is induced. These results may be relevant to a variety of disorders—such as renal failure associated with intravascular hemolysis, hemorrhagic injury to the central nervous system, and, perhaps, atherogenesis—in which hemoglobin-derived heme may promote the formation of fatty acid hydroperoxides.
Introduction
Aerobic organisms are well endowed with enzymatic oxidant defense systems, which provide protection against activated oxygen species. Damage caused by reactive oxygen can be greatly amplified by “free” redox active iron.1,2 For example, iron-rich Staphylococcus aureus is 3 orders of magnitude more susceptible to killing by hydrogen peroxide than are iron-poor staphylococci.3 Conversely, depletion of cellular iron powerfully protects eukaryotic and prokaryotic cells against oxidant challenge.4,5 One abundant source of potentially toxic iron is heme, and both exogenous and endogenous heme can synergistically enhance oxidant-mediated cellular damage.6-10
Heme, a ubiquitous iron-containing compound, is quite hydrophobic, readily enters cell membranes, and greatly increases cellular susceptibility to oxidant-mediated killing.8 Heme also acts as a catalyst for the oxidation of low-density lipoprotein (LDL), generating products toxic to endothelium.9,11,12 The toxic effects of heme may be important in a number of pathologies. These include not only acute conditions such as intravascular hemolysis (which can lead to renal failure) but also more insidious processes such as atherogenesis in which intralesional deposits of iron (perhaps derived from erythrocytes, which are known to intrude into atherosclerotic lesions) have been observed.13
As a defense against such toxicity, normal cells upregulate heme oxygenase-1 (HO-1) and ferritin. HO is a heme-degrading enzyme that opens the porphyrin ring, producing biliverdin and carbon monoxide and releasing iron.14,15 Three genes encode for 3 isoenzymes for HO.16 HO-1, identified as a 32.8-kd stress protein,17 is transcriptionally inducible by a variety of agents, such as heme, oxidants, and cytokines.17-21Ferritin, a multimeric protein with a very high capacity for storing iron, consists of 24 subunits of 2 types (heavy chain and light chain).22 The central importance of HO-1 was recently highlighted by the discovery of a child with HO-1 deficiency who exhibited extensive endothelial damage.23 Similar damage to endothelium, as well as hepatic and renal cytotoxicity, has been observed in transgenic knockout mice deficient in HO-1.24In both the human patient and mice, very high concentrations of circulating heme were present.
Free hemoglobin (Hb) in plasma, when oxidized, can provide heme to endothelium, which greatly enhances cellular susceptibility to oxidant-mediated cell injury.25,26 We hypothesized that oxidation of free Hb in plasma could threaten vascular endothelial cell integrity via oxidative modification of LDL and that oxidized LDL might also induce cytoprotectants such as HO-1 and ferritin. The present investigations represent an attempt to determine whether the cytotoxic effects of circulating Hb might, in fact, derive from oxidized species of LDL. The results indicate that components of LDL, primarily lipid hydroperoxides, are distinctly cytotoxic and probably account for a substantial portion of the observed toxic effects of free Hb in plasma. Intravascular hemolysis associated with persistent endothelial damage was shown to be the main characteristic in a child diagnosed with HO-1 deficiency.23 We suggest that the endothelial damage observed in this case was mediated in part by oxidation of LDL catalyzed by metHb-derived heme.
Materials and methods
Materials
Medium 199, fetal calf serum, and Hanks balanced salt solution (HBSS) were purchased from Life Technologies (Vienna, Austria); dispase II was from Boehringer Mannheim (Vienna, Austria); and hydroxyethyl starch was from Du Pont (Wilmington, DE). Plasma samples of the child with HO-1 deficiency, which was diagnosed by Yachie et al,23 were obtained from Department of Laboratory Sciences of Kanazawa University (Japan). The child was in relatively stable condition when blood samples were taken over a period of 2 months. The child's plasma samples were stored for 10 to 11 months at −70°C and transported to the University of Debrecen within 48 hours on dry ice. All other reagents used were purchased from Sigma (St Louis, MO) unless otherwise specified.
Endothelial cell isolation and culture
As in our previous studies,25 human umbilical vein endothelial cells (HUVECs) were removed from human umbilical veins by exposure to dispase and cultured in medium 199 containing 15% fetal calf serum, penicillin (100 U/mL), streptomycin (100 U/mL), and heparin (5 U/mL) supplemented with l-glutamine, sodium pyruvate, and endothelial cell growth factor. Endothelial cells were identified by their morphology and by the presence of von Willebrand factor.
Preparation of human neutrophils
Polymorphonuclear leukocytes (PMNs) were isolated from human volunteers after informed consent (following guidelines of the Committee on the Use of Human Subjects in Research of the University of Debrecen, Hungary).9
Hb preparation
Purified Hb was prepared from fresh blood drawn from volunteers by using ion-exchange chromatography on diethylaminoethanol-sepharose CL-6B column.25,26Hb was assessed for purity by means of isoelectric focusing. MetHb was formed by incubation of Hb with 1.5-fold molar excess K3Fe(CN)6 over heme followed by dialysis. CyanometHb was prepared by the addition of 2-fold excess NaCN to metHb followed by gel filtration. For spectral analysis of Hb in plasma, samples were diluted 6-fold with saline and scanned with the use of diluted Hb-free plasma as blank. Oxyhemoglobin Hb, metHb, and hemichrome concentrations were determined as described by Winterbourn.27
Preparation of human LDL
LDL was isolated from plasma derived from EDTA-anticoagulated venous blood of healthy volunteers28 who had fasted overnight; 1 mg/mL EDTA was used. Before LDL separation, plasma was incubated with 80 μM heme, 20 μM ferrohemoglobin (ferroHb), 20 μM metHb, 20 μM metHb liganded to haptoglobin, 20 μM cyanometHb, 80 μM metmyoglobin, or 80 μM cytochrome c for 2 hours at 37°C. In separate experiments, plasma anticoagulated with 5 U/mL heparin was incubated with PMNs (107 cells per milliliter), either resting or activated by 500 ng/mL phorbol myristate acetate (PMA), in the presence or absence of ferroHb (80 μM in heme) at 37°C for 90 minutes; PMNs were then removed by centrifugation at 400g for 5 minutes at room temperature, followed by a 2-hour incubation at 37°C in the presence of 1 mg/mL EDTA before the isolation of LDL. Density of plasma was adjusted to 1.3 g/mL with potassium bromide (KBr), and a 2-layer gradient was made in a Quick-Seal polyallomer ultracentrifuge tube (Beckman Instruments, Palo Alto, CA) by layering 0.9% NaCl on 10 mL density-adjusted plasma, which was then centrifuged at 302 000g for 3 hours at 4°C (VTi 50.2 rotor) (Beckman Instruments). For the small plasma samples from the HO-1–deficient child (1.5 mL), the density was adjusted to 1210 g/L with KBr, and after the 2-layer gradient was made in a 5.1-mL Quick-Seal tube, a single spin-gradient ultracentrifugation was performed at 228 000g for 90 minutes at 4°C (VTi 65.2 rotor; Beckman Instruments) to isolate LDL.29 Purity of the LDL fraction was checked by agarose gel electrophoresis. The LDL samples were kept at 4°C and protected from light, and the protein content was determined by the BCA protein assay (Pierce, Rockford, IL).
Measurement of oxidative resistance and detection of oxidative modification of LDL
We used a microassay, based on the kinetics of heme-catalyzed lipid peroxidation of LDL, to assess the resistance of lipoprotein to oxidative modification.29 Briefly, in heme-catalyzed oxidation of LDL, heme degradation occurs inversely with formation of lipid oxidation products, including conjugated dienes and lipid hydroperoxide; thus, heme degradation functions as a probe for the lipid peroxidation process. The kinetics of heme disappearance is monitored spectrophotometrically at 405 nm in an automated microplate reader (Bio-Tek Instruments, Winooski, VT). The oxidative resistance of LDL was characterized by change in time (ΔT) at maximum velocity (Vmax) in seconds (the time period until the maximal velocity of heme degradation as defined by the maximum change in absorbance of heme in the propagation phase of lipid peroxidation). The shortening of ΔT at Vmax indicates the decrease in oxidative resistance of LDL. Importantly, because the HO-1–deficient child's plasma was stored for 10 to 11 months, we established that storage of plasma samples of healthy individuals (n = 54) for 12 months at −70°C does not alter the oxidative resistance of LDL (ΔT = 3017 ± 1194 versus 3131 ± 1381 seconds before and after storage, respectively). The conjugated diene formation and generation of thiobarbituric acid–reactive substances (TBARSs) in LDL (50 μg/mL) were measured as in our previous studies.9 Lipid hydroperoxide (LOOH) was detected by means of the ferrous oxidation in xylenol orange assay.30 The α-tocopherol content of LDL was determined as described.28 Reactive amino groups in LDL were estimated with fluorescamine with the use of lysine as a standard.9The electrophoretic mobility of lipoprotein was determined by agarose gel electrophoresis by means of the Hydragel LIPO plus Lp(a) kit (Sebia, Issy-les-Moulineaux, France).
Determination of fatty acids
Lipids extracted from LDL samples (500 μg/mL) were hydrolyzed, and fatty acids methylated. After extraction of fatty acid methylesters with n-hexane, the fatty acid analysis was performed with the use of a gas chromatograph (Hewlett Packard, Palo Alto, CA) coupled to a mass selective detector.31
Iron and heme determinations
Iron was measured spectrophotometrically as ferrozine-iron complex in reducing environment.9 LDL-associated heme was determined spectrophotometrically at 398 nm after the addition of 300 μL formic acid to LDL samples (100 μL) at a concentration of 1.5 mg/mL with the use of an extinction coefficient of 1.5 × 105M−1 × cm−1.32Apolipoprotein B-100 was measured by the Unimate 3 ApoB immunoturbidometric assay (F Hoffmann–La Roche, Basel, Switzerland) to calculate the molar ratios of iron and heme to apolipoprotein B.
Endothelial cell cytotoxicity assay
Confluent endothelial cell monolayers grown in 24-well tissue-culture plates were washed 3 times with HBSS and then exposed to LDL samples (200 μg/mL) treated with 5 μM heme in medium 199. In some experiments, LDL was pretreated with 4 mM glutathione (GSH) and/or 2 U/mL GSH peroxidase. In the experiments with organic lipid–hydroperoxides, endothelial cells were treated with cumene hydroperoxide at a concentration of 5 to 100 μM. After a 4-hour incubation, the test solutions were replaced with 500 μL 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl-tetrazolium bromide (MTT) solution (0.5 mg/mL) in HBSS, and monolayers were incubated for an additional 6 hours. The reduced MTT was measured spectrophotometrically at 570 nm after the formazan was dissolved in 100 μL 10% sodium dodecyl sulfate and 500 μL hot isopropanol containing 20 mM HCl.
Measurement of intracellular GSH
HUVEC monolayers were treated with LDL or organic lipid–hydroperoxide test solutions, and intracellular GSH content was determined by using a kinetic assay.33
HO enzyme activity assay
HO activity in endothelial cell microsomes was measured by bilirubin generation34 after exposure of the cells to LDL isolated from variously treated plasma in medium 199 for 60 minutes followed by an 8-hour incubation with complete medium alone. HO activity is expressed as picomoles of bilirubin formed per milligram of cell protein per 60 minutes.
Ferritin assay
Endothelial cell ferritin content was measured after treatment of cells with control medium or LDL test solutions in medium 199 for 1 hour. After a further 16 hours of incubation in complete medium, the cells were solubilized34 and analyzed for ferritin content with the use of the IMx ferritin enzyme immunoassay (Abbott Laboratories, Abbott Park, IL). The results are expressed as nanograms of ferritin per milligram of cell protein.
HO messenger RNA analysis
HO-1 messenger RNA (mRNA) content was analyzed in confluent endothelial cells incubated with control medium or LDL test solutions as described for the measurement of HO enzyme activity. After 4 hours of exposure, total cellular RNA was isolated, electrophoresed, and transferred to nylon membrane. The position of 28S and 18S ribosomal RNA and the equal loading of samples were checked by ethidium bromide staining. After hybridization with biotin-labeled (Bioprime DNA Labeling System) (Life Technologies) complementary DNA (cDNA) for HO-1,25 34 the probes were detected by a chemiluminescent detection system (Photogene System 2.0) (Life Technologies). Autoradiographs were quantified by computer-assisted videodensitometry.
Statistical analysis
We should note that, in the healthy subjects group, SD stands for the between-subjects SD, whereas in the HO-1–deficient child it represents the within-subject SD. The distributions of the LDL α-tocopherol and lipid peroxidation parameters were approximated by normal distributions, with parameter estimates taken from the empirical frequency distributions. The probabilities of observing values as extreme as those observed in the HO-1–deficient child in healthy subjects were calculated from these distributions.
Results
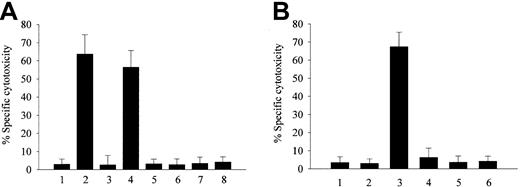
LDL isolated from plasma preincubated with either metHb or heme was found to be markedly cytotoxic (Figure1A). In contrast, LDL isolated from plasma preincubated with ferroHb or other heme proteins such as metmyoglobin or cytochrome c, all of which avidly bind heme,35-37 failed to harm endothelial cell monolayers. These results suggest that the release of heme from metHb is an important precedent event in generating toxic (presumably oxidized) LDL. Therefore, we conducted similar experiments using various strategies to stabilize the heme moiety.35,38,39Haptoglobin or cyanide was shown by Bunn and Jandl35 to strengthen heme-globin liganding, preventing heme release from metHb. Preincubation of metHb with sodium cyanide or stoichiometric amounts of haptoglobin prevented the generation of toxic species of LDL.
Effect of LDL derived from heme-, metHb-, or ferroHb- and activated PMN–containing plasma on endothelial cell cytotoxicity.
LDL derived from heme-, metHb-, or ferroHb- and activated PMN–containing plasma provoke endothelial cell cytotoxicity. (A) Confluent endothelial cells were incubated with native LDL (200 μg/mL protein) (first bar) or LDL isolated from plasma containing 80 μM heme (second bar), 20 μM ferroHb (third bar), 20 μM metHb (fourth bar), 80 μM metmyoglobin (fifth bar), 80 μM cytochrome c(sixth bar), 20 μM cyanometHb (seventh bar), or 20 μM metHb liganded to haptoglobin (eighth bar) in medium 199 for 4 hours followed by MTT assay as described in “Materials and methods.” (B) Endothelial cells were exposed to native LDL (first bar); to LDL derived from plasma containing 20 μM ferroHb (second bar); 20 μM ferroHb and 500 ng/mL PMA-activated PMNs (107/mL) (third bar); or PMA-activated PMNs alone (fourth bar); to LDL from plasma containing 20 μM ferroHb and resting PMNs (fifth bar); or to LDL from plasma containing 20 μM ferroHb and PMA (sixth bar). After a 4-hour incubation with LDL (200 μg/mL protein), MTT assays were performed. Results represent the percentage of specific cytotoxicity (mean ± SE) of at least 3 experiments done in duplicate.
Effect of LDL derived from heme-, metHb-, or ferroHb- and activated PMN–containing plasma on endothelial cell cytotoxicity.
LDL derived from heme-, metHb-, or ferroHb- and activated PMN–containing plasma provoke endothelial cell cytotoxicity. (A) Confluent endothelial cells were incubated with native LDL (200 μg/mL protein) (first bar) or LDL isolated from plasma containing 80 μM heme (second bar), 20 μM ferroHb (third bar), 20 μM metHb (fourth bar), 80 μM metmyoglobin (fifth bar), 80 μM cytochrome c(sixth bar), 20 μM cyanometHb (seventh bar), or 20 μM metHb liganded to haptoglobin (eighth bar) in medium 199 for 4 hours followed by MTT assay as described in “Materials and methods.” (B) Endothelial cells were exposed to native LDL (first bar); to LDL derived from plasma containing 20 μM ferroHb (second bar); 20 μM ferroHb and 500 ng/mL PMA-activated PMNs (107/mL) (third bar); or PMA-activated PMNs alone (fourth bar); to LDL from plasma containing 20 μM ferroHb and resting PMNs (fifth bar); or to LDL from plasma containing 20 μM ferroHb and PMA (sixth bar). After a 4-hour incubation with LDL (200 μg/mL protein), MTT assays were performed. Results represent the percentage of specific cytotoxicity (mean ± SE) of at least 3 experiments done in duplicate.
Although ferroHb in plasma does not itself provoke oxidation of LDL, we25 and others40,41 have demonstrated that it can readily be oxidized to heme-releasing metHb in the presence of inflammatory cell–derived oxidants. Under the conditions shown in Figure 1B, PMNs, when activated with PMA, markedly oxidize Hb (approximately 70%) in plasma within 30 minutes. Concordantly, as shown in Figure 1B, if endothelial cells were exposed to LDL isolated from plasma containing ferroHb and PMA-activated PMNs, endothelial cell toxicity was observed. Importantly, neither PMA-activated PMNs alone nor ferroHb alone caused the generation of cytotoxic LDL. Oxidation of ferroHb by PMA-activated PMNs in plasma was significantly inhibited by catalase; concomitantly, LDL isolated from plasma containing ferroHb, PMA-activated PMNs, and catalase caused reduced endothelial cell cytotoxicity (data not shown).
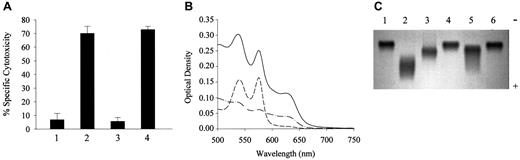
Evidence that the same toxic species of LDL might accumulate in vivo derived from additional experiments involving LDL isolated from the plasma of the HO-1–deficient child reported earlier.23 As shown in Figure 2A, LDL isolated from the plasma of this child showed the same cytotoxic effects as were obtained with LDL isolated following preincubation of normal plasma with metHb. In this child, chronic intravascular hemolysis and severe endothelial cell damage were present.23 Spectral analysis of plasma from this child (Figure 2B) revealed that plasma Hb was predominantly metHb. The proportion of total Hb present as metHb was around 80% (approximately 60 μM). Intriguingly, the child's LDL had increased electrophoretic mobility (Figure 2C), suggesting the loss of net positive charge, which was confirmed by measurement of fluorescamine-titratable free amino groups on the LDL particle; fluorescamine-reactive amino group content fell to 732 mol/molapolipoprotein B-100 as compared with control (978 mol/mol apolipoprotein B-100). Similar alterations in the anodal mobility of LDL occurred when normal plasma was exposed to metHb in vitro, whereas ferroHb had no effect (Figure 2C).
Effect of HO-1–deficient child's LDL on endothelial cells and on electrophoretic mobility.
HO-1–deficient child's LDL is cytotoxic to endothelial cells and has increased electrophoretic mobility accompanied by oxidation of plasma Hb. (A) Exposure of endothelial cells to the child's LDL. Endothelial cells from healthy subjects were treated with native LDL (first bar), LDL from patient (second bar), LDL from healthy individuals' plasma samples that were handled similarly to those from the patient with HO-1 deficiency (third bar), or LDL from metHb-pretreated plasma (fourth bar). After a 4-hour incubation with LDL (200 μg/mL protein), MTT assays were performed. Results represent the percentage of specific cytotoxicity (mean ± SE) of 2 experiments done in triplicate. (B) Spectral analysis of plasma-Hb. Plasma samples were diluted with saline and scanned with the use of diluted Hb-free plasma as blank. Solid line represents the wavelength scan of the child's plasma; also represented are wavelength scans of diluted plasma from healthy subjects containing 2.5 μM ferroHb (long dash) or 2.5 μM of metHb (dash-dot). (C) Electrophoretic mobility of the child's LDL. Native LDL (3 μg) (lanes 1 and 6); oxidatively modified LDL (lane 2); patient's LDL (lane 3); and LDL from plasma containing 20 μM ferroHb (fourth lane) or 20 μM metHb (lane 5) were electrophoresed on agarose gel.
Effect of HO-1–deficient child's LDL on endothelial cells and on electrophoretic mobility.
HO-1–deficient child's LDL is cytotoxic to endothelial cells and has increased electrophoretic mobility accompanied by oxidation of plasma Hb. (A) Exposure of endothelial cells to the child's LDL. Endothelial cells from healthy subjects were treated with native LDL (first bar), LDL from patient (second bar), LDL from healthy individuals' plasma samples that were handled similarly to those from the patient with HO-1 deficiency (third bar), or LDL from metHb-pretreated plasma (fourth bar). After a 4-hour incubation with LDL (200 μg/mL protein), MTT assays were performed. Results represent the percentage of specific cytotoxicity (mean ± SE) of 2 experiments done in triplicate. (B) Spectral analysis of plasma-Hb. Plasma samples were diluted with saline and scanned with the use of diluted Hb-free plasma as blank. Solid line represents the wavelength scan of the child's plasma; also represented are wavelength scans of diluted plasma from healthy subjects containing 2.5 μM ferroHb (long dash) or 2.5 μM of metHb (dash-dot). (C) Electrophoretic mobility of the child's LDL. Native LDL (3 μg) (lanes 1 and 6); oxidatively modified LDL (lane 2); patient's LDL (lane 3); and LDL from plasma containing 20 μM ferroHb (fourth lane) or 20 μM metHb (lane 5) were electrophoresed on agarose gel.
As might be expected, the oxidative resistance of the HO-1–deficient boy's LDL was virtually zero (Table 1), and there was only 0.2 mol/mol apolipoprotein B-100 α-tocopherol in his LDL particles. As shown in Table 1, the oxidative modification of the child's LDL was demonstrated by several independent assays for lipid peroxidation. Conjugated dienes, LOOHs, and TBARSs accumulated in his lipoprotein. Data for lipid peroxidation parameters of LDL isolated from healthy individuals' (n = 62) plasma processed similarly to HO-1–deficient child's plasma are also listed in Table 1. The plasma samples were stored for 11 to 14 months at −70°C and then placed on dry ice for 48 hours before they were assayed. The differences between values for LDL α-tocopherol and lipid peroxidation in healthy subjects versus in the HO-1–deficient child are quite enormous. The observation of such values of LDL α-tocopherol and lipid peroxidation parameters in healthy subjects that we found in the HO-1–deficient child is extremely unlikely; for most parameters, this probability is practically zero. Even the highest probability (for α-tocopherol) was very low, less than 10−5. Iron content of the HO-1–deficient child's LDL was 8 mol/mol apolipoprotein B-100. A comparable amount of LDL-associated heme was obtained if plasma from healthy subjects was exposed to 60 μM metHb for 2 hours (3.2 ± 0.2 heme molecules per LDL particle), and within 72 hours of incubation the heme in LDL particles was degraded while the iron content of LDL increased to 2.9 ± 0.3 mol/mol apolipoprotein B-100. Heme was not detectable in the child's LDL. The results of several independent assays for LDL lipid peroxidation support the conclusion that metHb-derived heme promotes LDL oxidation. As shown in Table2, shortening of ΔT at Vmaxby metHb is paralleled by a rapid decrease in the α-tocopherol content of LDL, which was followed by the formation of conjugated dienes, LOOHs, and TBARSs. In contrast, metHb complexed with haptoglobin or cyanomethemoglobin did not alter either ΔT at Vmax or the α-tocopherol content of LDL. This also prevented the generation of conjugated dienes, lipid hydroperoxides, and thiobarbituric acid–reactive substances in LDL. Finally, ferroHb in plasma did not have the capacity to increase the susceptibility of LDL to oxidative modification (Table 2).
Low-density lipoprotein from 62 healthy subjects and a heme oxygenase-1–deficient child compared for oxidative resistance, conjugated dienes, thiobarbituric acid—reactive substances, total lipid hydroperoxide, and α-tocopherol content
| Parameter . | LDL from healthy subjects (n = 62) . | LDL from HO-1–deficient child . | ||||
|---|---|---|---|---|---|---|
| Mean . | SD . | Min . | Max . | Mean . | SD . | |
| ΔT at Vmax, s | 3040.6 | 515.2 | 2220 | 4740 | 120 | 57 |
| Conj dienes, OD at 234 nm | 0.1794 | 0.0156 | 0.1471 | 0.2203 | 0.436 | 0.055 |
| TBARSs, nmol/mg LDL | 0.316 | 0.094 | 0.128 | 0.577 | 1.75 | 0.31 |
| Total LOOHs, nmol/mg LDL | 4.689 | 1.417 | 2.170 | 8.140 | 32.6 | 2.53 |
| α-tocopherol, mol/mol ApoB-100 | 7.91 | 1.85 | 4.32 | 14.56 | 0.20 | 0.11 |
| Parameter . | LDL from healthy subjects (n = 62) . | LDL from HO-1–deficient child . | ||||
|---|---|---|---|---|---|---|
| Mean . | SD . | Min . | Max . | Mean . | SD . | |
| ΔT at Vmax, s | 3040.6 | 515.2 | 2220 | 4740 | 120 | 57 |
| Conj dienes, OD at 234 nm | 0.1794 | 0.0156 | 0.1471 | 0.2203 | 0.436 | 0.055 |
| TBARSs, nmol/mg LDL | 0.316 | 0.094 | 0.128 | 0.577 | 1.75 | 0.31 |
| Total LOOHs, nmol/mg LDL | 4.689 | 1.417 | 2.170 | 8.140 | 32.6 | 2.53 |
| α-tocopherol, mol/mol ApoB-100 | 7.91 | 1.85 | 4.32 | 14.56 | 0.20 | 0.11 |
Conj indicates conjugated; OD, optical density.
Oxidative resistance, conjugated dienes, thiobarbituric acid—reactive substances, total lipid hydroperoxide, and α-tocopherol content of low-density lipoprotein from normal plasma treated with heme or hemoglobin
| Parameter . | Plasma . | Plasma + heme (80 μM) . | Plasma + ferroHb (20 μM) . | Plasma + metHb (20 μM) . | Plasma + metHb + Hp (20 μM) . | Plasma + cyanometHb (20 μM) . |
|---|---|---|---|---|---|---|
| ΔT at Vmax, s | 3130 ± 272 | 150 ± 118 | 3190 ± 346 | 300 ± 189 | 3721 ± 762 | 3480 ± 722 |
| Conj dienes, OD at 234 nm | 0.175 ± 0.011 | 0.483 ± 0.099 | 0.170 ± 0.05 | 0.321 ± 0.026 | 0.189 ± 0.027 | 0.166 ± 0.005 |
| TBARSs, nmol/mg LDL | 0.32 ± 0.09 | 14.22 ± 1.46 | 0.34 ± 0.21 | 6.65 ± 0.52 | 0.21 ± 0.12 | 0.31 ± 0.14 |
| Total LOOHs, nmol/mg LDL | 5.34 ± 2.28 | 142.82 ± 25.93 | 7.50 ± 4.49 | 101.5 ± 35.02 | 5.04 ± 1.29 | 4.72 ± 3.17 |
| α-tocopherol, mol/mol ApoB-100 | 7.71 ± 2.26 | 0.80 ± 0.86 | 7.59 ± 0.93 | 0.71 ± 0.78 | 6.88 ± 0.39 | 7.29 ± 1.09 |
| Parameter . | Plasma . | Plasma + heme (80 μM) . | Plasma + ferroHb (20 μM) . | Plasma + metHb (20 μM) . | Plasma + metHb + Hp (20 μM) . | Plasma + cyanometHb (20 μM) . |
|---|---|---|---|---|---|---|
| ΔT at Vmax, s | 3130 ± 272 | 150 ± 118 | 3190 ± 346 | 300 ± 189 | 3721 ± 762 | 3480 ± 722 |
| Conj dienes, OD at 234 nm | 0.175 ± 0.011 | 0.483 ± 0.099 | 0.170 ± 0.05 | 0.321 ± 0.026 | 0.189 ± 0.027 | 0.166 ± 0.005 |
| TBARSs, nmol/mg LDL | 0.32 ± 0.09 | 14.22 ± 1.46 | 0.34 ± 0.21 | 6.65 ± 0.52 | 0.21 ± 0.12 | 0.31 ± 0.14 |
| Total LOOHs, nmol/mg LDL | 5.34 ± 2.28 | 142.82 ± 25.93 | 7.50 ± 4.49 | 101.5 ± 35.02 | 5.04 ± 1.29 | 4.72 ± 3.17 |
| α-tocopherol, mol/mol ApoB-100 | 7.71 ± 2.26 | 0.80 ± 0.86 | 7.59 ± 0.93 | 0.71 ± 0.78 | 6.88 ± 0.39 | 7.29 ± 1.09 |
Hp indicates haptoglobin. Other abbreviations are explained in Table 1.
Further evidence for ongoing in vivo heme-catalyzed LDL oxidation derives from measurements of the fatty acid composition of the HO-1–deficient child's LDL. It contained 1.7 times more saturated and monounsaturated fatty acids and a lower percentage (20.18% versus 54.86%) of polyunsaturated fatty acids as compared with control LDL. There was an accumulation of palmitic acid (28.71% versus 11.49%) and a relative lack of linoleic acid (3.43% versus 41.04%) and arachidonic acid (1.65% versus 6.88%). Interestingly, and for unexplained reasons, the percentages of the highly oxidizable polyunsatured fatty acids and of eicosatrienoic and docosahexaenoic acids were higher in his LDL (5.25% versus 0% and 9.85% versus 2.84%, respectively).
These results raised the question of what kinds of cytotoxic materials might be present in oxidized LDL. LDL is a complex mixture that includes triglycerides, cholesterol esters, phospholipids, unesterified cholesterols, lysophosphatidylcholine, phosphatidylethanolamine, diacylglycerol, ceramide, and some phosphatidylinositol. Oxidation leads to formation of a wide range of biologically active products, and some of these, such as 7-oxysterols, have been reported to be highly cytotoxic.42 However, we suspected that the majority of the toxicity of oxidized LDL might derive from the high concentrations of LOOH (Table 2), which is chemically very similar to organic hydroperoxides, such as cumene hydroperoxide. In support, preincubation of oxidized LDL with reduced GSH or GSH peroxidase (which will relatively specifically reduce the LOOH to the alcohol) abolished almost 100% of the cytotoxic effects of the LDL. In further support of the toxicity of the LOOH per se, when endothelial cells were exposed to a concentration of cumene hydroperoxide approximately equal to the LOOH content of the toxic LDL, almost identical cytotoxicity was observed. Finally, if endothelial cells were exposed to either LOOH-containing LDL or cumene hydroperoxide, we observed a precipitous decline in intracellular GSH content as previously described.33
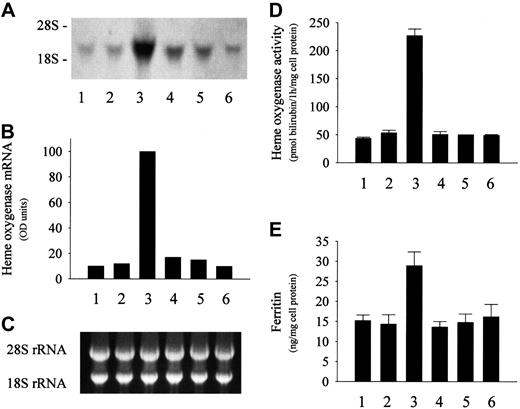
Exposure of endothelial cells to sublethal amounts of oxidized LDL isolated from plasma containing metHb markedly induced HO-1 mRNA (Figure 3A), a sensitive marker for oxidative stress. Accompanying this mRNA induction, HO activity was also significantly enhanced (Figure 3D). A similar increase in the expression of HO-1 mRNA and enzyme activity was observed in endothelial cells exposed to LDL preincubated with heme instead of metHb (Figure3A,D). In contrast, LDL isolated from ferroHb-treated plasma did not alter the HO-1 mRNA level and enzyme activity in endothelial cells (Figure 3A,D). Similar effects were observed in the case of ferritin; LDL from plasma exposed to metHb or heme caused a doubling of endothelial ferritin content, whereas ferroHb failed to induce ferritin synthesis (Figure 3E). In keeping with a posttranscriptional control of ferritin synthesis, ferritin light- and heavy-chain mRNA levels were not affected in these experiments (data not shown).
Effect of LDL isolated from heme- or metHb-containing plasma on endothelial HO-1 and ferritin.
LDL isolated from heme- or metHb-containing plasma induces endothelial HO-1 and ferritin. (A) For HO-1 mRNA Northern analysis, endothelial cells were incubated with native 50 μg/mL LDL (first lane) or with LDL derived from plasma containing 80 μM heme (second lane), 20 μM ferroHb (third lane), 20 μM metHb (fourth lane), or 80 μM metmyoglobin (fifth lane) as described in “Materials and methods.” (B) Densitometry tracings of HO-1 mRNA bands expressed as arbitrary OD units. (C) The corresponding 28S and 18S ribosomal RNA of the Northern blot in panel A. (D) HO enzyme activity measured at 8 hours after exposure of endothelial cell monolayers to the same LDLs as above. (E) Ferritin protein was measured at 16 hours in endothelium treated as in panel A. Results represent mean ± SE of at least 3 experiments performed in duplicate.
Effect of LDL isolated from heme- or metHb-containing plasma on endothelial HO-1 and ferritin.
LDL isolated from heme- or metHb-containing plasma induces endothelial HO-1 and ferritin. (A) For HO-1 mRNA Northern analysis, endothelial cells were incubated with native 50 μg/mL LDL (first lane) or with LDL derived from plasma containing 80 μM heme (second lane), 20 μM ferroHb (third lane), 20 μM metHb (fourth lane), or 80 μM metmyoglobin (fifth lane) as described in “Materials and methods.” (B) Densitometry tracings of HO-1 mRNA bands expressed as arbitrary OD units. (C) The corresponding 28S and 18S ribosomal RNA of the Northern blot in panel A. (D) HO enzyme activity measured at 8 hours after exposure of endothelial cell monolayers to the same LDLs as above. (E) Ferritin protein was measured at 16 hours in endothelium treated as in panel A. Results represent mean ± SE of at least 3 experiments performed in duplicate.
These changes in HO and ferritin synthesis cannot be ascribed to heme per se because, in the course of catalyzing the oxidation of LDL, heme itself undergoes degradation.9 Furthermore, the addition of antioxidants to LDL prior to its exposure to heme prevents the oxidation of lipoprotein and the induction of both mRNA and enzyme activity for HO in endothelium, in spite of the fact that the heme content of LDL remains high.32 43 In support, 200 μM α-tocopherol added to plasma prior to its exposure to metHb or heme also abrogated the induction of endothelial HO activity by isolated LDL (46.8 ± 8.2 and 52.5 ± 16.4 versus 302.5 ± 18.7 and 324.2 ± 34.7 pmol bilirubin formed per milligram cell protein per 60 minutes, respectively). Treatment of endothelial cells with 25 μM tin mesoporphyrin IX (an inhibitor of HO) before their exposure to LDL isolated from metHb-containing plasma significantly reduced the increase in ferritin content by 60%, suggesting that the induction of ferritin synthesis was in part due to iron liberated from endogenous heme.
Further experiments suggest that conditions in circulating plasma may permit the oxidation of Hb to metHb. Thus, if ferroHb-containing plasma is exposed to PMA-activated PMNs for 90 minutes and the LDL is then isolated and added to endothelial monolayers, induction of HO-1 mRNA and enzyme activity occur (Figure 4A,D). Under the same conditions, endothelial ferritin likewise accumulates (Figure 4E). In contrast, LDL from ferroHb-free plasma exposed to PMA-activated PMNs does not increase either HO-1 mRNA or enzyme activity (Figure 4A,D) and has no effect on ferritin content (Figure4E). In addition, LDL derived from ferroHb-containing plasma incubated with resting (nonstimulated) PMNs also has no effect on either HO-1 or ferritin expression (Figure 4).
Effect of LDL derived from ferroHb- and activated PMN–containing plasma on HO-1 and ferritin in endothelial cells.
LDL derived from ferroHb- and activated PMN–containing plasma induces HO-1 and ferritin in endothelial cells. (A) For HO-1 mRNA analysis, endothelium was treated with native LDL (first lane); LDL isolated from plasma containing 20 μM ferroHb (second lane), 20 μM ferroHb and PMA (500 ng/mL)–activated PMNs (107/mL) (third lane), PMA-activated PMNs alone (fourth lane), or 20 μM ferroHb and PMA (fifth lane); or LDL from plasma containing 20 μM ferroHb and resting PMNs (sixth lane) for 60 minutes in medium 199 followed by a 4-hour incubation in medium alone. Total cell RNA was isolated, and Northern blots of endothelial cell RNA were probed with biotin-labeled HO-1 cDNA probe. (B) Autoradiograph was quantified by video densitometry. (C) The corresponding 28S and 18S ribosomal RNA of the Northern blot in panel A. (D) HO enzyme activity measured at 8 hours. (E) Ferritin content was measured for the same groups as in panel A. Results represent mean ± SE of at least 3 experiments performed in duplicate.
Effect of LDL derived from ferroHb- and activated PMN–containing plasma on HO-1 and ferritin in endothelial cells.
LDL derived from ferroHb- and activated PMN–containing plasma induces HO-1 and ferritin in endothelial cells. (A) For HO-1 mRNA analysis, endothelium was treated with native LDL (first lane); LDL isolated from plasma containing 20 μM ferroHb (second lane), 20 μM ferroHb and PMA (500 ng/mL)–activated PMNs (107/mL) (third lane), PMA-activated PMNs alone (fourth lane), or 20 μM ferroHb and PMA (fifth lane); or LDL from plasma containing 20 μM ferroHb and resting PMNs (sixth lane) for 60 minutes in medium 199 followed by a 4-hour incubation in medium alone. Total cell RNA was isolated, and Northern blots of endothelial cell RNA were probed with biotin-labeled HO-1 cDNA probe. (B) Autoradiograph was quantified by video densitometry. (C) The corresponding 28S and 18S ribosomal RNA of the Northern blot in panel A. (D) HO enzyme activity measured at 8 hours. (E) Ferritin content was measured for the same groups as in panel A. Results represent mean ± SE of at least 3 experiments performed in duplicate.
Since endothelial cytolysis was induced by the HO-1–deficient child's LDL, we wondered whether it was also capable of enhancing the expression of HO-1 and ferritin in endothelial cells of healthy subjects. Exposure of endothelial cells derived from healthy subjects to sublethal stress of the child's LDL led to marked increase in enzyme activity for HO (35 ± 3 versus 163 ± 18 pmol bilirubin formed per milligram of cell protein per 60 minutes) and doubled ferritin content (15.4 ± 2.3 versus 26 ± 7.5 ng/mg cell protein). The increase in ferritin synthesis after exposure of endothelium to the HO-1–deficient child's LDL was significantly blunted (−45%) by tin mesoporphyrin IX.
Discussion
The pronounced vascular pathologies described for both an HO-1–deficient human and mice in which this enzyme has been knocked out suggest that defective heme catabolism (and, by implication, heme itself) can have serious pathologic effects. Whereas heme may be directly cytotoxic, the present investigations were an attempt to determine whether the observed in vivo effects of HO-1 deficiency might, at least in part, represent an indirect process. Specifically, we hypothesized that extensive, heme- or heme iron–mediated oxidation of LDL might produce oxidized forms of LDL with appreciable cytotoxicity.
In support of this concept, LDL isolated from plasma preincubated with either heme or metHb is markedly cytotoxic to cultured endothelial cells. Furthermore, similarly toxic LDL was present in the plasma of the HO-1–deficient child. Conversion of ferroHb to metHb appears to be essential for the ensuing oxidation of LDL, presumably because metHb readily releases heme, whereas ferroHb does not. That metHb and not ferroHb readily releases free heme was first demonstrated by Bunn and Jandl.35 This observation prompted us to hypothesize that, following dissociation of heme from metHb, the free heme may readily enter lipoprotein particles. Indeed, LDL particles successfully compete for heme released from metHb in plasma despite the presence of haptoglobin, hemopexin, and albumin. In fact, it has been estimated that, when heme is added to whole plasma, approximately 80% binds immediately to LDL and high-density lipoprotein.44
Once heme is lodged within the LDL, spontaneous oxidative reactions involving small amounts of preformed LOOH (or other oxidizing equivalent) will lead to oxidative lysis of the heme group and release of heme iron45 within the LDL particle. Most likely, it is this heme iron that catalyzes the further oxidative breakdown of heme as well as the accelerated oxidation of polyunsaturated fatty acids and other components of the LDL. This is supported by the observation that inclusion of the iron chelator, desferrioxamine, in incubations containing heme and LDL prevents the loss of intact heme and also stops the appearance of oxidation products such as LOOH and conjugated dienes.9
In plasma-free solutions,9,11 heme was earlier reported to catalyze LDL oxidation, generating products toxic to endothelial cells. In diluted serum (20%) as well, heme was shown to bind to LDL leading to its oxidation in the presence of hydrogen peroxide.12However, in the latter case, the possible formation of cytotoxic products was not investigated. Although a number of heme proteins—such as hemoglobin,46,47 myoglobin,48 horseradish peroxidase,49 and myeloperoxidase50—have been reported to act as oxidants of LDL, the mechanisms involved are by no means clear. In a plasma-free model, hemoglobin reacting with hydrogen peroxide was shown to induce lipid peroxidation of LDL accompanied by oxidative cross-linking of apolipoprotein B-100 via the formation of ferryl Hb and the subsequent generation of radicals on the globin surface.51 The authors of that study concluded that negligible heme transfer from Hb to LDL, or none at all, occurred under the oxidative conditions they employed. Oxidation of Hb to the ferryl state by peroxides has been reported to be accompanied by tyrosyl radical formation.52,53 In end-stage renal failure patients on chronic hemodialysis therapy, a high degree of apolipoprotein B-100 modification resulted from covalent association of hemoglobin with LDL was observed.54 Authors postulated that a tyrosyl radical species of Hb that forms by oxidation of metHb with hydrogen peroxide to ferryl-Hb induces cross-linking of LDL accompanied by an increase in dityrosine formation, and the modification of lipoprotein occurs through a mechanism independent of lipid peroxidation.
Our studies offer an alternative pathway for modification of LDL by Hb in plasma involving heme release from metHb. The results reported here generally support such a mechanism insofar as maneuvers that restrict heme transfer to LDL uniformly diminish or block LDL oxidation.
These observations raised the question of the nature of the toxic substances that might arise from heme- or heme iron–mediated LDL oxidation. LDL contains a number of components, and oxidized forms of several of these have been proposed as responsible for the toxicity of oxidized LDL. However, our results suggest that an accumulation of LOOH is the predominant toxic species within oxidized LDL because specific enzymatic reduction of LOOH to LOH yields LDL with minimal toxic effects. Furthermore, we find that, on an equimolar basis, LOOH within oxidized LDL and an organic hydroperoxide, cumene hydroperoxide, have very similar toxic effects on endothelial cells.
We have shown earlier that endothelial cells exposed to oxidized LDL increase the expression of HO-1 as well as ferritin.43Here, we present evidence that this induction probably involves LDL-associated hydroperoxides (or secondary oxidation events caused by these peroxides). LDL derived from metHb-containing plasma induces endothelial cells to increase HO-1 and ferritin synthesis. In contrast, LDL from plasma containing ferroHb fails to alter the expression of HO-1 and ferritin in endothelial cells. Since oxidation of Hb to metHb is essential for endothelial perturbation by LDL, we sought to model oxidant conditions that might be relevant to vascular pathophysiology. Previous studies have demonstrated that activated PMNs efficiently oxidize Hb contained in erythrocytes to metHb.40,41 In the present studies, we demonstrate that ferroHb in plasma is rapidly oxidized when exposed to activated, but not resting, PMNs. Moreover, LDL derived from plasma containing ferroHb and activated PMNs enhances the expression of both HO-1 and ferritin in endothelium. Such oxidized LDL can also threaten vascular endothelial cell integrity depending on its concentration.
In addition to being a possible marker of exposure to oxidizing events, HO-1 and ferritin have been demonstrated to be cytoprotective in various models.25,32,34,55-67 Iron-driven oxidative damage of endothelial cells, such as cytotoxicity provoked by oxidized LDL or inflammatory cells, can be prevented by induction of ferritin synthesis.32,34,55 The protective role of ferritin against iron-driven oxidative stress is attributable to high sequestering capacity for inorganic iron and ferroxidase activity of its H chain.34 Intracellular ferritin was shown to abolish iron-catalyzed oxidation of LDL.61 Increased expression of HO-1 in endothelial cells was associated with inhibition of monocyte transmigration induced by oxidized LDL65 and may also protect neurons from oxidative injury.66,67 To what extent the products (bilirubin and carbon monoxide) might explain such protection is a matter of current debate.68-71
These results may have further relevance to other pathologic conditions in which extracellular metHb, heme, and iron are present. These include renal failure, which can occur in instances of acute intravascular hemolysis, as well as the progression of atherosclerosis. Oxidation of LDL is recognized as one of the early events in atherogenesis.72 In fact, in an animal model, intravascular hemolysis increases the atherogenicity of diet-induced hypercholesterolemia.73 Interestingly, up-regulation of HO-1 and ferritin genes in endothelium may also occur in the early phase of progression of atherosclerotic lesions55 74possibly reflecting cellular responses to heme- or iron-generated lipid peroxidation products.
We think it is likely that the interactions between Hb/heme and LDL in plasma may explain some of the pathologies observed in the human patient and in mice deficient in HO-1. In the HO-1–deficient child, both intravascular hemolysis and endothelial cell injury were prominent features,23 and at least the latter pathology is also quite evident in HO-1 knockout mice.24 In the present studies, spectral analysis revealed that oxidation of Hb to metHb occurred in the child's plasma and demonstrated that iron was accumulating in his LDL. Several independent assays for LDL oxidation—conjugated diene formation, LOOH generation, production of TBARSs, loss of net positive charge, and loss of α-tocopherol content—all suggested that oxidative modification of lipoprotein was taking place in the HO-1–deficient child's plasma. Overall, our findings may not fully explain the pathologic effects observed in deficiency of HO-1. However, the present results do suggest that products of reactions between heme/heme iron and LDL are distinctly cytotoxic and could well account for some, perhaps most, of the endothelial damage observed in this condition.
MetHb represents a hazard to vascular endothelial cells by not only catalyzing the oxidation of LDL but sensitizing endothelium to oxidant damage.25 MetHb releases free heme to endothelial cells; this heme initially sensitizes endothelium to oxidant stress but later induces the cytoprotectants HO-1 and ferritin.25 In the present studies, we also observed an up-regulation of HO-1 and ferritin after endothelial cells derived from healthy humans were exposed to the LDL of the HO-1–deficient child. Inhibition of HO enzyme activity in endothelium blunted the rapid ferritin response to the child's LDL, suggesting that the induction of ferritin synthesis was in part due to iron liberated from endogenous heme. It is tempting to speculate that endothelial cells of the HO-1–deficient child were prone to oxidative damage arising from both heme-mediated oxidation of LDL and, perhaps, an associated lack of adaptive response (ie, induction of HO-1 and ferritin synthesis). Overall, our results suggest that heme derived from free Hb in plasma may threaten vascular endothelial cell integrity via oxidative modification of LDL; this lipoprotein, in turn, induces the cytoprotectants heme oxygenase and ferritin.
We thank Zoltán Vokó for his contribution to the statistical analysis and Alice Dobolyi for technical assistance.
Supported in part by Hungarian government grants OTKA-029558/037883, ETT-041/037, FKFP-0617, and NKFP-1/007 and by Grant-in-Aid for Scientific Research from the Ministry of Education, Science and Culture of Japan (A.Y.). J.W.E. is supported by The Commonwealth of Kentucky Research Challenge Trust Fund and by National Institutes of Health grant DK 58882.
V.J. and J.B. contributed equally to these studies.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
József Balla, Pf 19, Nagyerdei krt 98, 4012 Debrecen, Hungary; e-mail: balla@ibel.dote.hu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal