Abstract
Generating lentiviral vectors pseudotyped with different viral glycoproteins (GPs) may modulate the physicochemical properties of the vectors, their interaction with the host immune system, and their host range. We have investigated the capacity of a panel of GPs of both retroviral (amphotropic murine leukemia virus [MLV-A]; gibbon ape leukemia virus [GALV]; RD114, feline endogenous virus) and nonretroviral (fowl plague virus [FPV]; Ebola virus [EboV]; vesicular stomatitis virus [VSV]; lymphocytic choriomeningitis virus [LCMV]) origins to pseudotype lentiviral vectors derived from simian immunodeficiency virus (SIVmac251). SIV vectors were efficiently pseudotyped with the FPV hemagglutinin, VSV-G, LCMV, and MLV-A GPs. In contrast, the GALV and RD114 GPs conferred much lower infectivity to the vectors. Capitalizing on the conservation of some structural features in the transmembrane domains and cytoplasmic tails of the incorporation-competent MLV-A GP and in RD114 and GALV GPs, we generated chimeric GPs encoding the extracellular and transmembrane domains of GALV or RD114 GPs fused to the cytoplasmic tail (designated TR) of MLV-A GP. Importantly, SIV-derived vectors pseudotyped with these GALV/TR and RD114/TR GP chimeras had significantly higher titers than vectors coated with the parental GPs. Additionally, RD114/TR-pseudotyped vectors were efficiently concentrated and were resistant to inactivation induced by the complement of both human and macaque sera, indicating that modified RD114 GP-pseudotyped lentiviral vectors may be of particular interest for in vivo gene transfer applications. Furthermore, as compared to vectors pseudotyped with other retroviral GPs or with VSV-G, RD114/TR-pseudotyped vectors showed augmented transduction of human and macaque primary blood lymphocytes and CD34+ cells.
Introduction
Vectors derived from retroviruses offer particularly flexible properties in gene transfer applications given the numerous possible associations of various viral surface glycoproteins (GPs; determining cell tropism) with different types of viral cores (determining genome replication and integration).1 For example, association of the vesicular stomatitis virus G (VSV-G) GP with viral cores derived from lentiviruses results in vector pseudotypes that have broad tropism and can integrate into nonproliferating target cells.2 They have proved useful for the transduction of several cell types ex vivo and in vivo.3-7 Yet there is considerable interest in exploring the properties of lentiviral vectors pseudotyped with alternative viral GPs.8-14 This parameter is likely to modulate the physicochemical properties of the vectors, their interaction with the host immune system, and their host range. Several studies have indeed shown that the transduction efficiency of target cells is dependent on the type of GP used to coat retroviral vectors.15-20 Additionally, some in vivo gene transfer applications will require vectors that are targeted for specific cell entry or gene expression (or both) after systemic administration.21 Due to the wide distribution of its receptor, a lipid component of the plasma membrane,22VSV-G pseudotypes may bind to the surface of all cells encountered after inoculation before reaching the target cells. Moreover, VSV-G–pseudotyped vectors are rapidly inactivated by human serum23 and this might impose a limitation on the use of VSV-G as a GP to pseudotype vectors for systemic gene delivery.
Lentiviral vectors derived from simian immunodeficiency virus (SIV) have been generated in several laboratories,1 including our own.24 Characterization of these vectors has indicated that they are similar to those derived from human immunodeficiency virus 1 (HIV-1) with respect to the insertion of transgenes in nonproliferating cells, although SIV vectors perform better than HIV-1 vectors in simian cells.24 Here, we report the properties of SIVmac-derived vectors pseudotyped with a panel of GPs derived from different membrane-enveloped viruses. In particular, we examined stability in human or macaque sera and gene transfer in primary hematopoietic cells including peripheral blood lymphocytes (PBLs) and CD34+ cells.
Materials and methods
Cells
The 293T human embryo kidney cell line (American Type Culture Collection, Rockville, MD, CRL-1573) and the TE671 human rhabdomyosarcoma cell line (ATCC CRL-8805) were grown in Dulbecco modified Eagle medium (DMEM; Life Technologies, Cergy-Pontoise, France) supplemented with 10% fetal calf serum (FCS).
Human and cynomolgus macaque (Macaca fascicularis) CD34+ cells were obtained according to the institutional guidelines of the ethic commission from mobilized blood and bone marrow samples, respectively, as described previously.25-27CD34+ cells were recovered after Ficoll-Paque (Amersham-Pharmacia Biotech, Orsay, France) gradient centrifugation and were purified with anti-CD34 M450 Dynabeads (Dynal, Compiegne, France). CD34+ cell purity was greater than 95%.
Human and cynomolgus macaque peripheral blood mononuclear cells (PBMCs) were separated from fresh blood of healthy donors using a Ficoll-Hypaque/Percoll gradient (Pharmacia), as described previously.28 Peripheral blood lymphocytes (PBLs) were enriched from the PBMC fraction by overnight adherence at 37°C to remove adherent monocytes and were monitored for CD3 marker expression (75%-85% were CD3+).
Packaging and transfer vectors constructs
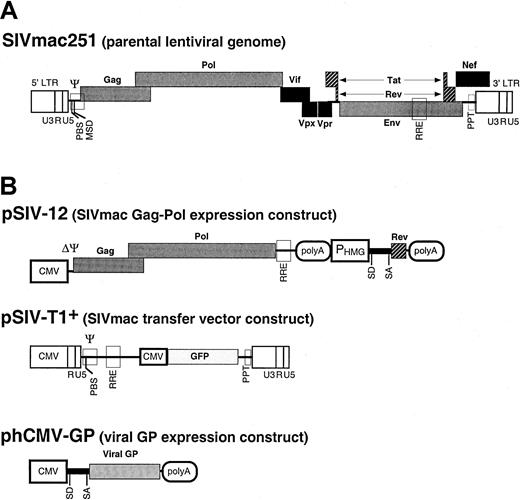
The pSIV-12 packaging plasmid (Figure1) is a derivative of pSIV824and expresses the SIVmac251 gag-polgenes under control of the human cytomegalovirus (hCMV) immediate-early promoter and an HIV-1 rev gene expression unit into which the 2 exons of rev have been fused and placed under control of the 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMG) promoter, HMG intron I, and the SV40 polyadenylation sequences. The pSIV-T1+ plasmid29 encodes a packaging-competent SIVmac251-based vector that expresses the enhanced green fluorescent protein (GFP) marker gene under control of the CMV promoter (Figure 1).
Generation of SIVmac251-derived vectors.
The genome of an infectious molecular clone of SIVmac (SIVmac251) (A) was used to derive constructs encoding the packaging functions and constructs carrying the transfer vector (B). Expression constructs expressing various viral GPs were also designed. The filled boxes represent the viral genes. The open boxes show thecis-acting sequences. LTR indicates long terminal repeat; CMV, human cytomegalovirus immediate-early promoter; PBS, primer binding site; MSD, major splice donor site; Ψ, packaging sequence; RRE, Rev-responsive element; PHMG, HMG promoter; polyA, polyadenylation site; SD, splice donor site; SA, splice acceptor site; SV40, simian virus 40 early promoter. Vector particles were produced by cotransfection of plasmids harboring the packaging functions, the viral GPs, and the transfer vector into 293T cells. The supernatants of transfected cells were collected during transient expression, concentrated by ultracentrifugation, and used for target cell transduction.
Generation of SIVmac251-derived vectors.
The genome of an infectious molecular clone of SIVmac (SIVmac251) (A) was used to derive constructs encoding the packaging functions and constructs carrying the transfer vector (B). Expression constructs expressing various viral GPs were also designed. The filled boxes represent the viral genes. The open boxes show thecis-acting sequences. LTR indicates long terminal repeat; CMV, human cytomegalovirus immediate-early promoter; PBS, primer binding site; MSD, major splice donor site; Ψ, packaging sequence; RRE, Rev-responsive element; PHMG, HMG promoter; polyA, polyadenylation site; SD, splice donor site; SA, splice acceptor site; SV40, simian virus 40 early promoter. Vector particles were produced by cotransfection of plasmids harboring the packaging functions, the viral GPs, and the transfer vector into 293T cells. The supernatants of transfected cells were collected during transient expression, concentrated by ultracentrifugation, and used for target cell transduction.
Viral GP expression constructs
The following plasmids, phCMV-G,30 EboV-GP (kind gift of V. Volchkov), phCMV-HA,31phCMV-10A1,32 and phCMV-GALV32 encode the VSV-G protein, the GP of the Zaire strain of Ebola virus (EboV), the fowl plague virus (FPV) H7-HA hemagglutinin, MLV-10A1, and the gibbon ape leukemia virus (GALV) envelope GPs, respectively. All GPs were expressed under control of the same cis-acting signals: CMV promoter, rabbit β-globin intron II, and polyadenylation sequences (Figure 1).
phCMV-G was used as a backbone to express the GPs derived from the feline endogenous virus RD114 (GenBank X87829)33and the 4070A strain of amphotropic murine leukemia virus (MLV-A).34 The phCMV-RD114 expression vector, expressing the RD114 virus envelope glycoprotein (RD114 GP), and the phCMV-GALV construct were further modified to express the RD114/TR (Figure2B) and GALV/TR8,13 14chimeric GPs carrying the MLV-A GP cytoplasmic tail (designated TR).
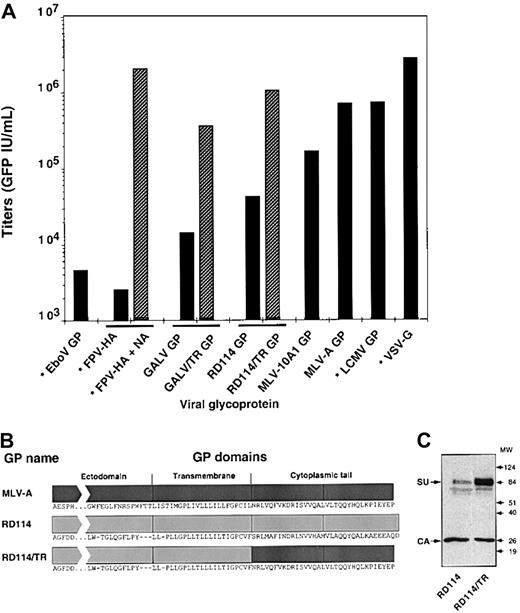
Infectious titers of SIVmac-derived vectors pseudotyped with different viral GPs.
Vectors carrying the GFP marker gene were generated with the indicated GPs of retroviral or nonretroviral (stars) origins. TE671 target cells were infected with dilutions of nonconcentrated vector preparations and the percentage of GFP+ cells was determined 3 days after infection. Infectious titers were calculated as GFP IU/mL. In duplicate experiments, vector producer cells expressing the FPV-HA were treated with 2 U Clostridium perfringensneuraminidase (Sigma-Aldrich, Saint Quentin, Fallavier, France) for 24 hours to induce the release of HA-pseudotyped particles from the surface of producer cells (FPV-HA + NA). (B) Schematic representation of the RD114/TR chimeric GP in which the cytoplasmic domain of the RD114 GP was replaced with that of the MLV-A GP. The sequences of the 3 topologic domains, ectodomain, transmembrane, and cytoplasmic tail, are shown. The GALV/TR chimeric GP was modified in a similar manner. (C) Incorporation of RD114 and RD114/TR GPs in virions was assessed in immunoblots of SIV vector particles pelleted through 20% sucrose cushions, using anti-RD114 SU and anti-CA antibodies. The position of the molecular weight markers is shown (kd).
Infectious titers of SIVmac-derived vectors pseudotyped with different viral GPs.
Vectors carrying the GFP marker gene were generated with the indicated GPs of retroviral or nonretroviral (stars) origins. TE671 target cells were infected with dilutions of nonconcentrated vector preparations and the percentage of GFP+ cells was determined 3 days after infection. Infectious titers were calculated as GFP IU/mL. In duplicate experiments, vector producer cells expressing the FPV-HA were treated with 2 U Clostridium perfringensneuraminidase (Sigma-Aldrich, Saint Quentin, Fallavier, France) for 24 hours to induce the release of HA-pseudotyped particles from the surface of producer cells (FPV-HA + NA). (B) Schematic representation of the RD114/TR chimeric GP in which the cytoplasmic domain of the RD114 GP was replaced with that of the MLV-A GP. The sequences of the 3 topologic domains, ectodomain, transmembrane, and cytoplasmic tail, are shown. The GALV/TR chimeric GP was modified in a similar manner. (C) Incorporation of RD114 and RD114/TR GPs in virions was assessed in immunoblots of SIV vector particles pelleted through 20% sucrose cushions, using anti-RD114 SU and anti-CA antibodies. The position of the molecular weight markers is shown (kd).
Production of retroviral vectors
Pseudotyped SIV-derived vectors were generated as previously described24 by transient transfection of 293T cells. The pSIV-T1+ vector construct (8.1 μg), the pSIV-12 packaging construct (8.1 μg), and the viral GP-expression construct (2.7 μg) were used to cotransfect 293T cells seeded the day before in 10-cm plates. The medium (12 mL/plate) was replaced 16 hours after transfection, and supernatant was harvested 24 hours later. Concentration of the vector particles was performed by pelleting the virions in 26-mL ultracentrifugation tubes, which were spun for 1 hour at 32 000 rpm at 4°C in a 70Ti Beckman rotor. Viral pellets were resuspended in serum-free DMEM supplemented with 1% bovine serum albumin (BSA) in 1/100 of the initial volume of the viral supernatant, aliquoted, and stored at −80°C.
Infection assays
Determination of transduction efficiencies and infectious titers was performed as detailed previously,24 using TE671 as target cells. Stability of vector pseudotypes in human or macaque sera was examined by titrating surviving viral particles after incubation in 1:1 mixtures (vol/vol) of virus preparations with fresh sera for 1 hour at 37°C, as previously described.35 Approximately 5 × 104 GFP infectious units (IU) of pseudotyped vector particles were used per point. Sera were harvested from healthy blood donors and conditioned as published.35Stability of virions was determined as the percentage of infectivity of primate serum-treated viruses versus FCS-treated viruses. Heat-inactivated sera (56°C, 1 hour) were used as controls.
Transduction of primary cells
Purified CD34+ cells were incubated overnight in 12-well plates at 2 × 106 cells/well in 2 mL StemSpan serum free expansion medium (SFEM) supplemented with antibiotics (Stem Cell Technologies, Meylan, France) and with 10 ng/mL thrombopoietin (TPO; Peprotech, London, United Kingdom). Preactivated CD34+ cells were then seeded in 96-well plates (104/well) and were transduced with the pseudotyped vectors in a total volume of 200 μL StemSpan medium containing TPO and 6 μg/mL polybrene. Variable multiplicities of infection (MOIs), determined using TE671 target cells, were applied to the target cells and were in the range of 0.5 to 60 infectious particles/target cell. Transduction in retronectin-coated wells (CH-296; Takara Shuzo, Shiga, Japan) was performed using the same protocol in 96-well plates precoated for 2 hours with 8 μg retronectin/well. After 16 hours, CD34+ cells were washed, suspended in 400 μL StemSpan medium supplemented with 10% FCS (Life Technologies), with antibiotics, with TPO, and with Stempan CC 100 cytokine cocktail (Stem Cell Technologies). GFP expression was analyzed by fluorescence-activated cell sorter (FACS) analysis 5 days after infection.
Human and macaque PBLs were preactivated for 24 hours before infection as described previously by adding 1 μg anti-CD3 (HIT3a, Pharmingen, Pont de Chaix, France) and anti-CD28 (CD28.2, Pharmingen) antibodies to 1 mL medium containing 2 × 106 human PBLs28 or by adding 5 ng/mL concanavalin A and 10 ng/mL interleukin 2 (IL-2) to 2 × 106 macaque PBLs.36 For transduction, 105 activated PBLs were mixed with the pseudotyped vectors in a total volume of 1 mL PBL medium supplemented with 6 μg/mL polybrene, for 4 hours at 37°C. After infection, cells were washed in phosphate-buffered saline (PBS) and incubated at 37°C for 5 days in RPMI 1640 (Life Technologies) supplemented with IL-2 until transduction efficiency was determined by FACS analysis.
Results
Ability of different viral GPs to pseudotype an SIV vector
We examined a panel of viral GPs for their ability to pseudotype lentiviral vectors derived from SIVmac251. These GPs were derived from type C mammalian retroviruses, such as the envelope GPs of the feline endogenous retrovirus RD114, MLV-A, the MLV-10A1, and GALV, or from membrane-enveloped viruses, such as the FPV-hemagglutinin (FPV-HA), LCMV, EboV, and VSV GPs. Pseudotyped SIV vectors were generated by transient expression in 293T cells transfected with 3 plasmids (Figure 1) encoding the SIV viral core proteins, an SIV-based transfer vector harboring the GFP marker gene, and the different GPs. Infection assays on TE671 human rhabdomyosarcoma cells indicated that titers higher than 105 IU/mL were obtained for vectors generated with the GPs of VSV, LCMV, MLV-A, and MLV-10A1 (Figure 2A). In contrast, vectors generated with the GPs of EboV and FPV had low titers, of less than 5 × 103 IU/mL. SIV vectors generated with the GPs of GALV and RD114 had intermediate titers, between 104 and 5 × 104 IU/mL. These relative differences in infectivity of the pseudotyped vectors were reproduced on other target cells such as 293T cells (data not shown), suggesting that determination of the infectious titers on TE671 cells reflected the capacity of the different GPs to pseudotype SIV cores.
The infectious titers obtained with SIV vectors generated with the GPs of FPV, GALV, and RD114 were surprisingly low in comparison to those achieved with MLV vectors pseudotyped with the same GPs.20,33,37 Because budding of lentiviral core particles is not dependent on the expression of viral GPs,38 this suggested that the virions could not efficiently incorporate these GPs or, alternatively, that they could not egress from producer cells after GP assembly. Indeed, when vector-producer cells expressing the FPV-HA were treated with neuraminidase, infectivity of HA-pseudotyped vectors was strongly increased by up to 1000-fold (Figure 2A). This enhancement correlated with a 50-fold increased production of viral particles in the supernatant of producer cells (data not shown). This was most likely induced by neuraminidase-mediated release of virions from the cell surface on which they were retained because of binding to sialic acid-containing cell surface molecules.39,40 However, such a defect in virion egress could not explain the lack of infectivity of SIV vectors generated with the GALV and RD114 GPs because the titers of MLV vectors pseudotyped with the latter GPs are generally high.20,33 This suggested, rather, a defect at the level of GP incorporation on the lentiviral cores. Previous studies have indicated that the cytoplasmic tail of mammalian type C retroviruses bears elements that control the formation or infectivity of pseudotypes with primate lentiviruses.8,13,14 Because the MLV-A GP efficiently pseudotypes lentiviral vectors (Figure 2A), we hypothesized that its cytoplasmic tail should contain all the elements required for optimal GP incorporation on lentiviral particles. Indeed, replacement of the cytoplasmic tail of RD114 (Figure 2B) and GALV GPs with that of MLV-A GP resulted in strongly increased incorporation of either GP on lentiviral cores, as shown in Figure 2C for the RD114 GP and elsewhere for the GALV GP.8,13 14 These chimeric GALV and RD114 GPs, named GALV/TR and RD114/TR, preserved the host range of the initial GPs, as assessed on receptor-interference assays (data not shown), and conferred 25-fold increased titers to the SIV vectors (Figure 2A).
Characterization of pseudotyped SIV-based vector stocks
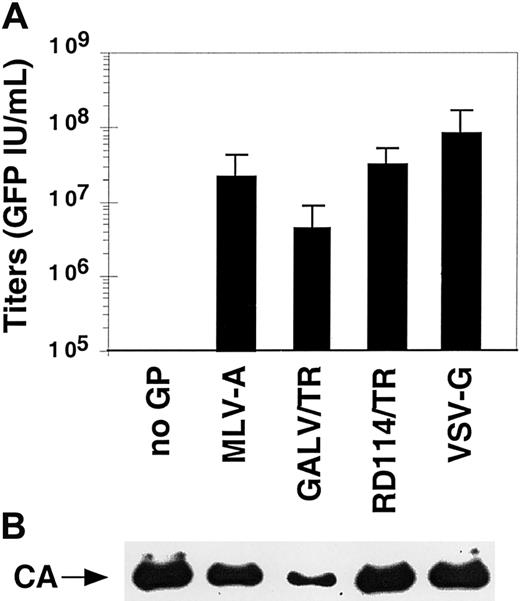
We sought to characterize the properties of vectors coated with the modified or unmodified viral GPs that efficiently pseudotyped the SIV vector particles. The SIV vector pseudotypes were concentrated by ultracentrifugation, resuspended in a storage buffer containing 1% BSA, aliquoted, and stored at −80°C prior to infection assays. Although vectors coated with MLV-10A1 GP had fair titers before concentration, they were not used in the further analyses because they could not be efficiently concentrated (data not shown). In contrast, vectors pseudotyped with FPV-HA, VSV-G, or with the GALV/TR, RD114/TR, MLV-A, and LCMV GPs were very efficiently concentrated, allowing recovery of more than 80%, on average, of the infectious particles after a 100-fold concentration of the physical particles (data not shown). Because vectors pseudotyped with the FPV-HA and LCMV GPs failed to transduce the primary hematopoietic cells tested here (ie, PBLs and CD34+ cells; data not shown), they were not analyzed further. Infectious titers of the concentrated stocks of vectors pseudotyped with the remaining GPs (ie, MLV-A, GALV/TR, RD114/TR, and VSV-G) were determined using TE671 target cells and were in the range of 5 × 106 for the less infectious pseudotypes, obtained with GALV/TR GP, to 1 × 108 IU/mL for the most infectious one, obtained with VSV-G (Figure3A). Similar differences in titers between the vector pseudotypes were detected on other human adherent cell lines (data not shown). This indicated that titer determination using the highly permissive TE671 cells reflected the evaluation of the specific infectivity of pseudotyped vectors. Importantly, the number of infectious particles correlated with the presence of physical particles. As shown in Figure 3B, within a given preparation of pseudotyped vectors, similar amounts of virion-associated capsid proteins were detected for the vector pseudotypes that gave the highest titers (VSV-G and MLV-A or RD114/TR GPs). Lower amounts of physical particles were reproducibly detected for virions pseudotyped with GALV/TR GPs, in agreement with their lower titers (Figure 3A). However, important differences in the absolute quantities of virion-associated capsid proteins were noticed when 2 independent vector preparations were compared, despite comparable infectious titers (data not shown). Thus, to minimize artifacts due to differences in the quality of vectors stocks, each subsequent evaluation experiment was conducted using pseudotyped vectors generated concurrently. Moreover, because the detection of virion-associated capsid proteins did not appear to be a valid indicator of infectious particles and precluded comparison of results, normalization of the pseudotyped vector stocks was performed using titers determined on TE671 cells.
Characterization of pseudotyped SIV-based vector stocks.
(A) Infectious titers of SIVmac-based vector stocks pseudotyped with the indicated GPs and concentrated by ultracentrifugation. The mean titers ± SD from 9 individual experiments performed on TE671 target cells are shown. (B) Detection of physical particles was performed by immunoblotting of representative purified vector stocks using anti-SIV-CA (capsid) antibodies.
Characterization of pseudotyped SIV-based vector stocks.
(A) Infectious titers of SIVmac-based vector stocks pseudotyped with the indicated GPs and concentrated by ultracentrifugation. The mean titers ± SD from 9 individual experiments performed on TE671 target cells are shown. (B) Detection of physical particles was performed by immunoblotting of representative purified vector stocks using anti-SIV-CA (capsid) antibodies.
Stability of vector pseudotypes in primate sera
Vectors suitable for in vivo gene delivery should be stable at 37°C and should retain high infectivity in primate sera. The stability of the vector pseudotypes was therefore determined by comparing titers of viral particles incubated for 1 hour at 37°C versus 4°C. Lentiviral vectors pseudotyped with RD114/TR GP or VSV-G were stable at 37°C, with more than 85% of the vector particles remaining infectious after incubation at 37°C (data not shown). In comparison, vectors pseudotyped with MLV-A and GALV/TR GPs lost more than 75% of infectivity following incubation at 37°C (data not shown), suggesting that the latter GPs incorporated into lentiviral core particles were sensitive to temperature.
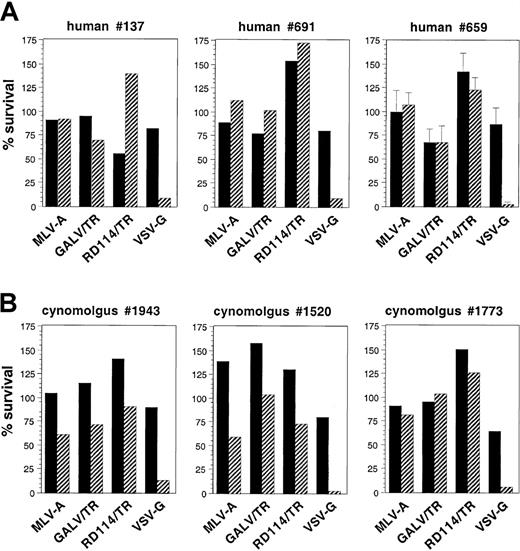
The stability of the pseudotyped vectors in human and cynomolgus macaque sera was evaluated. The same quantities of pseudotyped infectious particles were mixed with fresh primate sera at a ratio of 50:50 (vol/vol) and incubated for 1 hour at 37°C. Heat-inactivated primate sera as well as FCS were used as controls. The results, represented as the percentages of residual infectivity after incubation in fresh or heat-inactivated primate sera relative to the infectivity of FCS-incubated virions (100%), are shown in Figure4. The VSV-G–pseudotyped vectors were inactivated by both human and macaque sera, resulting in more than 90% degradation of viral particles. Vectors pseudotyped with the retroviral GPs were significantly more resistant in human sera, although their levels of resistance were variable according to the serum sample tested and the type of retroviral GP. Vectors pseudotyped with MLV-A GPs were stable in human serum but were relatively sensitive to inactivation by macaque serum. Vectors coated with GALV/TR GP displayed variable levels of stability in human and macaque sera. In contrast, lentiviral vectors pseudotyped with the RD114/TR GP exhibited complete stability in all human sera tested (Figure 4A) and presented good stability in macaque sera (Figure 4B).
Stability of pseudotyped SIV-vector virions in human and macaque sera.
Infectious pseudotyped SIV-vector particles (50 000 GFP IU in 50 μL suspension buffer) were mixed with 50 μL fresh (diagonal bars) or heat-inactivated (solid bars) human (A) or macaque (B) sera. As a reference, virions were mixed with 50 μL heat-inactivated FCS. Virion-sera mixtures were incubated at 37°C for 1 hour and then used to transduce TE671 target cells. Values show the titers of primate sera-incubated virions relative to the titers of the same virions incubated in FCS (%). The results of experiments performed with sera of 3 different individual donors are shown. The experiments with human serum no. 659 were performed in triplicate and are displayed as mean values ± SD.
Stability of pseudotyped SIV-vector virions in human and macaque sera.
Infectious pseudotyped SIV-vector particles (50 000 GFP IU in 50 μL suspension buffer) were mixed with 50 μL fresh (diagonal bars) or heat-inactivated (solid bars) human (A) or macaque (B) sera. As a reference, virions were mixed with 50 μL heat-inactivated FCS. Virion-sera mixtures were incubated at 37°C for 1 hour and then used to transduce TE671 target cells. Values show the titers of primate sera-incubated virions relative to the titers of the same virions incubated in FCS (%). The results of experiments performed with sera of 3 different individual donors are shown. The experiments with human serum no. 659 were performed in triplicate and are displayed as mean values ± SD.
Transduction of human and macaque primary hematopoietic cells
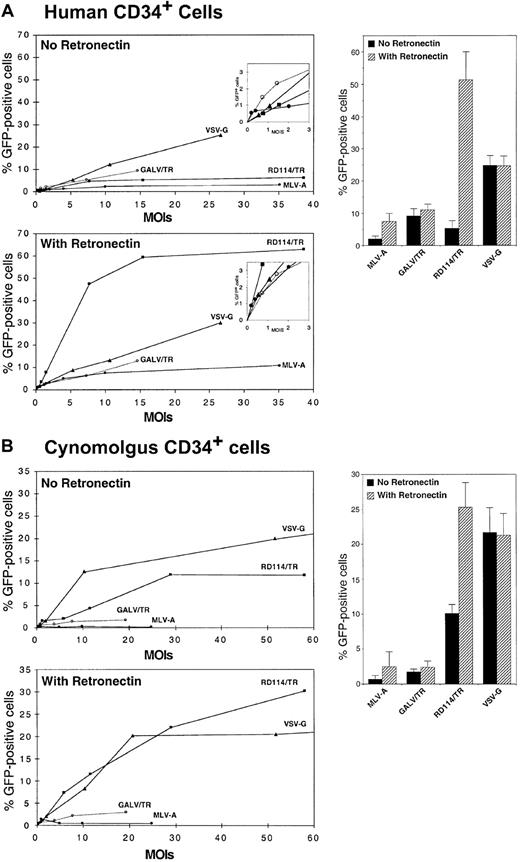
We next compared the different vector pseudotypes for their capacity to transduce primary hematopoietic cells such as CD34+ cells and PBLs. Human CD34+ cells derived from mobilized blood were preactivated overnight in serum-free medium supplemented with TPO and were transduced for 16 hours with a single-hit of SIV vectors pseudotyped with the MLV-A, GALV/TR, RD114/TR, or VSV-G GPs. Variable MOIs, as determined using infectious titers assessed on TE671 cells, were used to transduce the CD34+ cells. Side-by-side transduction experiments were performed in the presence, or in the absence, of CH-296 retronectin fragment.41-43 After infection, cells were grown for 5 days in the presence of TPO, SCF, IL-6, IL-3, and Flt3-L. GFP expression was readily detected in the transduced cells by flow cytometry, allowing us to evaluate the influence of the MOIs and the pseudotyping GP on transduction efficiency (Figure5A). For transduction in the absence of retronectin, the percentage of GFP+ cells initially increased as a direct function of the MOI and the curves flattened at MOIs comprised between 2 and 20, reaching a maximum of 25% GFP+ cells. These moderate transduction efficiencies were likely due to the suboptimal infection protocol and, specifically, the single and short incubation of target cells with virions. In these experimental conditions, the most efficient vectors were those pseudotyped with the VSV-G GPs (mean GFP+ cells, 24.75% ± 3.23%; n = 5), although, at MOIs lower than 2, SIV vectors pseudotyped with GALV/TR and MLV-A GPs exhibited a transduction efficiency higher than that of VSV-G–pseudotyped vectors (Figure 5A, inset). However, at the most efficient MOIs tested, vectors generated with the MLV-A, GALV/TR, and RD114/TR GPs achieved 5- to 12-fold lower transduction efficiencies than VSV-G pseudotypes (Figure 5A). The relatively low titers of vectors generated with the GALV/TR GP (Figure 3A) did not allow transduction efficiency to be evaluated at high MOIs. Divergent results were obtained when infections of CD34+ cells were performed on retronectin-coated plates (Figure 5A). Under these conditions, the VSV-G–pseudotyped vectors retained the same maximal transduction efficiency (24.56% ± 3.27% GFP+ cells; n = 5) than in the absence of retronectin, in agreement with results of others.44 In contrast, the RD114/TR-pseudotyped vectors exhibited a 10-fold increased transduction efficiency, reaching up to 65% GFP+ cells (mean, 51.30% ± 8.74%; n = 5), indicating that the combined use of RD114/TR GP and retronectin synergistically enhanced infection. The retronectin also enhanced the transduction efficiency of vectors pseudotyped with GALV/TR and MLV-A GPs, yet with a much lower magnitude compared to vectors pseudotyped with RD114/TR GP (Figure 5A).
Transduction of human and macaque CD34+ cells.
CD34+ cells derived from human mobilized blood (A) and from cynomolgus macaque bone marrow (B) were prestimulated by overnight incubation with TPO and were transduced for 16 hours at different MOIs with SIV vectors pseudotyped with VSV-G (triangles), MLV-A GP (closed circles), GALV/TR GP (open circles), or RD114/TR GP (closed squares). For each sample of CD34+ cells, transductions were performed in duplicate: in the absence or in the presence of CH-296 retronectin polypeptides coated on the plates. After infection, cells were washed in PBS and cultured in the presence of Flt3-L, TPO, and SCF for an additional 3 days until transduction efficiency was assessed. The dose-response curves of representative experiments are shown for the same batches of CD34+ cells as well as the statistical analyses of the maximal transduction efficiencies of at least 4 experiments performed with CD34+ cells derived from different donors and stocks of pseudotyped vectors.
Transduction of human and macaque CD34+ cells.
CD34+ cells derived from human mobilized blood (A) and from cynomolgus macaque bone marrow (B) were prestimulated by overnight incubation with TPO and were transduced for 16 hours at different MOIs with SIV vectors pseudotyped with VSV-G (triangles), MLV-A GP (closed circles), GALV/TR GP (open circles), or RD114/TR GP (closed squares). For each sample of CD34+ cells, transductions were performed in duplicate: in the absence or in the presence of CH-296 retronectin polypeptides coated on the plates. After infection, cells were washed in PBS and cultured in the presence of Flt3-L, TPO, and SCF for an additional 3 days until transduction efficiency was assessed. The dose-response curves of representative experiments are shown for the same batches of CD34+ cells as well as the statistical analyses of the maximal transduction efficiencies of at least 4 experiments performed with CD34+ cells derived from different donors and stocks of pseudotyped vectors.
We then transduced macaque CD34+ cells derived from bone marrow with the pseudotyped vectors (Figure 5B). In the absence of retronectin, the best pseudotyping GP was VSV-G, allowing transduction of up to 26% GFP+ cells (21.7% ± 3.51%; n = 5). SIV vectors pseudotyped with the GALV/TR and with the MLV-A GPs were the less efficient to transduce macaque CD34+ cells (maximal transduction efficiency of 3% GFP+ cells). Compared to vectors pseudotyped with VSV-G, the RD114/TR GP–pseudotyped vectors resulted in about 2-fold less efficient transduction (10.12% ± 1.26%, n = 4). The presence of retronectin during transduction did not improve the efficiency of transduction by VSV-G–pseudotyped vectors (Figure 5B). However, in a manner similar to transduction of the human CD34+ cells, retronectin enhanced transduction of macaque CD34+ cells by lentiviral vectors pseudotyped with MLV-A and RD114/TR GPs. Under these conditions, maximal levels of transduction of up to 30% GFP+ cells (24.23% ± 4.15%, n = 5) could be obtained with RD114/TR-pseudotyped vectors (Figure 5B).
We then determined the transduction efficiencies of the pseudotyped SIV vectors in human and macaque PBLs. PBLs isolated from fresh blood were incubated for 4 hours with the vectors in the absence of retronectin. Preactivation of the PBLs for 24 hours with soluble anti-CD3 and anti-CD28 antibodies was necessary for transduction with lentiviral vectors, as previously reported.25,28,45 As a result of these experimental conditions that favored stimulation and survival of CD3+ cells, transduction of PBLs was oriented to T cells. GFP expression, determined at 5 days after infection (Figure6), showed that transduction of the PBLs was dependent on the MOI. At low MOIs, the percentages of GFP+ cells steadily increased for the different vector pseudotypes until reaching plateaus. The MOIs required for reaching these plateaus varied with the vector pseudotype. The plateaus of transduction were quickly reached at MOIs of less than 5 infectious particles per cell for lentiviral vectors pseudotyped with VSV-G, with MLV-A GP, or with GALV/TR GP (Figure 6A). In contrast, the threshold MOI necessary to reach a plateau with RD114/TR GP–pseudotyped virions was about 5 to 10 infectious particles per cell (Figure 6A). Interestingly, the maximal transduction levels also varied with the vector pseudotype tested. VSV-G–pseudotyped vectors only transduced a maximum of 10% to 23% of T cells (mean, 16.87% ± 6.53%; n = 4). This somewhat low level of transduction is in agreement with our previous results25 obtained with a VSV-G–pseudotyped HIV-1–derived vector of the same generation and design as the SIV-T1+ vector used in this report. In contrast, much higher levels of transduction, reaching 50% to 75%, were achieved with vectors pseudotyped with the RD114/TR chimeric GP (55.04% ± 11.74%; n = 4). Maximal transduction efficiencies obtained with the other pseudotyped vectors remained low although, as mentioned above, the low titers of vectors coated with the GALV/TR chimeric GP did not allow us to assay for MOIs higher than 2. Additionally, for some vector preparations, the transduction efficiency was found to decrease when high MOIs of MLV-A GP or GALV/TR GP–pseudotyped SIV vectors were used to transduce the human PBLs (Figure 6A). This effect was probably due to competition for receptor binding induced by an excess of defective particles or by soluble GP “shed” from viral particles, as suggested in recent studies.43 46
Transduction of human and macaque PBLs.
PBLs of human (A) or cynomolgus macaque (B) origins were transduced with the indicated SIV vector pseudotypes at different MOIs. Human PBLs were activated with soluble anti-CD3 and anti-CD28 antibodies for 24 hours. Macaque PBLs were activated with concanavalin A and recombinant human IL-2 for 2 days prior to infection. Activated PBLs were infected for 4 hours with SIV vectors pseudotyped with VSV-G (triangles), MLV-A GP (closed circles), GALV/TR GP (open circles), or RD114/TR GP (closed squares). Infected cells were washed in PBS and grown in PBL culture medium, and transduction efficiency was assessed 5 days after infection. The results of experiments performed with PBLs from different donors are shown, as well as the statistical analyses of the maximal transduction efficiencies of at least 4 experiments performed with PBLs derived from different donors and stocks of pseudotyped vectors.
Transduction of human and macaque PBLs.
PBLs of human (A) or cynomolgus macaque (B) origins were transduced with the indicated SIV vector pseudotypes at different MOIs. Human PBLs were activated with soluble anti-CD3 and anti-CD28 antibodies for 24 hours. Macaque PBLs were activated with concanavalin A and recombinant human IL-2 for 2 days prior to infection. Activated PBLs were infected for 4 hours with SIV vectors pseudotyped with VSV-G (triangles), MLV-A GP (closed circles), GALV/TR GP (open circles), or RD114/TR GP (closed squares). Infected cells were washed in PBS and grown in PBL culture medium, and transduction efficiency was assessed 5 days after infection. The results of experiments performed with PBLs from different donors are shown, as well as the statistical analyses of the maximal transduction efficiencies of at least 4 experiments performed with PBLs derived from different donors and stocks of pseudotyped vectors.
Similar results were obtained for transduction of macaque PBLs, although the threshold MOIs necessary to reach the plateaus of infection seemed higher than those necessary for human PBLs and the maximal levels of transduction were lower than those obtained with human PBLs (Figure 6B). Transduction efficiencies obtained with vector particles pseudotyped with GALV/TR or MLV-A GPs remained very low (< 4%-12% GFP+ cells) and were found to decrease at MOIs higher than 1. In comparison to vectors pseudotyped with these latter GPs or with VSV-G (15.32% ± 10.06%; n = 4), PBL transduction with RD114/TR GP-pseudotyped vectors was facilitated. Up to 40% of GFP+ cells could be transduced (26.86% ± 8.07%; n = 4) although higher transduction levels might clearly be expected when using MOIs superior to those applied in these experiments.
Altogether these results indicated that the RD114/TR GP was particularly potent to allow transduction of primate CD34+cells and PBLs with pseudotyped SIV vectors, although the RD114/ TR GP-pseudotyped SIV vectors required the retronectin CH-296 fragment for optimal transduction of short-term stimulated CD34+ cells.
Discussion
Pseudotyping of lentiviral vectors
Protein incorporation on retroviruses is not specific to the homologous viral GPs. Over 40 different host cell–derived proteins have been identified on the exterior of HIV-1 viral particles, including major histocompatibility complex class I and class II molecules, adhesion molecules, costimulation molecules, and complement control proteins.47 Additionally, many heterologous viral GPs can be incorporated into retrovirus particles and mediate infectivity.48 This process, known as pseudotyping, allows retroviral vectors to transduce a broader range of cells and tissues. Engineering of lentiviral vectors with the VSV-G GP exemplifies the ability of a heterologous GP to extend the tropism of a vector.2 However, coexpression of a given GP with a heterologous viral core will not necessarily give rise to highly infectious viral particles8,13,14,49 (Figure 2C). Functional associations between GPs and viral cores are rather unpredictable, in large part because of our insufficient knowledge of the mechanisms that dictate assembly of retroviral particles. It is currently admitted that at least 2 types of mechanisms lead to assembly of homologous and heterologous, viral or cellular, GPs on viral particles. The passive model of GP incorporation implies nonobligatory interactions between the pseudotyping GP and the viral core, provided that the former is sufficiently abundant at the site of virus budding50 and that its cytoplasmic tail does not bear determinants that are sterically incompatible with viral assembly or virion morphology.48 In this respect, heterologous GPs harboring short cytoplasmic tails such as those of FPV, LCMV, and VSV (Figure 2) are likely to be incorporated on lentiviral particles via a passive mechanism. On the other hand, in the active model of GP incorporation, interactions between the cytoplasmic tail of the pseudotyping GP and components of the virion core dictate assembly of viral particles. Ample evidence in the literature supports the critical role of such interactions in viral assembly (for reviews, see Freed38 and Swanstrom and Wills48), at least for lentiviruses.51-54
Pseudotyping of lentiviral core particles with the GPs of type C and D mammalian retroviruses may involve an alternative pathway of assembly (V.S., B.B., and F.-L.C., manuscript in preparation, 2002). The GPs of some of these retroviruses, like the GALV8,13,14 and the RD114 (Figure 2C) viruses, have been shown to harbor in their cytoplasmic tail a determinant that restricts incorporation on lentiviral cores. The relatively short cytoplasmic tails of type C/D mammalian retrovirus GPs, of about 30 to 40 amino acids long, harbor a 15 to 20 amino acid–long carboxy-terminal peptide, named R for MLVs, whose cleavage by the homologous viral core protease is required to activate the fusion potential of the GP.55-57 The cytoplasmic tail of MLV contains all the elements required for optimal pseudotyping of lentiviral cores (V.S., B.B., and F.-L.C., manuscript in preparation, 2002). Based on these observations, we have generated efficient SIV-derived vectors pseudotyped with chimeric GPs derived from GALV8 and RD114 (Figure 2). These mutant GPs, named GALV/TR and RD114/TR (Figure 2), respectively, harbor the cytoplasmic tail of the MLV-A GP whose cleavage site is compatible with the HIV-1 and SIV proteases. It is likely due to its features that they are efficiently incorporated on lentiviral particles (Figure 2C).
Stability of lentiviral vector pseudotypes in primate sera
The VSV-G–pseudotyped lentiviral vectors have proved useful to transduce several cell types in vivo or in vitro.3-7 Yet their high sensitivity to human23 and nonhuman primate (Figure 4) complement may preclude their utility for in vivo systemic administration. In contrast to VSV-G pseudotypes, vectors generated with retroviral GPs were stable in human and macaque sera, with RD114/TR-pseudotyped SIV vectors being constantly resistant to human sera, suggesting that the latter vectors could be particularly suitable for systemic gene delivery (Figure 4). Several factors contribute in determining complement sensitivity and depend on sera from different individuals, the type of producer cells,33,35 the presence of α1-3 galactose sugar epitope in GP,58-60 or the type of pseudotyping GP.33,35,61 Retroviruses produced by human cells are usually resistant in human serum,33,35with the exception of VSV-G–pseudotyped vectors.23However, in a recent study, it was found that oncoretroviral vectors coated with MLV GPs and produced by human cells were differentially sensitive to complement inactivation in sera from nonhuman Old World primates in a manner that correlated with increasing evolutionary distance from humans.62 Sensitivity to macaque sera resulted in more than 99% vector degradation.62 Thus, in apparent disagreement with these latter results obtained with oncoretroviral vectors, here we found that lentiviral vectors pseudotyped with retroviral GPs are relatively stable in macaque sera (Figure 4B). A factor that could modulate response to sera and explain the discrepancy between oncoretroviral and lentiviral particles may be the incorporation of the CD46, CD55, and CD59 complement inhibitory molecules into lentiviral particles, as reported for HIV and SIV.47 63
Transduction of primary cells with pseudotyped SIV vectors
The broad tropism of VSV-G–pseudotyped lentiviral vectors may not be suitable for particular gene transfer applications where cell type-specific gene delivery would be required. More selective tropisms could be achieved by taking advantage of the natural tropisms of GPs derived from some membrane-enveloped viruses or, alternatively, by engineering the host range of incorporation-competent GPs (eg, MLV, GALV/TR, or FPV-HA).64,65 For instance, the use of surface GPs derived from viruses that cause lung infection and infect via the airway epithelia, like EboV or influenza virus, may prove useful for gene therapy of the human airway.10 Nevertheless, it should be noted that lentiviral vector pseudotypes might not always retain the host range of the parental viruses from which the pseudotyping GPs were derived. For example, although the GP of the Mokola virus, a neurotropic lyssavirus, efficiently pseudotypes HIV-1 vectors,12 the pseudotyped vectors do not reproduce the specific neurotropism of the parental virus.9
Recent reports have demonstrated that oncoretroviral vectors pseudotyped with the RD114 GP efficiently transduce human and canine CD34+ cells.15-17,20 Transduced cells could repopulate nonobese diabetic–severe combined immunodeficiency mice and dogs with an efficiency similar to that of nontransduced cells and displayed multilineage expression.15-17 From these studies, it was suggested that, in human CD34+ cells, the “major barrier to gene transfer is at the receptor level and is not due to the quiescence of the target cells.”17 We attempted to test this hypothesis with lentiviral vectors pseudotyped with the MLV-A, GALV/TR, RD114/TR, and VSV-G GPs. In contrast to the former studies, we used conditions of infection that would minimize the influence of factors that may affect virus-receptor interactions or transduction, that is, no reiterated infections, absence of retronectin or stromal cells, and only minimal cytokine treatment. Thus, human CD34+ cells were transduced by a single and short virus/cell exposure under cytokine treatment that would not allow MLV vectors to transduce the CD34+ cells.25Because of these suboptimal conditions, the maximal levels of gene transfer were relatively low; yet they allowed reliable comparison of the specific influence of the pseudotyping GPs in CD34+cells transduction. The best GPs under these conditions were clearly the VSV-G and GALV/TR GPs (Figure 5A). Compared to VSV-G, much lower transduction levels were achieved with vectors pseudotyped with the MLV-A and RD114/TR GPs. These results may reflect differences in the pattern of receptor expression on the CD34+ cells for the different GPs and seem to contradict those previously reported with oncoretroviral vectors.17 However, in agreement with the previous studies,15-17 the combined use of the RD114/TR GP and retronectin strongly increased transduction of human cells, allowing RD114/TR-pseudotyped lentiviral vectors to surpass those pseudotyped with VSV-G (Figure 5). The mechanisms by which CH-296 retronectin fragment enhances infection may involve the colocalization of retroviral particles and target cells,42 owing to the property of CH-296 to bind both the cell surface, through its attachment to α4/5β1 integrins, and the viral GP, through a high-affinity heparin II domain.41Although alternative explanations, involving inhibition of apoptosis and stimulation of cell division, have been proposed,66our results are in favor of the former mechanism because differential effects of CH-296 were detected according to the type of GP used to pseudotype the lentiviral core particles. Proteins of the extracellular matrix, such as heparan sulfate proteoglycans, play a major role in the initial steps of infection and perhaps are more important to mediate viral/cell attachment67 than the viral receptors themselves, which primarily serve to trigger membrane fusion.68,69 Motives that differentially influence binding to extracellular matrix proteins have been identified in GPs of several enveloped viruses.70 71 They may be particularly efficient in the RD114 GP and stimulate CH-296–mediated attachment to cells.
RD114/TR-pseudotyped SIV vectors very efficiently transduced human and macaque PBLs (Figure 6), in the absence of retronectin. Indeed, in these cells, there was a striking difference in the transduction efficiencies observed with vectors pseudotyped with either VSV-G or MLV-A GP and those coated with RD114/TR GP. The reasons for this discrepancy may lie in difference in expression of the receptors for these GPs. Alternatively these results may not necessarily involve differences in receptor density or initial virus-receptor interaction parameters. Several reports have shown that transduction efficiency does not correlate with the level of receptor expression16,72 but rather establish the importance of postbinding events such as receptor clustering, membrane fusion mechanism, site of fusion, uncoating, and migration of the viral particle from the site of uncoating and the nucleus.73 74It can therefore be surmised that, for transduction of PBLs with SIV vectors, the RD114 receptor modulates postbinding events in a more efficient fashion than the VSV-G or MLV-A receptors.
We are indebted to Naomi Taylor and Anne Dubart for stimulating discussions and critical reading of the manuscript. We thank Dorothee vonLaer, Wolfgang Garten, and Viktor Volchkov for providing the complementary DNAs of the LCMV, FPV, and EboV GPs, respectively. We thank Vincent Kindler (HUG, Geneva, Switzerland) and Naomi Taylor (IGM, Montpellier, France) for providing human CD34+ cells from mobilized blood.
Prepublished online as Blood First Edition Paper, May 17, 2002; DOI 10.1182/blood-2001-11-0042.
Supported by the Agence Nationale pour la Recherche contre le SIDA (ANRS), the European Community (QLK3-1999-00859), Association Franco-Israélienne pour la Recherche Scientifique et Technologique (AFIRST), Association Française contre les Myopathies (AFM), Association pour la Recherche contre le Cancer (ARC), Centre National de la Recherche Scientifique (CNRS), Institut National de la Santé et de la Recherche Médicale (INSERM), Institut Clayton de la Recherche, and Swiss National Science Foundation.
V.S. and B.B. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
François-Loı̈c Cosset, LVRTG, INSERM U412, ENS de Lyon, 46 Allée d'Italie, 69364 Lyon Cedex 07, France; e-mail: flcosset@ens-lyon.fr.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal