Abstract
Myelodysplastic syndrome (MDS) is a disease characterized by ineffective hematopoiesis. There are significant biologic and clinical differences between MDS and acute myeloid leukemia (AML). We studied a cohort of 802 patients, 279 (35%) with newly diagnosed MDS and 523 (65%) with newly diagnosed AML, and compared clinical and biologic characteristics of the 2 groups. Complete clinical and cytogenetic data were available on all patients, and a subgroup of patients was studied for apoptosis, angiogenesis, proliferation, and growth factors. Our results demonstrate that MDS is a discrete entity that is different from AML and is characterized primarily by increased apoptosis in early and mature hematopoietic cells. Using cell sorting and loss of heterozygosity, we demonstrate that the leukemic cells from MDS patients are capable of differentiation into mature myeloid cells and monocytes. We also demonstrate that there is a significant overlap between AML and MDS when MDS is defined on the basis of an arbitrary percentage of blasts of 20% or 30%. These data suggest that despite similarities between AML and MDS in their responses to treatment and outcomes, MDS is biologically and clinically different from AML and should not be considered an early phase of AML. The data indicate that MDS must be better defined on the basis of its biology rather than the percentage of blasts; further, the data suggest that there is a need to develop therapeutic approaches that specifically address the biologic abnormalities of MDS.
Introduction
Myelodysplastic syndromes (MDS) are a heterogeneous group of diseases characterized by active but ineffective hematopoiesis leading to pancytopenia.1-5 MDS has been recognized for more than 50 years and has been called preleukemia, smoldering leukemia, oligoblastic leukemia, and refractory anemia. Most patients with this syndrome die without progressing to overt acute leukemia.1-5 The term MDS reflects the presence of dysplasia in bone marrow and peripheral blood. Dysplasia may reflect disordered maturation and fragmentation of the nuclear structures, both of which are signs of increased apoptosis.6
There is significant clinical variability in MDS.7-10Patients with severe cytopenia, increased percentage of blasts, or cytogenetic abnormalities have clinical outcomes that are not significantly different from those seen in acute myeloid leukemia (AML) (P = .1, log-rank test).10 On the other hand, patients with none of these features are likely to live several years.
According to the French-American-British (FAB) classification, MDS is said to be present in patients who have less than 30% blasts in bone marrow and peripheral blood and have evidence of ineffective hematopoiesis.11,12 If 30% blasts are present, AML is diagnosed. The 30% cut-off rate is arbitrary. A new classification proposed by the World Health Organization (WHO) reduces the maximum percentage of blasts from 30% to 20%, taking into consideration the fact that patients with 20% to 30% blasts (previously called refractory anemia with excess blasts in transformation [RAEB-T]) might have AML.13-15 The proposed new classification was based on several reports suggesting that in addition to similarities in the natural history of RAEB-T and AML, RAEB-T responds to combination chemotherapy in a fashion similar to that of AML. However, it is important to note that this similarity in outcome does not necessarily imply that AML and MDS are biologically similar. Here we compare the biologic characteristics of AML and MDS. We hypothesized that the clinical differences between MDS and AML reflect biologic differences. We investigated the basis for the peripheral pancytopenia and confirmed that apoptosis in bone marrow prevents cells from reaching peripheral blood. We also hypothesized that the leukemic cells are capable of differentiation. Using loss of heterozygosity (LOH) and X-chromosome activation, we demonstrated that malignant cells in MDS patients could differentiate to mature hematopoietic cells. We hypothesized that the biologic differences between MDS and AML are clinically relevant, and we studied the clinical impact of these biologic markers when MDS is treated as AML. We also found that there is significant overlap between MDS and AML when the 2 diseases are separated based on the percentage of blasts. Although our data suggest that the separation of the 2 diseases as recommended by the FAB classification is helpful, classification based on the biology of MDS is needed.
Patients and methods
Eight hundred two patients with newly diagnosed AML or MDS who were treated at The University of Texas MD Anderson Cancer Center between 1994 and 1998 were reviewed. Included were 133 patients with RAEB-T, 85 with RAEB; 38 with chronic myelomonocytic leukemia (CMML), 15 with refractory anemia (RA), 6 with refractory anemia with ring sideroblasts (RARS), and 523 with AML. The diagnosis of RAEB-T was based on the presence of Auer rods in 13.3% of the patients, more than 5% blasts in peripheral blood in 26% of the patients, and more than 20% blasts in bone marrow in the rest of the patients. All patients classified as having RAEB-T based on the presence of Auer rods had increased blasts (more than 5%). Patients with the t(15;17) translocation were excluded from the analysis because of the specific molecular abnormality and clinical course. Patients with inversion 16 and t(8;22) are automatically classified as AML in our institution regardless of their percentage of blasts. Patients who had a diagnosis of MDS that did not require immediate therapy were not included in this study. MDS patients were treated if they required transfusion, had platelet counts less than 50 000/μL, had infection or bleeding, or had blast counts in bone marrow greater than 10%. This group of patients is heterogeneous, and diagnoses could not always be established with certainty. Therefore, these patients were excluded from our analysis. When the disease progressed, they were re-evaluated and treated with chemotherapy. Based on recent evaluation of the International Prognostic Scoring System (IPSS), the population of MDS patients seen at MD Anderson may be different than what is diagnosed in general hospital populations or even in other referral centers because the IPSS system does not confirm the clear separation of IPSS groupings reported by many other studies in the literature.16 CMML patients are overall different from MDS patients, and the new WHO classification suggests separating CMML from MDS. However, CMML patients show high levels of apoptosis, and it remains controversial whether CMML can be divided into dysplastic disease and proliferative disease. All data and studies were analyzed after excluding CMML, and we found no change in our conclusion. For these reasons, we did not separate patients with CMML from the rest of the MDS patients. All MDS patients underwent AML therapy, which was based on ara-C. Therapy in these patients can be divided into 3 arms: idarubicin + ara-C (IA), topotecan + ara-C (TA), and fludarabine + ara-C + idarubicin (FAI).
Clinical and laboratory data were collected from the Leukemia Department database. Plasma, serum, and bone marrow samples were collected on subgroups of patients in random fashion without specific selection. To eliminate any possible confounding in study results by freezing and thawing of samples, additional patient samples were analyzed prospectively for apoptosis and proliferation without freezing and thawing. These patients were diagnosed and treated fairly recently and had only short follow-up; thus, they were not included in most of the clinical analysis. The distribution of overall values was similar using all methods.
Antecedent hematologic disease (AHD) is defined as a history of abnormal blood count (hemoglobin less than 12 g/dL, or neutrophils less than 1500/μL, or WBC greater than 10 000/μL or less than 4000/μL, or platelet count less than 150 000/μL) documented to be present for at least 1 month before patient evaluation at our center. AHD is considered 0 when there is no history of AHD. Clinical remission (CR) is defined as a marrow sample showing less than 5% blasts, peripheral platelet count more than 100 000/μL, and peripheral neutrophil count more than 1000/μL.
Enzyme-linked immunoadsorbent assays
Enzyme-linked immunoadsorbent assays for various cytokines, such as tumor necrosis-α (TNF-α), hepatocyte growth factor (HGF), and interleukin-6 (IL-6), in the patients' plasma were performed using kits commercially available from R&D Systems (Minneapolis, MN).17 We followed the protocols recommended by the manufacturer.
Protein extraction
Protein was extracted from cells by a method previously described.18-20 Briefly, after Ficoll-Hypaque separation, cells were lysed in TENN buffer (50 mM Tris-HCl at pH 7.4; 5 mM EDTA; 0.5% NP-40; 150 mM NaCl; 1 mM phenylmethylsulfonyl fluoride; 2 μg/mL leupeptin, and 2 μg/mL pepstatin) for 30 minutes on ice with frequent vortexing. The lysate was then centrifuged at 14 000 rpm for 1 hour. The supernatant was separated and saved. Protein concentration was determined by the Bradford method, and 200 μg of each extract was analyzed by 7.5% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and stained with Coomassie blue R-250 to check the protein profile and to confirm the concentration and preservation of proteins.
Measurement of caspase-3 activity
Caspase-3 was measured using a tetrapeptide Ac-DEVD-pNA (prepared by Calbiochem, San Diego, CA). As recommended by the manufacturer,21 100 μL reaction mixture consisted of 50 μg cellular protein extracts and 200 μM Ac-DEVD-pNA in 1× assay buffer (100 mM NaCl, 50 mM HEPES,10 mM dithiothreitol, 1 mM EDTA, 10% glycerol, 0.1% CHAPS at pH 7.4). A positive control consisted of the same components plus 30 U human recombinant caspase-3 (1 U enzyme is the amount required to release 1 pmol pNA from 200 μM DEVD-pNA per minute at 25°C). Two negative controls were also used, in which either the cell extract or the substrate was not added to the reaction mixture. An additional negative control was included in which cell extracts were treated with caspase inhibitor before the reaction. All reactions were allowed to proceed for 3 hours at 25°C, and optic density at 405 nm was measured every 30 minutes using a spectrometer (Elx808; BioTek Instruments, Winooski, VT). Optical densities were plotted as a function of time, and the slope of the initial linear portion of the curve was used as a measurement of the amount of caspase-3 activity. Mean caspase-3 activity of peripheral blood mononuclear cells from 22 healthy controls was assigned a value of 1. Activity in the leukemic and MDS samples was normalized to the mean of controls. Spontaneous hydrolysis of substrate in negative controls was negligible (less than 0.01).
Caspase-3 activity testing was repeated on 60 samples on 2 different days. No significant differences in results were found for the repeated tests.
Mitochondrial potential measurement (DePsipher assay)
Bone marrow samples were collected in EDTA tubes (minimum of 106 cells), and the red cells were lysed and washed twice. An aliquot of 0.5 μL DePsipher assay (5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolocarbocyanine++ + iodide) (Trevigen, Gaithersburg, MD) was added, and the mixture was incubated at 37°C in 5% CO2 for 20 to 30 minutes. Cells were washed with phosphate-buffered saline (PBS) then analyzed on FACScalibur (Becton Dickinson, Mansfield, MA) immediately.22-25
Measurement of annexin V
Cells were isolated using double-density Histopaque 1119 and 1077 to capture mononuclear and polymorphonuclear cells. Both cell populations were mixed, washed, and stained with annexin V and propidium iodide as recommended by the manufacturer (Becton Dickinson, Mansfield, MA).26 27 Cells were also costained with CD14 and CD34. Briefly, phosphate-buffered saline (PBS)–washed cells were incubated with propidium iodine and fluorescein isothiocyanate–conjugated annexin V antibodies for 15 minutes, washed, processed, and acquired by FACScalibur within 5 minutes of staining.
Measurement of bromodeoxyuridine incorporation
The commercial kit provided by PharMingen/Becton Dickinson (San Diego, CA) was used. Briefly, cells were washed twice, and 0.5 mL 1× PBS w/NaAz was added with 4 mL RPMI. Cells in similar number were prepared similarly in a different well. Bromodeoxyuridine (BrdU) was then added (1 μL/mL) to one of each pair of wells and was incubated for 45 minutes. Cells were then washed and costained with CD34 according to a standard procedure.28 29
Loss of heterozygosity and X-chromosome activation studies
Various cell subpopulations (CD34+, CD14+, CD19+, CD3+) were sorted using magnetic beads and AutoMACS columns as recommended by the manufacturer (Miltenyi Biotec, Auburn, CA).30 31 Sorted cells fractions were more than 50% pure when analyzed using CD34, CD64, CD20, and CD7. Maturing myeloid cells and polymorphonuclear cells were separated by negative selection.
DNA was isolated using standard techniques as previously described.32 Microsatellite markers were purchased from Applied Biosystems (Foster City, CA). In our study of X-chromosome activation, we amplified the human androgen receptor locus (HUMARA) using primers and a method described by Busque et al.33All primers were labeled with FAM, HEX, or TAMRA fluorescent dye (Perkin-Elmer, Norwalk, CT). In the HUMARA assay, the DNA was digested with HpaII and RsaI using a standard procedure. DNA was amplified using standard techniques.33-38 Briefly, samples were activated at 95°C for 12 minutes, then amplified at 94°C for 30 seconds and at 60°C for 30 seconds for 30 cycles on a 9700 Perkin-Elmer thermal cycler in a total volume of 25 μL. Polymerase chain reaction (PCR) was performed using AmpliTaq Gold DNA polymerase. Aliquots (0.6 μL) of the PCR reaction were mixed with 0.1 μL of size standard (GENESCAN 2500-ROX) and analyzed using the ABI 310 machine. Automatically collected data were analyzed by using GENESCAN software (version 1.2) as described in the manufacturer's manual.
Statistical analysis
Wilcoxon rank-sum tests were used to compare baseline clinical and biologic characteristics of the MDS and AML groups for continuous risk factors, whereas χ2 analysis (or Fisher exact test) was used for categorical variables. Survival distribution curves were estimated by the method of Kaplan and Meier. The univariate Cox proportional hazard model was used to evaluate a possible association between survival duration and each risk factor.
Univariate analysis was used to identify adverse risk factors for achieving complete remission (CR) by using χ2 (or Fisher exact) test and Wilcoxon tests. All P values presented are 2-sided, and P < .05 was regarded as statistically significant. Statistical analyses were carried out using SPLUS3.4 (MathSoft, Seattle, WA).
Results
Clinical features
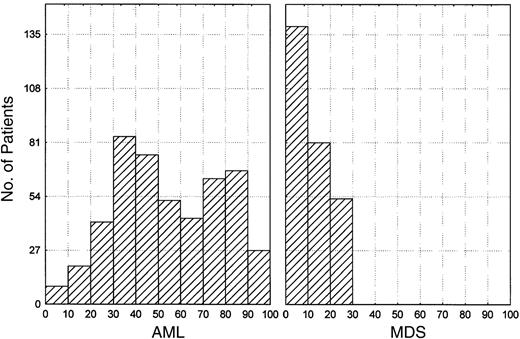
Of the 802 patients for whom clinical data were available, 279 (35%) had MDS and 523 (65%) had AML. Approximately 80% of patients with advanced MDS were dead within 2 years of induction therapy, a mortality rate not significantly different from that seen in patients with AML. Despite this similarity in overall survival rates, MDS in these patients represented a distinct disease that is clinically different from AML. In fact, most of our MDS patients lived with and died of MDS without it transforming to AML. MDS evolved to acute leukemia (30% or more blasts) in only 36 (13%) of the patients. However, these patients were on therapy, and most died of infection or bleeding. In contrast, significant numbers of AML patients had high percentages of blasts despite the fact that they died of infection or bleeding. The possibility remains that the low percentage of transformation was caused by the death of cells through chemotherapy. Regardless of transformation, MDS is an aggressive and deadly disease. Table 1 compares the clinical and laboratory characteristics of the MDS and AML groups. The MDS patients were older, more frequently had poor prognosis cytogenetics (−5, −7, 11q23, +8), and had lower platelet, bone marrow blast, and WBC counts. The initial distinction between AML and MDS for this study was based on the presence of less than 30% blasts in the bone marrow and peripheral blood. However, evaluation of the percentage of blasts in the bone marrows of these patients clearly shows gradual changes in number without clustering (Figure1).
Characteristics of MDS and AML groups
| Variable . | MDS . | AML . | P . | ||
|---|---|---|---|---|---|
| N . | Median (range) . | N . | Median (range) . | ||
| Age, y | 279 | 63 (19-84) | 523 | 59 (16-87) | .0005 |
| WBC count, × 109/L | 279 | 4.7 (0.4-124.5) | 523 | 10.4 (0.2-437) | < .00001 |
| Platelets, × 109/L | 279 | 40 (2-492) | 523 | 49 (1-2292) | .001 |
| Hemoglobin, g/dL | 279 | 7.8 (1.7-15.1) | 523 | 7.9 (2.8-15) | .54 |
| BM cellularity, % | 255 | 60 (5-100) | 502 | 75 (5-100) | .001 |
| Absolute lymphocytes, × 109/L | 279 | 1378 (54-34 486) | 523 | 1900 (0-28 425) | .00005 |
| HGF, pg/mL | 42 | 843.5 (192.3-8 657.4) | 59 | 854.8 (101.9-12 819.5) | .5 |
| TNF-α, pg/mL | 42 | 8.7 (7.1-48.2) | 59 | 9.1 (7.2-18.8) | .32 |
| AHD, mo | 279 | 3 (0-96) | 523 | 0 (0-168) | < .00001 |
| β2-microglobulin, mg/L | 181 | 2.7 (0.8-12) | 333 | 2.6 (0-31.3) | .72 |
| BM blasts, % | 279 | 10 (0-29) | 519 | 50 (2-97) | < .00001 |
| Telomerase | 29 | 948 (0-25 625) | 50 | 876 (0-13 868) | .67 |
| Caspase 3 | 36 | 4.1 (0-16.6) | 54 | 1.2 (0-22.3) | .04 |
| Performance status 0-2 | 279 | 93% | 523 | 88% | .01 |
| Poor prognosis cytogenetics | 279 | 48% | 523 | 35% | .001 |
| Variable . | MDS . | AML . | P . | ||
|---|---|---|---|---|---|
| N . | Median (range) . | N . | Median (range) . | ||
| Age, y | 279 | 63 (19-84) | 523 | 59 (16-87) | .0005 |
| WBC count, × 109/L | 279 | 4.7 (0.4-124.5) | 523 | 10.4 (0.2-437) | < .00001 |
| Platelets, × 109/L | 279 | 40 (2-492) | 523 | 49 (1-2292) | .001 |
| Hemoglobin, g/dL | 279 | 7.8 (1.7-15.1) | 523 | 7.9 (2.8-15) | .54 |
| BM cellularity, % | 255 | 60 (5-100) | 502 | 75 (5-100) | .001 |
| Absolute lymphocytes, × 109/L | 279 | 1378 (54-34 486) | 523 | 1900 (0-28 425) | .00005 |
| HGF, pg/mL | 42 | 843.5 (192.3-8 657.4) | 59 | 854.8 (101.9-12 819.5) | .5 |
| TNF-α, pg/mL | 42 | 8.7 (7.1-48.2) | 59 | 9.1 (7.2-18.8) | .32 |
| AHD, mo | 279 | 3 (0-96) | 523 | 0 (0-168) | < .00001 |
| β2-microglobulin, mg/L | 181 | 2.7 (0.8-12) | 333 | 2.6 (0-31.3) | .72 |
| BM blasts, % | 279 | 10 (0-29) | 519 | 50 (2-97) | < .00001 |
| Telomerase | 29 | 948 (0-25 625) | 50 | 876 (0-13 868) | .67 |
| Caspase 3 | 36 | 4.1 (0-16.6) | 54 | 1.2 (0-22.3) | .04 |
| Performance status 0-2 | 279 | 93% | 523 | 88% | .01 |
| Poor prognosis cytogenetics | 279 | 48% | 523 | 35% | .001 |
Telomerase activity was measured by an arbitrary unit as defined in Verstovsek et al43. Caspase 3 activity was normalized to the average detected in normal control, which was assigned a value of 1.
Distribution of percentage of bone marrow blasts in patients with AML and MDS.
There is no bimodal distribution for the percentage of blasts. Patients with acute progranulocytic (M3) leukemia or monocytic leukemia (M5) were excluded.
Distribution of percentage of bone marrow blasts in patients with AML and MDS.
There is no bimodal distribution for the percentage of blasts. Patients with acute progranulocytic (M3) leukemia or monocytic leukemia (M5) were excluded.
The lack of bimodal distribution suggests that the percentage of blasts is a continuous variable and may not be the best way to distinguish MDS from AML, thus raising questions regarding the validity of using a cut-off point, whether 20%, 30%, or another rate. Better criteria based on the biology of the disease should be used to distinguish MDS from AML.
Increased apoptosis in MDS
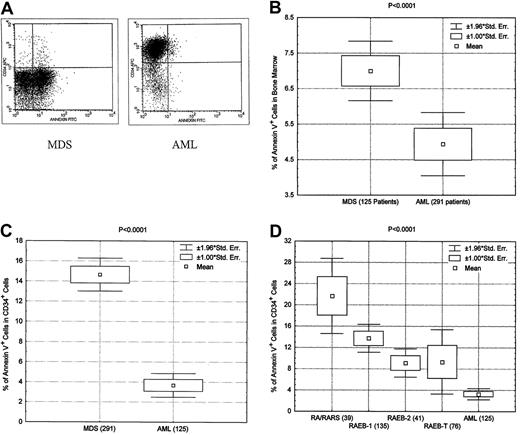
MDS is characterized by the presence of dysplasia in myeloid, erythroid, and megakaryocyte cells. Close examination suggests that the dysplastic changes may represent increased apoptosis. Condensation and fragmentation of the nucleus and clumping of the chromatin seen in MDS are all known characteristics of apoptosis. Raza et al39-42 reported increased apoptosis in MDS using a methodology based on in situ end-labeling. We used annexin V analysis, mitochondrial membrane potential, and caspase 3 activity to compare apoptotic activity in AML and MDS samples. Annexin V and mitochondrial potential analyses were performed in prospective fashion on patients with newly diagnosed disease seen at our institution. As shown in Figure 2, the expression of annexin V was significantly higher in patients with MDS than in those with AML (Wilcoxon rank-sum test, P < .0001) (Figure 2). By costaining with CD34, we demonstrated that the increase in apoptosis was not restricted to mature cells but was also seen in CD34+ immature blasts (Figure 2) (Wilcoxon rank-sum test,P < .0001). Further analysis showed that increased apoptosis in CD34+ cells can be demonstrated in RA, RARS, RAEB-1 (5%-9% blasts), RAEB-2 (10%-19% blasts), and RAEB-T (20%-29% blasts) compared with AML.
Increased apoptosis in MDS as measured by annexin V.
(A) Representative example of annexin V analysis showing greater annexin V level in a bone marrow sample in a patient with MDS than in a patient with AML. (B) Box plot showing significantly greater levels of annexin V expression in MDS patients than in AML patients. (C) Box blot showing significantly greater apoptotic activity in CD34+ cells from patients with MDS than in those from patients with AML. (D) Box blot showing significantly greater apoptotic activity in CD34+ cells from patients with various subtypes of MDS than in those from patients with AML.
Increased apoptosis in MDS as measured by annexin V.
(A) Representative example of annexin V analysis showing greater annexin V level in a bone marrow sample in a patient with MDS than in a patient with AML. (B) Box plot showing significantly greater levels of annexin V expression in MDS patients than in AML patients. (C) Box blot showing significantly greater apoptotic activity in CD34+ cells from patients with MDS than in those from patients with AML. (D) Box blot showing significantly greater apoptotic activity in CD34+ cells from patients with various subtypes of MDS than in those from patients with AML.
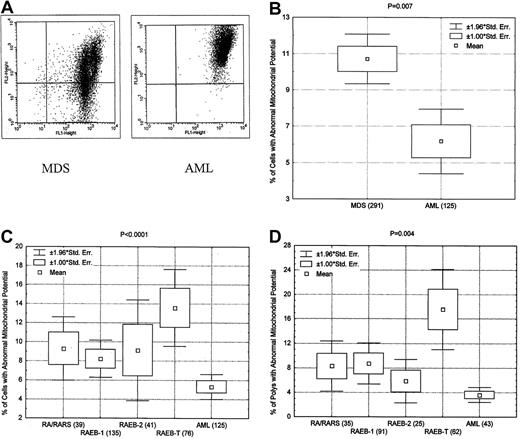
We also evaluated mitochondrial membrane potential as a means of measuring apoptosis. We used a dye (DePsipher) that aggregates and turns orange-red when mitochondrial membrane is polarized (Figure3) but remains monomeric green when the membrane potential is disturbed. Disturbance of the mitochondrial membrane has been demonstrated to be a sign of apoptosis. Upon analyzing bone marrow samples from patients with MDS and AML, Wilcoxon rank-sum analysis showed a significantly greater loss of mitochondrial potential in MDS than in AML (P = .007) (Figure 3). Further analysis showed increased apoptosis in RA, RARS, RAEB-1, RAEB-2, and RAEB-T as compared with AML. Similar results were also obtained when we gated only the polymorphonuclear cells, suggesting that the difference in apoptosis between AML and various subgroups of MDS are not simply caused by higher percentages of blasts in AML (Figure 3). As shown in Figures 2 and 3, there is some difference between annexin V and mitochondrial potential in the RAEB-T group. This may reflect the fact that mitochondrial potential measures early apoptosis, whereas annexin V measures late apoptosis. Overall there was excellent correlation between annexin V and mitochondrial potential (P < .0001) when all samples were considered and when only RAEB-T patients were considered.
Increased apoptosis in MDS as measured by mitochondrial potential.
(A) Representative example of mitochondrial potential analysis showing greater apoptotic activity in bone marrow of a patient with MDS (green, detected on FL2, instead of orange, detected on FL1) than in that of a patient with AML. (B) Box plot showing overall significantly higher apoptotic activity (disturbance in mitochondrial potential) in patients with MDS than in those with AML. (C) Box blot showing significantly greater percentage of cells with disturbance in mitochondrial potential from patients with various subtypes of MDS than in those from patients with AML. (D) Box blot showing significantly greater percentage of polymorphonuclear cells with disturbance in mitochondrial potential from patients with various subtypes of MDS than in those from patients with AML.
Increased apoptosis in MDS as measured by mitochondrial potential.
(A) Representative example of mitochondrial potential analysis showing greater apoptotic activity in bone marrow of a patient with MDS (green, detected on FL2, instead of orange, detected on FL1) than in that of a patient with AML. (B) Box plot showing overall significantly higher apoptotic activity (disturbance in mitochondrial potential) in patients with MDS than in those with AML. (C) Box blot showing significantly greater percentage of cells with disturbance in mitochondrial potential from patients with various subtypes of MDS than in those from patients with AML. (D) Box blot showing significantly greater percentage of polymorphonuclear cells with disturbance in mitochondrial potential from patients with various subtypes of MDS than in those from patients with AML.
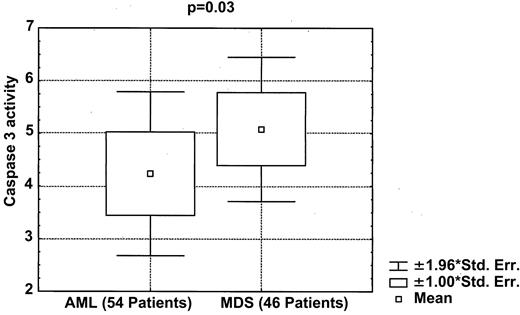
Apoptosis was also measured using caspase 3 activity in cell lysates from 36 patients with MDS and 54 with AML; this assay showed significantly greater caspase 3 activity in MDS than in AML (P = .04, Wilcoxon test) (Figure4). When high or low caspase 3 activity levels (using the median as a cut-off point) was used in predicting diagnosis, the predictive association was significant (P = .01). These data confirm that increased apoptosis is one of the characteristics distinguishing MDS from AML.
Box plot showing significantly higher caspase 3 activity in patients with MDS than in those with AML.
Box plot showing significantly higher caspase 3 activity in patients with MDS than in those with AML.
Increased proliferation in MDS patients
Using BrdU incorporation to measure DNA synthesis, we demonstrated that cell proliferation was greater in MDS than in AML (Figure5) (P = .03). Analysis of CD34+ cells also showed increased proliferation in CD34+ cells in RA, RARS, RAEB-1, RAEB-2, and RAEB-T compared with AML (P = .01) (Figure 5).
Increased proliferation in MDS.
(A) Representative example demonstrating greater BrdU incorporation in CD34+cells of a patient with MDS than in those of a patient with AML. (B) Box plot showing significantly greater BrdU incorporation in cells of patients with MDS than in those of patients with AML. (C) Box plot showing significantly greater BrdU incorporation in CD34+cells of patients with various subtypes of MDS than in those of patients with AML. The number of patients in the RAEB-T group is small (4 patients), and the apparent increase in BrdU incorporation in this group may not be accurate.
Increased proliferation in MDS.
(A) Representative example demonstrating greater BrdU incorporation in CD34+cells of a patient with MDS than in those of a patient with AML. (B) Box plot showing significantly greater BrdU incorporation in cells of patients with MDS than in those of patients with AML. (C) Box plot showing significantly greater BrdU incorporation in CD34+cells of patients with various subtypes of MDS than in those of patients with AML. The number of patients in the RAEB-T group is small (4 patients), and the apparent increase in BrdU incorporation in this group may not be accurate.
MDS cells are capable of differentiation
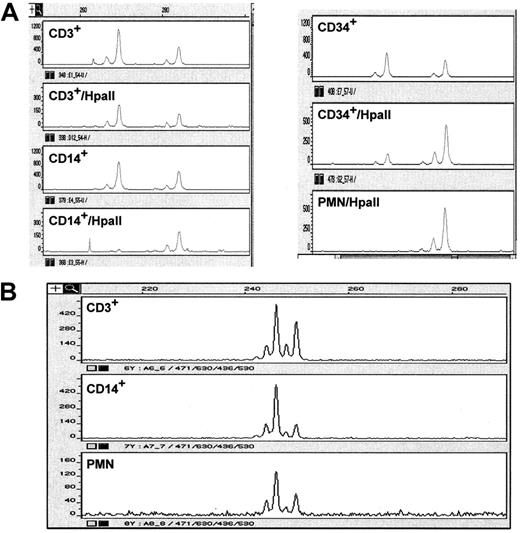
Several fluorescence in situ hybridization studies in patients with MDS with cytogenetic abnormalities have demonstrated the capability of MDS cells to differentiate to mature myeloid and erythroid cells.1 Clonality assays using the X chromosome also showed the ability of MDS cells to differentiate. We used magnetic beads to sort blasts (CD34+), monocytes (CD14+), T cells (CD3+), and polymorphonuclear cells from 10 female patients who had MDS and cytogenetic abnormalities involving chromosome 5 or 7, or both, and studied clonality using X chromosome activation and LOH (Figure 6). As shown in Table 2, mature polymorphonuclear cells always showed clonality, confirming the ability of leukemic cells to differentiate. Interestingly, monocytes in some patients with MDS without monocytosis can also be clonal. The possibility of residual normal mature polymorphonuclear cells cannot be ruled out using this methodology. All studied patients had either −5 (or 5q−) or −7 (7q−) to demonstrate LOH. Interestingly, in 2 patients we found clonality in T cells using X-chromosome activation, but we did not find clonality using LOH, which may represent X-chromosome usage bias inactivation (the tendency to inactivate one and not the other X chromosome) rather than actual clonality. In that regard, most of the patients with AML had too few mature cells for isolation and clonality study. We were able to study mature polymorphonuclear cells in 2 AML patients and found no clonality by LOH in mature cells in one patient. The second patient demonstrated clonality in mature cells, and the possibility of contamination by immature cells cannot be ruled out.
Representative example demonstrating clonality in various subpopulations of cells in patients with MDS.
(A) LOH (D5S471). (B) X-chromosome activation. Two peaks represent the 2 alleles. Loss of one peak (or significant reduction in its intensity) represents a loss of an allele. In the X-chromosome analysis, clonality is present when one peak disappears after digestion with the restriction enzyme Hpa1 because of lack of amplification products caused by the enzymatic digestion.
Representative example demonstrating clonality in various subpopulations of cells in patients with MDS.
(A) LOH (D5S471). (B) X-chromosome activation. Two peaks represent the 2 alleles. Loss of one peak (or significant reduction in its intensity) represents a loss of an allele. In the X-chromosome analysis, clonality is present when one peak disappears after digestion with the restriction enzyme Hpa1 because of lack of amplification products caused by the enzymatic digestion.
Differentiation of patients with neoplastic cells and those with MDS
| Diagnosis . | CD3 . | CD34 . | CD14 . | PMN . | ||||
|---|---|---|---|---|---|---|---|---|
| X . | LOH . | X . | LOH . | X . | LOH . | X . | LOH . | |
| RAEB | NC | NC | C | C | NC | NC | C | C |
| CMML | C | NC | C | C | C | C | C | C |
| RA | NC | NC | C | C | C | C | C | C |
| CMML | NC | NC | C | C | C | C | C | C |
| RAEB-T | C | NC | C | C | C | C | C | C |
| RAEB | NC | NC | C | C | C | C | C | C |
| RAEB-T | NC | NC | C | C | NC | NC | C | C |
| CMML | NC | NC | C | C | C | C | C | C |
| CMML | NC | NC | C | C | C | C | C | C |
| RAEB | C | NC | C | C | C | C | C | C |
| Diagnosis . | CD3 . | CD34 . | CD14 . | PMN . | ||||
|---|---|---|---|---|---|---|---|---|
| X . | LOH . | X . | LOH . | X . | LOH . | X . | LOH . | |
| RAEB | NC | NC | C | C | NC | NC | C | C |
| CMML | C | NC | C | C | C | C | C | C |
| RA | NC | NC | C | C | C | C | C | C |
| CMML | NC | NC | C | C | C | C | C | C |
| RAEB-T | C | NC | C | C | C | C | C | C |
| RAEB | NC | NC | C | C | C | C | C | C |
| RAEB-T | NC | NC | C | C | NC | NC | C | C |
| CMML | NC | NC | C | C | C | C | C | C |
| CMML | NC | NC | C | C | C | C | C | C |
| RAEB | C | NC | C | C | C | C | C | C |
NC indicates not clonal; C, clonal; and PMN, polymorphonuclear.
Inverse correlation between apoptosis and percentage of blasts
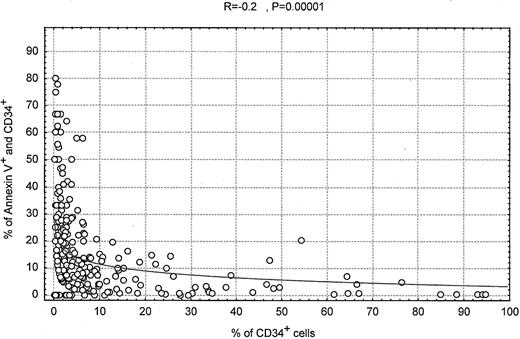
To investigate the relationship between percentage of blasts and apoptosis, we grouped the AML and MDS patients and correlated apoptosis in the CD34+ cells (blasts) as measured by annexin V with the number of CD34+ cells counted. As shown in Figure7, the extent of apoptosis decreased significantly with an increased percentage of CD34+ cells (R = −0.2; P = .00001). However, a significant number of patients were identified who had low numbers of blasts and low apoptotic activity (Figure 7). At the same time, some patients had apoptotic activity and high percentages of blasts. Furthermore, the changes in apoptotic activity appear to be gradual and show no sharp change at the blast counts of 10%, 20%, or 30%. An inverse correlation between percentage of blasts in the bone marrow and caspase 3 activity was also identified by the Spearman correlation test (P = .002; R = −0.21). A low percentage of blasts was associated with low caspase 3 activity, and, in rare patients, a high percentage of blasts was associated with high caspase 3 activity. These data suggest that there is some overlap between AML and MDS when the division is based on blast count only. Clearly, apoptotic activity is a dominant feature that distinguishes MDS from AML, and the exceptions (MDS patients without increased apoptosis) may represent patients with early AML discovered while the percentage of blasts is still low.
Scatter plot showing increased numbers of apoptotic CD34+ cells in AML and MDS patients when the percentage of total CD34+ cells is low.
We gated here on all cells in the aspirate samples rather than on mononuclear cells. Therefore, these samples are diluted by peripheral blood, and the percentage of CD34+ cells is overall less than the percentage of blasts in the bone marrow.
Scatter plot showing increased numbers of apoptotic CD34+ cells in AML and MDS patients when the percentage of total CD34+ cells is low.
We gated here on all cells in the aspirate samples rather than on mononuclear cells. Therefore, these samples are diluted by peripheral blood, and the percentage of CD34+ cells is overall less than the percentage of blasts in the bone marrow.
Clinical relevance of the biologic differences between AML and MDS
The data described above demonstrate that significant differences exist between AML and MDS. Major differences between the group of patients with MDS and those with AML are listed in Table 1. We evaluated whether these variables have a different prognostic value in AML than in MDS. Table 3 shows the results of the univariate survival analysis of these factors in AML and MDS. Overall, the prognostic values of most of these factors are similar in AML and MDS, which reflects the lack of significant difference in survival between AML and MDS using the current therapeutic approaches. These patients were treated using 1 of 3 arms: idarubicin + ara-C (IA), topotecan + ara-C (TA), and fludarabine + ara-C + idarubicin (FAI). There were significant differences in survival between the 3 arms when univariate analysis was used. However, this difference was not significant when adjusted for age. Multivariate analysis showed no difference between AML and MDS in survival regardless of the treatment arm or age. Multivariate analysis using the logistic regression model was performed and established that only age, TNF-α, and cytogenetics were significant (P = .0002, .003, and .03, respectively).
Univariate survival analysis in MDS and AML patients
| Variable . | MDS . | AML . | ||
|---|---|---|---|---|
| N . | P . | N . | P . | |
| Age | 279 | .02 | 523 | < .00001 |
| Poor prognosis cytogenetics (-5, -7, 11q23) | 279 | < .00001 | 523 | < .00001 |
| Platelets | 279 | .0017 | 523 | .5 |
| BM cellularity | 279 | .2 | 523 | .66 |
| Absolute lymphocytes | 279 | .4 | 523 | .20 |
| AHD | 279 | .089 | 523 | < .00001 |
| WBC | 279 | .5 | 523 | .08 |
| BM blasts | 279 | .66 | 523 | .5 |
| Caspase 3 | 36 | .9 | 54 | .8 |
| IL-6 | 43 | .83 | 71 | .0004 |
| Treatment arm | 278 | .0005 | 520 | .0002 |
| Variable . | MDS . | AML . | ||
|---|---|---|---|---|
| N . | P . | N . | P . | |
| Age | 279 | .02 | 523 | < .00001 |
| Poor prognosis cytogenetics (-5, -7, 11q23) | 279 | < .00001 | 523 | < .00001 |
| Platelets | 279 | .0017 | 523 | .5 |
| BM cellularity | 279 | .2 | 523 | .66 |
| Absolute lymphocytes | 279 | .4 | 523 | .20 |
| AHD | 279 | .089 | 523 | < .00001 |
| WBC | 279 | .5 | 523 | .08 |
| BM blasts | 279 | .66 | 523 | .5 |
| Caspase 3 | 36 | .9 | 54 | .8 |
| IL-6 | 43 | .83 | 71 | .0004 |
| Treatment arm | 278 | .0005 | 520 | .0002 |
Except for platelets, all significant variables had negative effects on survival. Treatment arms were IA, TA, and FAI. Longer survival was observed in the IA arm, whereas no difference was observed between the TA and FAI arms.
When we compared these variables in patients who achieved CR with those who did not achieve CR, some differences in the levels of these variables were seen (Table 4). Patients who did not achieve CR had a greater tendency toward high levels of caspase 3 activity. In addition, when we analyzed annexin V in CD34+ cells, patients with higher percentages of annexin V+/CD34+ cells had lower chances for achieving CR. This suggests a distinct clinical behavior for patients with increased apoptosis. Nonresponders also had higher levels of β2-microglobulin, IL-6, TNF-α, interleukin-1β (IL-1β), and IL-1 receptor antagonist (IL-1ra). Older age was also associated with no response. Older age and increased apoptosis are generally associated with MDS as shown in Table 1.
Comparison of patients who did and did not achieve CR
| Variable . | CR . | Non-CR . | P . | ||
|---|---|---|---|---|---|
| N . | Median (range) . | N . | Median (range) . | ||
| Age | 463 | 59.0 (18-84) | 334 | 63.5 (16-87) | .0002 |
| HGF | 65 | 805.4 (101.9-4176.1) | 36 | 1119.6 (298.7-12 819.5) | .06 |
| TNF-α | 65 | 8.6 (7.1-8.6) | 36 | 9.3 (7.2-48.2) | .05 |
| β2M | 301 | 2.4 (0.0-13.3) | 211 | 3.0 (0.8-31.3) | < .00001 |
| WBC | 463 | 6.4 (0.4-262.5) | 334 | 9.2 (0.2-266.0) | .17 |
| IL-1β | 60 | 2.5 (1.9-177.9) | 35 | 2.7 (2.1-25.9) | .01 |
| IL-1Ra | 60 | 471.3 (0-8 456.3) | 35 | 875.5 (53-7852.7) | .01 |
| IL-6 | 73 | 4.9 (2.3-261.1) | 41 | 5.6 (2.6-449.7) | .56 |
| Caspase 3 | 57 | 1.95 (0-22.3) | 33 | 3.47 (0-18.9) | .05 |
| Annexin in CD34+ | 10 | 6.48% (0%-33%) | 58 | 13.5% (0%-100%) | .03 |
| Variable . | CR . | Non-CR . | P . | ||
|---|---|---|---|---|---|
| N . | Median (range) . | N . | Median (range) . | ||
| Age | 463 | 59.0 (18-84) | 334 | 63.5 (16-87) | .0002 |
| HGF | 65 | 805.4 (101.9-4176.1) | 36 | 1119.6 (298.7-12 819.5) | .06 |
| TNF-α | 65 | 8.6 (7.1-8.6) | 36 | 9.3 (7.2-48.2) | .05 |
| β2M | 301 | 2.4 (0.0-13.3) | 211 | 3.0 (0.8-31.3) | < .00001 |
| WBC | 463 | 6.4 (0.4-262.5) | 334 | 9.2 (0.2-266.0) | .17 |
| IL-1β | 60 | 2.5 (1.9-177.9) | 35 | 2.7 (2.1-25.9) | .01 |
| IL-1Ra | 60 | 471.3 (0-8 456.3) | 35 | 875.5 (53-7852.7) | .01 |
| IL-6 | 73 | 4.9 (2.3-261.1) | 41 | 5.6 (2.6-449.7) | .56 |
| Caspase 3 | 57 | 1.95 (0-22.3) | 33 | 3.47 (0-18.9) | .05 |
| Annexin in CD34+ | 10 | 6.48% (0%-33%) | 58 | 13.5% (0%-100%) | .03 |
These data suggest that a biologic difference exists between patients with high apoptotic activity and those with low apoptotic activity. Because outcomes in AML and advanced MDS using the current therapy were no different, delineating significant clinical differences between the 2 diseases is difficult.
Discussion
The concept of MDS as a preleukemic or early leukemic process may not be accurate. Despite the similarities between MDS and AML, most patients with MDS die without their disease evolving to leukemia. Currently, treatment outcomes for AML and advanced stages of MDS remain poor, without significant differences in survival rates between the 2 diseases. Although further studies using large numbers of patients are needed, our data suggest that patients with high apoptosis are more likely not to respond to current therapy. Similarity between AML and MDS in survival using the current therapeutic approaches does not imply that the 2 diseases are the same. For example, survival in small cell lung cancer is similar to that in AML, but we do not consider the 2 diseases the same because there are biologic differences.
Diagnosis of MDS based on the percentage of blasts allows for significant overlap between AML and MDS, making assessment of differences in clinical characteristics and responses to therapy between the 2 diseases more difficult. When we grouped AML and MDS patients together and investigated whether any of the biologic markers make a difference in achieving CR, caspase 3 activity and annexin V positivity in CD34+ cells—the major biologic markers that distinguish AML from MDS—also enabled distinguishing patients with higher chances for achieving CR. This suggests that apoptosis, which is the main biologic characteristic that distinguishes AML from MDS, may have some clinical importance and perhaps allows better separation of AML from MDS based on biology rather than solely on percentage of blasts and can be clinically useful. MDS is best defined as ineffective hematopoiesis. In fact, the data presented here raise questions regarding the inclusion of the RAEB-T patients with the AML patients because most of the RAEB-T patients show high levels of apoptosis in immature and mature cells. As others and we have reported, the ineffective hematopoiesis (peripheral neutropenia) in MDS (despite the increase in bone marrow cellularity) appears to result from increased apoptosis and increased proliferation in most patients and the ability of neoplastic cells to mature.39-42 These constitute statistically significant biologic differences between MDS and AML. However, further studies are needed to actually test the significance of these differences. Clearly these data also indicate that there is significant overlap between AML and MDS, as currently classified, in their biologic characteristics. Perhaps these differences are important only in conjunction with specific therapy, but until these differences are carefully considered and investigated, we cannot neglect them. These biologic differences, rather than an arbitrary cut-off point (20% or 30% blasts), may provide more reliable criteria to distinguish AML from MDS. This calls for a better classification system that allows more accurate differentiation of MDS and AML. This classification system should be based on the levels of apoptosis, proliferation, and differentiation (including percentage of blasts) rather than solely on the number of blasts. Only then can therapeutic approaches that specifically address the biologic abnormalities of MDS result in clinical outcomes for MDS that are distinguishable from those for AML. Without proper separation of MDS from AML, it might be difficult to delineate the effectiveness of a therapy that addresses the specific biologic abnormalities of MDS.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Maher Albitar, Department of Hematopathology, The University of Texas MD Anderson Cancer Center, Box 72, 1515 Holcombe Blvd, Houston, TX 77030-4095; e-mail: malbitar@mdanderson.org.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal