Most patients with BCR-ABL–positive chronic myeloid leukemia (CML) express e13a2 (b2a2) or e14a2 (b3a2) mRNAs that result from a rearrangement of the major breakpoint cluster region (M-BCR), while rearrangements of the minorBCR with e1a2 transcripts are rare in CML.1Variation of the breakpoint within ABL is uncommon, but occasional CML and acute lymphoblastic leukemia (ALL) patients with fusions between BCR and ABL exon 3 have been described, most of whom expressed e13a3 or e14a3, respectively.2,3 E1a3 transcripts were detected in 3 cases of ALL4,5 and 1 case of CML.6 No particular phenotype has been ascribed to CML or ALL lacking exon a2.
The diagnosis of the breakpoint is usually made by reverse transcriptase–polymerase chain reaction (RT-PCR). Many laboratories have adopted the multiplex protocol established by Cross et al, which allows one to distinguish between the 3 common types of fusion transcripts based on the size of the amplified bands.7 However, atypical transcripts of similar size as 1 of the 3 major types might be missed. For example, the e1a3 transcript (307 base pair [bp]) and e13a2 transcripts (310 bp) are indistinguishable by size, even on polyacrylamide gels.5
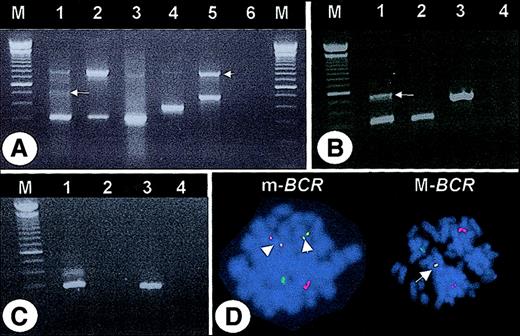
After the observation of a CML patient (case #1) with an apparent e13a2 transcript but an m-BCR rearrangement by fluorescence in-situ hybridization (FISH), we reanalyzed 65 CML patients who had been diagnosed as e13a2 by multiplex PCR7 (Figure1A). In 63 (96.8%) of the 65, the e13a2 fusion was confirmed with M-BCR–specific primers. In 2 (3.2%) of the 65, no M-BCR–specific products were generated, even by 2-step nested PCR (not shown). However, m-BCR–specific primers amplified a band of approximately 340 bp that was confirmed as e1a3 by sequencing. An additional 540-bp band was amplified from case #1 that containedBCR exon e1 fused to ABL exon a2, separated by an intervening sequence (derived from BCR intron 1) that maintained the open reading frame (Figure 1B). Amplification with primers from BCR exon e1 and ABL exon a2 confirmed this result, while no product was generated in case #2 (Figure 1C). Metaphases from 40 patients were available for FISH. The 2 patients with e1a3 fusions were found to have an m-BCRrearrangement in all metaphases (Figure 1D), while an M-BCR breakpoint was seen in all other specimens. This indicates that the e1a3 transcripts are not derived from alternative splicing of an M-BCR–derived mRNA.
PCR amplification from cDNA and FISH.
(A) Multiplex PCR. Lane 1 = case #1, lane 2 = case #2, lane 3 = e13a2-positive patient, lane 4 = K562 cell line (e14a2 positive), lane 5 = SD1 cell line (e1a2 positive), lane 6 = “blank.” Note the faint band of approximately 500 bp (arrow) and the 800-bp band corresponding to BCR(arrowhead). (B) PCR with primers corresponding to BCR exon e1 and ABL exon a3. Lane 1 = case #1, lane 2 = case #2, lane 3 = SD1 cell line, lane 4 = “blank.” Sequencing of the 540-bp PCR product (arrow) revealed 51 bp ofBCR intron 1 (nt 75253-75302, accession U07000) fused between e1 and a3. (C) PCR with primers corresponding to BCRexon e1 and ABL exon a2. Lane 1 = case #1, lane 2 = case #2, lane 3 = SD1 cell line, lane 4 = “blank.” M indicates molecular weight marker. (D) FISH (LSI bcr/abl probe, Vysis, Downer's Grove, IL). Left panel (case #1): two yellow fusion signals (arrowheads) indicate an m-BCR rearrangement. Right panel: one yellow fusion signal (arrow) indicates an M-BCRrearrangement (patient with confirmed e13a2 transcript).
PCR amplification from cDNA and FISH.
(A) Multiplex PCR. Lane 1 = case #1, lane 2 = case #2, lane 3 = e13a2-positive patient, lane 4 = K562 cell line (e14a2 positive), lane 5 = SD1 cell line (e1a2 positive), lane 6 = “blank.” Note the faint band of approximately 500 bp (arrow) and the 800-bp band corresponding to BCR(arrowhead). (B) PCR with primers corresponding to BCR exon e1 and ABL exon a3. Lane 1 = case #1, lane 2 = case #2, lane 3 = SD1 cell line, lane 4 = “blank.” Sequencing of the 540-bp PCR product (arrow) revealed 51 bp ofBCR intron 1 (nt 75253-75302, accession U07000) fused between e1 and a3. (C) PCR with primers corresponding to BCRexon e1 and ABL exon a2. Lane 1 = case #1, lane 2 = case #2, lane 3 = SD1 cell line, lane 4 = “blank.” M indicates molecular weight marker. (D) FISH (LSI bcr/abl probe, Vysis, Downer's Grove, IL). Left panel (case #1): two yellow fusion signals (arrowheads) indicate an m-BCR rearrangement. Right panel: one yellow fusion signal (arrow) indicates an M-BCRrearrangement (patient with confirmed e13a2 transcript).
Both of our patients had a relatively benign clinical course with chronic phases of 56 (case #1) and 62 (case #2) months on interferon-alpha (IFN-alpha) before they were started on imatinib, where both achieved complete cytogenetic responses (Table1). Monocytosis was noted in both cases, similar to e1a2-positive CML.1 In contrast, our cases did not consistently exhibit the other features of such cases, namely, failure to respond to IFN-alpha and absence of splemomegaly at diagnosis.
Patient characteristics
| Patient . | Age at diagnosis, y . | Phase at diagnosis . | Splenomegaly . | WBC count at diagnosis, × 109/L . | Differential count at diagnosis . | Platelet count at diagnosis, × 109/L . | Maximal monocyte count, × 109/L (%) . | Therapy prior to imatinib . | Best response prior to imatinib . | Duration of chronic phase prior to imatinib, mo . | Cytogenetics at initiating imatinib . | Best response to imatinib . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case #1 (male) | 41 | Chronic | Yes | 189.5 | 60% mature neutrophils, 32% immature neutrophils, 2% blasts, 4% eosinophils, 2% lymphocytes | 184 | 11 (13) | IFN-alpha (9 MU/d) + hydrea | Complete hematologic response | 56 | 100% Ph-positive | Complete cytogenetic remission |
| Case #2 (female) | 64 | Chronic | No | 53.2 | 72% mature neutrophils, 22% immature neutrophils, 1% blasts, 3% lymphocytes, 2% monocytes | 156 | 3.6 (27) | IFN-alpha (3-12.5 MU/wk) + hydrea | Major cytogenetic response (1/16 metaphases Ph-positive) | 62 | 50% Ph-positive | Complete cytogenetic remission |
| Case reported by Roman et al6 | 75 | Chronic | No | 18.5 | 76% mature neutrophils, 1% basophils, 17% lymphocytes, 6% monocytes | 257 | 1.1 (6) (at diagnosis) | NA | NA | 66 (at last reporting) | NA | NA |
| Patient . | Age at diagnosis, y . | Phase at diagnosis . | Splenomegaly . | WBC count at diagnosis, × 109/L . | Differential count at diagnosis . | Platelet count at diagnosis, × 109/L . | Maximal monocyte count, × 109/L (%) . | Therapy prior to imatinib . | Best response prior to imatinib . | Duration of chronic phase prior to imatinib, mo . | Cytogenetics at initiating imatinib . | Best response to imatinib . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case #1 (male) | 41 | Chronic | Yes | 189.5 | 60% mature neutrophils, 32% immature neutrophils, 2% blasts, 4% eosinophils, 2% lymphocytes | 184 | 11 (13) | IFN-alpha (9 MU/d) + hydrea | Complete hematologic response | 56 | 100% Ph-positive | Complete cytogenetic remission |
| Case #2 (female) | 64 | Chronic | No | 53.2 | 72% mature neutrophils, 22% immature neutrophils, 1% blasts, 3% lymphocytes, 2% monocytes | 156 | 3.6 (27) | IFN-alpha (3-12.5 MU/wk) + hydrea | Major cytogenetic response (1/16 metaphases Ph-positive) | 62 | 50% Ph-positive | Complete cytogenetic remission |
| Case reported by Roman et al6 | 75 | Chronic | No | 18.5 | 76% mature neutrophils, 1% basophils, 17% lymphocytes, 6% monocytes | 257 | 1.1 (6) (at diagnosis) | NA | NA | 66 (at last reporting) | NA | NA |
WBC indicates white blood cell; NA, not applicable.
Deletion of the ABL exon a2 from the fusion transcript is expected to result in a protein that lacks the N-terminal two thirds of the Src homology 3 (SH3) domain. The latter is required for full leukemogenic potential in vivo. Bcr-Abl mutants lacking SH3 induce growth-factor independence and transform murine bone marrow, but leukemic cell proliferation in vivo is retarded as a result of reduced tissue invasiveness,8 and SH3 and SH2 domains are required for STAT5 activation byBcr-Abl.9 Thus, a benign clinical course would fit well to a CML patient with a Bcr-Ablprotein lacking SH3.
Diagnosing CML patients with e1a3 fusions may be important for 2 reasons. First, if the benign clinical course is confirmed in more cases, it might have an impact on the choice of treatment. Second, if M-BCR–specific primers are used in such patients for monitoring, a false-negative result will be obtained. Indeed, this happened in our patient (case #2) during follow-up on imatinib and was initially interpreted as evidence of molecular remission.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal