Abstract
Platelet-derived growth factor β receptor (PDGFβR) fusion genes have been shown to be critical transforming oncogenes in a subset of patients with chronic myelomonocytic leukemia (CMML). The sensitivity of dysregulated tyrosine kinase oncogenes to the tyrosine kinase inhibitor STI571 (imatinib mesylate) makes it a potentially attractive treatment option in this subset of patients. We have recently cloned a novel member of the PDGFβR fusion oncogene family, rabaptin-5-PDGFβR. A patient with CMML carrying the rabaptin-5-PDGFβR fusion gene underwent allogeneic stem cell transplantation (SCT) and was monitored closely with a sensitive reverse transcriptase–polymerase chain assay to detect the novel fusion gene transcript. After achieving a molecular remission at 5 months after transplantation, 15 months after SCT the patient showed persistent and progressive evidence of molecular relapse. After demonstrating in vitro that cells transformed with this specific fusion oncogene are efficiently killed by STI571, the patient was started on STI571. The patient responded rapidly and entered molecular remission after 6 weeks of therapy, and he continues to be in remission 6 months later. These results suggest that STI571 may be an effective targeted therapy in patients with CMML related to PDGFβR fusion oncogenes.
Introduction
No treatment other than allogeneic stem cell transplantation (SCT) has been demonstrated to alter the natural history of chronic myelomonocytic leukemia (CMML). The utility of STI571 (imatinib mesylate) in chronic myelogenous leukemia (CML)1,2 likely depends both on the high sensitivity and relative specificity of the agent against the tyrosine kinase fusion oncogene, bcr-abl, as well as the critical importance of this dysregulated tyrosine kinase activity in the pathogenesis of CML. A small subset of patients with CMML carry balanced translocations involving chromosome band 5q33, resulting in fusion of the platelet-derived growth factor β receptor (PDGFβR) to a variety of fusion partners, leading to constitutive activation of the tyrosine kinase function of the PDGFβR.3-6 We have recently cloned a novel member of this family, rabaptin-5-PDGFβR (RAB5EP-PDGFβR). We have further shown that, when expressed in primary murine bone marrow cells, this fusion gene leads to a rapidly fatal myeloproliferative disease in a mouse leukemia model,6 closely mimicking the human counterpart. The nontransforming properties of a kinase inactive mutant of RAB5EP-PDGFβR emphasize the importance of the tyrosine kinase domain, similar to what is seen with bcr-abl in CML.
Along with the Abelson tyrosine kinase activated inbcr-abl, STI571 has also been shown to efficiently inhibit 2 family members of the class III receptor tyrosine kinase family, that is, PDGF receptors (both α and β) and c-kit.7 The critical importance of the PDGFβR fusion protein in this subcategory of CMML, as shown by mouse models, and the sensitivity of PDGFβR to STI571, makes STI571 an attractive targeted therapy in these patients. We provide here the first clinical evidence supporting this hypothesis, by showing evidence of a response to STI571 after molecular relapse following stem cell transplantation, in a patient carrying theRAB5EP-PDGFBR fusion oncogene.
Study design
Patient history
The patient is a previously healthy Hispanic man who presented at age 29 with constitutional symptoms, massive splenomegaly, and leukocytosis (173 000/mL), with elevated absolute counts of both neutrophils and monocytes, and left-shifted granulocytic maturation. His marrow was hypercellular, with left-shifted granulocytic maturation, increased promonocytes (10%), and dysplastic megakaryocytes, consistent with CMML. Cytogenetic analysis revealed a t(5;17)(q33;p13.3), with normal T-cell cytogenetics, ruling out a constitutional abnormality. Fluorescent in situ hybridization and reverse transcriptase-polymerase chain reaction (RT-PCR) tests for thebcr-abl oncogene were negative. The RAB5EP-PDGFBRfusion oncogene was cloned from the patient's blood cells as previously reported.6
Minimal residual disease monitoring
Total mRNA from patient peripheral blood mononuclear cells (Ficoll separated) and normal controls was extracted using RNA-STAT kit (Tel-Test, Friendswood, TX). Minimal residual disease monitoring was performed using RT-PCR for the RAB5EP-PDGFBR fusion transcript. Nested PCR primers spanning the fusion-breakpoint were: RP2151F (5′-AAGCACAGCCTGCATGTGTC-3′), RP2574R (5′-GGTCCACGTAGATGTACTCA-3′; outer), and RP2269F 5′-CAGCAGACCACGTAGAAGAA-3′), RP2433R (5′-CTGAGATCACCACCACCTTA-3′; inner). Patient sample, pretransplantation positive control, negative control total RNA (1 μg), and negative control diethyl pyrocarbonate (DEPC)–treated water were reverse transcribed using MuLV Reverse Transcriptase and Random Hexamers primers (Perkin-Elmer, Norwalk, CT). For semiquantitation, positive control cDNA was diluted in negative control cDNA in sequential 10-fold dilutions up to a dilution of 10−6. Samples were amplified using Taq DNA polymerase (Perkin-Elmer) in a nested PCR using the following cycle conditions for both outer and inner cycles: 95°C for 2 minutes followed by 25 cycles (outer)/30 cycles (inner) of 95°C for 1 minute, 60°C for 1.5 minutes, 72°C for 2 minutes, and final extension of 72°C for 8 minutes.
In vitro STI571 sensitivity
The murine hematopoietic interleukin 3 (IL-3)–dependent cell line Ba/F3 was retrovirally infected with a murine stem cell virus (MSCV) bicistronic plasmid carrying theRAB5EP-PDGFBR or the bcr-abl fusion oncogene along with enhanced green fluorescent protein (eGFP), as previously described.6 Cells were incubated in RPMI 1640 media with 10% fetal calf serum, with or without murine IL-3 (1 ng/mL), in the presence or absence of STI571 (0.01-10 μM) and counted daily to evaluate cell growth.
Western blotting
Western blots (as previously described6) were run on 12% Tris-Glycine gels and immunoblotted using a rabbit anti–p-STAT1 antibody, 1:1000 (specific for Tyr-701 phosphorylated Stat1 p91 and Stat1 p84; Santa Cruz Biotechnology, Santa Cruz, CA), followed by a secondary antirabbit horseradish peroxidase–conjugated antibody (1:20 000).
Results and discussion
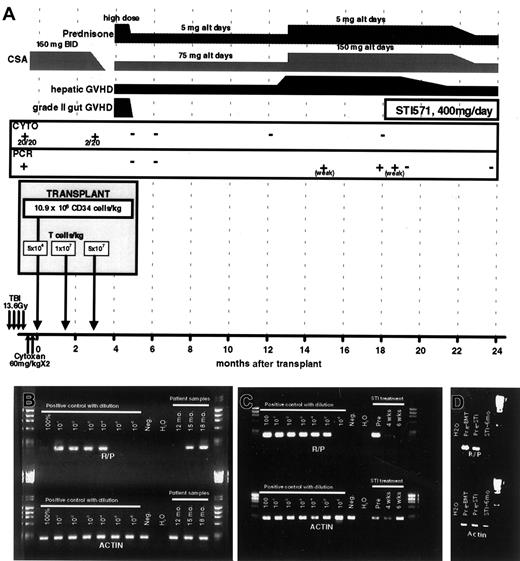
A patient carrying a PDGFRβR fusion oncogene (RAB5EP-PDGFBR), presenting with CMML (see “Patient history”) underwent T-depleted allogeneic SCT from an HLA-matched sibling, with scheduled T-cell add-back (National Heart, Lung, and Blood Institute-Institutional Review Board approved protocol no. 99-H-0046).8 Figure 1A summarizes the conditioning regimen, scheduled T-cell infusion, and the posttransplantation course. At day 100 after SCT, when the patient still had positive cytogenetics (in 2 of 20 metaphases) and splenomegaly, he received a scheduled donor lymphocyte infusion (DLI), resulting in grade 2 graft-versus-host disease (GVHD) of the gut. This was promptly followed by normalization of cytogenetics and resolution of splenomegaly. A sensitive RT-PCR assay was developed to detect theRAB5EP-PDGFBR fusion gene transcript and allow monitoring for minimal residual disease. After the day 100 DLI, the patient achieved a stable hematologic and molecular remission.
Transplantation regimen and posttransplantation course.
(A) A schematic representing the SCT regimen, and posttransplantation course, detailing GVHD, immunosuppression, disease status (by both cytogenetics and RT-PCR), and STI571 treatment. (B,C) Results of RT-PCR for RAB5EP-PDGFBR (R-P) and actin control transcripts. Pretransplantation bone marrow mRNA was diluted in negative control mRNA for semiquantitative purposes. In panel B, the patient's test samples are labeled by time from transplantation (12-18 months); in panel C the patient samples are labeled by time from initiating STI571 therapy (pre, immediately before initiating therapy). (D) Results of RT-PCR for RAB5EP-PDGFBR (R-P) and actin control transcripts, 6 months after initiation of STI571 therapy compared to pretransplantation and pre-STI571 time points.
Transplantation regimen and posttransplantation course.
(A) A schematic representing the SCT regimen, and posttransplantation course, detailing GVHD, immunosuppression, disease status (by both cytogenetics and RT-PCR), and STI571 treatment. (B,C) Results of RT-PCR for RAB5EP-PDGFBR (R-P) and actin control transcripts. Pretransplantation bone marrow mRNA was diluted in negative control mRNA for semiquantitative purposes. In panel B, the patient's test samples are labeled by time from transplantation (12-18 months); in panel C the patient samples are labeled by time from initiating STI571 therapy (pre, immediately before initiating therapy). (D) Results of RT-PCR for RAB5EP-PDGFBR (R-P) and actin control transcripts, 6 months after initiation of STI571 therapy compared to pretransplantation and pre-STI571 time points.
At 13 months after SCT the patient developed liver GVHD, requiring increased immunosuppression. Two months later (15 months after peripheral blood SCT), RT-PCR for the RAB5EP-PDGFBRfusion transcript became weakly positive in circulating mononuclear cells (Figure 1B). At 18 months, the patient remained dependent on immunosuppression to control GVHD and was found to have strongly positive RT-PCR assays for RAB5EP-PDGFBR transcripts (Figure1B), although he remained in hematologic and cytogenetic remission. Due to the persistent and progressive molecular evidence of early relapse, and an inability to reduce immunosuppression due to active chronic liver GVHD, we sought nonimmunologic means to prevent progression of his disease.
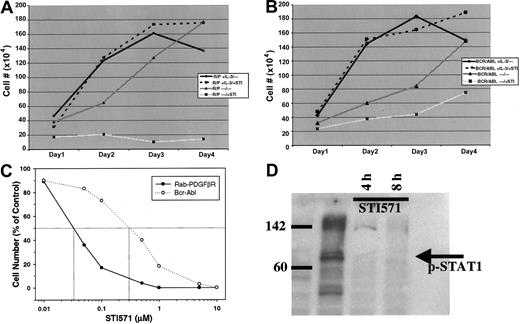
Because STI571 has been shown to be a potent PDGFβR tyrosine kinase inhibitor,7 we tested the effect of this drug on growth of the murine hematopoietic cell line Ba/F3 cells transformed with the RAB5EP-PDGFBR oncogene, compared tobcr-abl–transformed Ba/F3 cells. This cell line is dependent on IL-3 for growth, whereas the transformed cells become IL-3 independent.6 STI571 at a dose of 10 μM effectively inhibited all growth of the Ba/F3 murine hematopoietic cell line transformed with the novel RAB5EP-PDGFBR oncogene orbcr-abl. The inhibition produced by STI571 exposure can be circumvented by the addition of IL-3, demonstrating that this effect is specific (Figure 2A,B). A dose response for STI571 reveals that RAB5EP-PDGFβR is even more sensitive to the drug than bcr-abl, with a 50% inhibitory concentration (IC50) of approximately 0.03 μM compared to 0.3 μM for bcr-abl (Figure 2C). Western blotting using an antibody specific for the phosphorylated (activated) form of the downstream signaling molecule STAT1,9 showed disappearance after 4 and 8 hours of STI571 of the phosphorylated STAT1, indicating blockade of downstream effectors (Figure 2D).
Effects of STI571 on RAB5EP-PDGFBR– andbcr-abl–transformed Ba/F3 cells.
RAB5EP- PDGFBR–transformed (A) andbcr-abl–transformed (B) BaF3 cells were incubated at 2 × 105 cells/mL, with or without murine IL-3 (1 ng/mL), in the presence or absence of STI571 (10 μM). Viable cells were counted daily to assess cell growth, with each treatment being done in triplicate. (C) Dose response for STI571 after 48 hours of incubation for bcr-abl and RAB5EP-PDGFBR. (D) Western blot for phosphorylated (activated) form of STAT1 at baseline and 4 and 8 hours after addition of STI571 (10 μM).
Effects of STI571 on RAB5EP-PDGFBR– andbcr-abl–transformed Ba/F3 cells.
RAB5EP- PDGFBR–transformed (A) andbcr-abl–transformed (B) BaF3 cells were incubated at 2 × 105 cells/mL, with or without murine IL-3 (1 ng/mL), in the presence or absence of STI571 (10 μM). Viable cells were counted daily to assess cell growth, with each treatment being done in triplicate. (C) Dose response for STI571 after 48 hours of incubation for bcr-abl and RAB5EP-PDGFBR. (D) Western blot for phosphorylated (activated) form of STAT1 at baseline and 4 and 8 hours after addition of STI571 (10 μM).
Given the effects of STI571 against RAB5EP-PDGFβR–transformed Ba/F3 cells, the patient was started on STI571, 400 mg daily. He tolerated therapy well, without any side effects. No myelosuppression was noted. Four weeks after initiation of STI571 therapy, molecular testing for RAB5EP-PDGFβR showed marked reduction in the expression of the RAB5EP-PDGFβR fusion transcript in peripheral blood cells, and by 6 weeks after starting the drug the patient had attained molecular remission (Figure 1C). During this time the patient continued on the same dose of immunosuppression, arguing against an immune effect mediating the molecular response. Six months after initiating STI571 therapy, he continues to be in molecular remission (Figure 1D). These results clearly demonstrate both an in vitro and an in vivo inhibitory effect of STI571 against leukemic cells harboring a PDGFBRfusion oncogene, in a clinically relevant situation. As STI571 has also been shown to be highly effective against cells transformed withtel-PDGFBR fusion oncogene,10 it would be predicted that patients with CMML carrying this fusion gene would also respond to STI571. Preliminary findings in 2 patients with CMML carrying the tel-PDGFBR fusion gene suggests a good response to STI571.11
CMML with a PDGFβR fusion gene, like CML, is an ideal disease for targeted therapy, where a key pathogenetic event is defined. The rapid development of a virtually identical phenotype in the mouse retroviral bone marrow transplant model emphasizes the critical importance of the activated tyrosine kinase in the disease phenotype. STI571 binds tightly into the adenosine triphosphate (ATP) binding pocket in the tyrosine kinase region ofbcr-abl,12 and would be predicted to similarly bind to the ATP binding area of the PDGFβR. The different PDGFβR oncogenes all share an identical breakpoint in the PDGFβR, and retain the tyrosine kinase region, including the ATP binding pocket.3-6 The different fusion partners are believed to mediate self-association, and thus constitutive activation.13 14 Given the identical tyrosine kinase domain in the various fusion proteins, they would all be predicted to share STI571 susceptibility. The clinical response we report here indicates that STI571 is active in vivo against leukemic cells harboring PDGFβR fusion oncogenes and is useful in the setting of molecular relapse. Furthermore, this response is further proof-of-principle for the application of selective tyrosine kinase inhibitors in hematologic malignancies and gives promise that this drug may be an effective therapeutic option in patients with CMML harboring PDGFβR fusion oncogenes.
Prepublished online as Blood First Edition Paper, June 7, 2002; DOI 10.1182/ blood-2002-01-0165.
References
Author notes
Magnus K. Magnusson, Hematology Branch, National Heart, Lung and Blood Institute, Bldg 10, Room 7C103, MSC1652, 9000 Rockville Pike, Bethesda, MD 20892; e-mail: magnussm@nhlbi.nih.gov.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal