Abstract
In most cases, the lack of Rh in Rhnull red cells is associated with RHAG gene mutations. We explored the role of RhAG in the surface expression of Rh. Nonerythroid HEK293 cells, which lack Rh and RhAG, or erythroid K562 cells, which endogenously express RhAG but not Rh, were transfected with RhD and/or RhAG cDNAs using cytomegalovirus (CMV) promoter–based expression vectors. In HEK293 cells, a low but significant expression of RhD was obtained only when RhAG was expressed at a high level. In K562 cells, as expected from the opposite effects of the phorbol ester 12-O-tetradecanoyl phorbol 13-acetate (TPA) on erythroid and CMV promoters, the levels of endogenous RhAG and recombinant RhD transcripts were substantially decreased and enhanced upon TPA treatment of RhD-transfected cells (K562/RhD), respectively. However, flow cytometry and fluorescence microscopy analysis revealed a decreased cell-surface expression of both RhAG and RhD proteins. Conversely, TPA treatment of RhAG-transfected cells increased both the transcript and surface expression levels of RhAG. When K562/RhD cells were cotransfected by the RhAG cDNA, the TPA-mediated induction of recombinant RhAG and RhD transcription was associated with an increased membrane expression of both RhAG and RhD proteins. These results demonstrate the role of RhAG as a strictly required posttranscriptional factor regulating Rh membrane expression. In addition, because the postulated 2:2 stoichiometry between Rh and RhAG observed in the native red cell membrane could not be obtained in cotransfected K562 cells, our study also suggests that as yet unidentified protein(s) might be involved for optimal membrane expression of Rh.
Introduction
The Rh antigens are defined by the association of membrane polypeptides, which are missing from or severely reduced in red blood cells (RBCs) of rare Rhnull individuals. These individuals suffer from a clinical syndrome characterized by abnormalities of the red cell shape, cation transport, and membrane phospholipid organization (reviewed by Cartron1 and Huang et al2). The core of this complex is thought to be a tetramer composed of 2 Rh and 2 RhAG subunits,3 with which accessory chains (CD47, LW, and glycophorin B [GPB]) are associated by noncovalent linkages.4-7 Rh and RhAG proteins are erythroid specific and are encoded by homologous genes.8,9 Furthermore, nonerythroid homologues of RhAG are present in other tissues, thus defining an “RH gene superfamily.”10 In addition, RhAG might play a role in RBCs by participating in the linkage of the membrane cytoskeleton to the phospholipid bilayer and by exhibiting ammonium transport activity.11
The RH locus is composed of the RHD andRHCE genes, which are organized in tandem on chromosome 1p34-p36.12 The RHD gene, however, is absent from most white individuals of the RhD-negative phenotype.13 RhD and RhCcEe polypeptides are nonglycosylated, fatty-acylated proteins that span the lipid bilayer 12 times.14 The RHAG locus (chromosome 6p11-p21) encodes a glycosylated protein with the same membrane topology as Rh proteins.15-17 Although the RH locus is highly polymorphic, the few mutations of the RHAG gene described so far all resulted in defective membrane expression of RhAG in Rhnull individuals.1,2,16 18
A current model suggests that the Rh complex is not assembled or transported to the cell surface when one subunit is missing. However, molecular analysis of Rhnull individuals has revealed that abnormalities occurred only at the RH and RHAGloci, without alteration of the genes encoding CD47, LW, and GPB.1,2,18 This correlates well with the description of rare individuals whose RBCs selectively lack GPB or LW glycoproteins and are not deficient in Rh, RhAG, and CD47 proteins. Human RBCs selectively deficient in CD47 have not been described, but RBCs from CD47−/− mice19 carry a normal amount of Rh and RhAG proteins.20 Thus, CD47 is not strictly required for Rh expression, at least in mice.
Although Rh and RhAG both appear critical for surface expression of Rh antigens, they differ notably by the factors that determine their cell-surface expression. Indeed, whereas Rh proteins have never been found in the absence of RhAG, Rh proteins may be partially dispensable for the routing of RhAG to the membrane, as suggested by the following evidence: (1) A significant level of RhAG was detected on Rhnull RBCs, which lack detectable Rh proteins21 from patients carrying RH gene mutations; and (2) erythroleukemic K562 cells that either lacked22 or expressed a very low amount of Rh antigen23 expressed 6 to 9 × 104 copies of RhAG24; and (3) RhAG was expressed before Rh proteins during the differentiation of erythroid progenitors in vitro.25 26 Therefore, there is a need to develop expression systems that may clarify how Rh and RhAG proteins may interact in vivo to build a cell-surface complex expressing Rh antigens.
Expression of recombinant human RhCE and RhD proteins in erythroleukemic K562 cells using a retroviral expression system has proved useful to demonstrate the genetic basis of the major Rh antigens.27-30 We report here a cellular model in which recombinant expression of Rh and RhAG was achieved after standard plasmid cell transfection. Most important, by coupling this heterologous expression system with the property of the phorbol ester TPA (12-O-tetradecanoyl phorbol 13-acetate) to repress or induce the transcriptional activity of genes under the control of erythroid and cytomegalovirus (CMV) promoters, respectively, we demonstrate the role of RhAG as a critical posttranscriptional factor regulating membrane expression of Rh polypeptides in K562 cells. We also show for the first time that a significant, albeit low, expression level of Rh can be achieved in a nonerythroid cell line cotransfected by RhD and RhAG cDNAs.
Materials and methods
Antibodies
Murine monoclonal antibodies (mMAbs) were obtained as follows: anti-Rh, BRIC69 (Dr Anstee, Bristol, United Kingdom); anti-RhAG, 2D10 and LA18.18 (Dr Von dem Borne, Amsterdam, The Netherlands); anti-LW, BS56 (Dr Sonneborn, Offenbach, Germany); anti-CD47, 6H9 (Dr Telen, Durham, NC); anti-GPA, R18 (Dr Edwards, Cambridge, United Kingdom); anti-Lub, LM342 (Dr Fraser, Glasgow, United Kingdom); anti-CD44, 9D6 and anti-CD55, 4D3 (Bioatlantic, Nantes, France); and anti-K2, F7 (Dr Rubinstein, New York, NY). Human monoclonal antibodies (hMAbs) specific for the 9 D epitopes were reported at the first, the second, and the third International Workshop on Monoclonal Antibodies Against Human Red Cells and Related Antigens.31 Two other anti-D hMAbs were used: LOR-15C9 (Dr Blancher, Toulouse, France)32 and F5 (from our institute).33 The other hMAbs used were anti-c, RaE11; anti-E, MCG8; and anti-G, H2G11 (from our institute)33 and anti-C (Diagast, France). The rabbit polyclonal antibody MPC8 raised against the C-terminal region of the Rh polypeptides was described earlier.34
Expression vectors
The RhD cDNA was previously described.35 The RhAG cDNA was amplified by polymerase chain reaction (PCR) from total reticulocyte RNAs between primers specific for the noncoding regions of the RhAG messenger.17 cDNAs were subcloned in the pcDNA3, pREP9, and pCEP4 (Invitrogen Cergy Pontocse, France) and pcDNA-hyg36 expression vectors. Nucleotide sequences were determined using an ALFexpress automated sequencer (Amersham Pharmacia Biotech, Orsay, France). The pcDNA3-K2 vector, leading to cell-surface expression of the Kell glycoprotein carrying the K2 blood group antigen, was a gift from E. Collec from our institute.
Cell culture, transfection, and cell sorting
K562 (human erythroleukemia) and HEK293 (human embryonal kidney) cells (American Type Culture Collection, Rockville, MD) were grown, respectively, in RPMI 1640 and Dulbecco modified Eagle medium containing 10% fetal calf serum (FCS). A total of 1 × 107 K562 cells were transfected with 35 μg pcDNA3-RhD vector using Lipofectin reagent (Life Technologies, Gaithersburg, MD) and were maintained in G418-containing medium (800 μg/mL Geneticin; Life Technologies). After 2 weeks, a neomycin-resistant pool of K562 cells was incubated with an anti-D (F5) hMAb and mixed with BioMag magnetic beads coated with anti-human IgG Fc-specific antibody (Bio Advance, Emerainville, France). Cells that were retained by the magnet after several washes were recultured in RPMI/FCS 10% supplemented with G418 (800 μg/mL) and then transferred to a 96-well microplate after limiting dilution. After 2 weeks, neomycin-resistant stably transfected clones were transferred to 24-well plates and expanded before flow cytometric analysis. These transfections generated the K562/RhD clones. A total of 5 × 106 HEK293 cells were transfected with 10 μg pCEP4-RhAG vector in 400 μL phosphate-buffered saline (PBS), HEPES 10 mM, by electroporation (Biorad; 960 μF and 0.230 V) and were maintained in culture medium supplemented with 300 μg/mL hygromycin (Life Technologies).
HEK293/RhAG cells transfected by the pREP9-RhD and K562 and K562/RhD cells transfected by the pcDNA-hyg-RhAG vector were maintained in hygromycin-containing (300 μg/mL) medium with or without G418 (800 μg/mL). After 2 weeks, hygromycin K562–resistant cells were grown to a density of approximately 6 × 105/mL, and TPA (Sigma, St Louis, MO) was added in culture medium at a final concentration of 0.05 μg/mL. After 24 hours, TPA-treated K562 cells were sorted by an anti-RhAG (2D10) MAb using a FACS Vantage cytometer (Becton Dickinson, San Jose, CA). TPA-treated K562/RhD cells were sorted by magnetic beads (CELLection pan Mouse IgG Kit; Dynal, Oslo, Norway) using an anti-Rh polypeptide mMAb (clone BRIC69). Sorted cells were recultured in RPMI/FCS 10% supplemented with hygromycin (300 μg/mL) with or without G418 (800 μg/mL). The cells were then subjected to limiting dilution cloning in 96-well microplates before expanding for flow cytometry analysis. These sequential transfections generated K562/RhAG and K562/RhD/RhAG clones. K562 or K562/RhD cells transiently transfected by the pcDNA-RhAG and pcDNA3-K2 (control) vectors were treated with TPA after 24 hours of culture. Flow cytometric analyses were performed 48 hours after the treatment on K562 cells and 48 hours after the transfection on HEK293 cells.
Flow cytometric analysis
K562 or HEK293 cells (5 × 105) were resuspended in 50 μL PBS/0.2% (wt/vol) bovine serum albumin (BSA) and incubated for 30 minutes at 4°C or 27°C with mMAbs or hMAbs, respectively. After several washes with PBS, cells were incubated for 30 minutes at 4°C or 22°C with fluorescein isothiocyanate (FITC)–conjugated F(ab′)2 fragment of goat anti-mouse immunoglobulins (Immunotech, Marseille, France) or with R-phycoerythrin (RPE)–conjugated F(ab′)2 fragment of goat anti-human immunoglobulins (Immunotech), respectively. In double immunostaining (2-color labeling) experiments, sequential incubations with human anti-D, anti-human RPE-F(ab′)2, mouse anti-RhAG, and anti-mouse FITC F(ab′)2 were performed in the conditions described above. After a common excitation at 488 nm, the fluorescence was measured at 515 to 545 nm for FITC and 564 to 606 nm for PE. With an additional excitation at 630 nm, a third fluorescence measurement at 653 to 669 nm using the TO-PRO-3 iodide (Interchim, Montluçon, France) allowed exclusion of dead cells from analysis. Fluorescence was measured on a FACSCalibur flow cytometer (Becton Dickinson). When using anti-mouse antibodies, we quantified the cell-surface antigen expression using calibration mouse IgG-coated beads (Qifikit; DAKO, Glostrup, Denmark) as standards, according to the manufacturer's instructions. The results were expressed as specific antibody-binding capacity (SABC) units, which proved to be directly proportional to the number of molecules bound per cell.
Immunofluorescence microscopy
K562 cells were washed in PHEM buffer, pH 6.9 (K-PIPES 60 mM, HEPES 25 mM, EGTA 1 mM, Mg-acetate 1 mM), spread on glass slides, and fixed with 4% paraformaldehyde for 20 minutes. After washes in PBS, the cells were treated by 50 mM NH4Cl to block aldehyde groups. After washes in PBS, cells were permeabilized with 0.5% Triton X-100 for 10 minutes. Cells were then blocked with a solution of 0.1% BSA in PBS for 10 minutes and incubated simultaneously with appropriate dilutions of mMAbs and hMAbs for 1 hour. After 3 washes in PBS containing BSA, the cells were stained with Alexa-Fluor 488-conjugated anti-mouse and Alexa-Fluor 568-conjugated anti-human IgG (Molecular-Probes, Interchim, Asnières, France). After washes in PBS, the stained cells were mounted in Slow Fade Light antifade (Molecular-Probes, Interchim). Fluorescence was observed with a Leica TCS (Leica, Rueil Malmaison, France) equipped with a krypto-argon laser, a 63/1.4 oil objective lens, and a simultaneous dual-channel detector.
Immunoprecipitation of RhD and RhAG polypeptides from K562 cells
K562 and K562/RhD/RhAG cells were labeled with [35S]-methionine (Amersham) (400 μCi [14.8 MBq]/107 cells) for 3 hours in 0.5 mL methionine-free RPMI. After 3 washes with ice-cold PBS, cells were incubated with 200 μL lysis buffer (PBS containing 1% Triton X-100 with complete EDTA-free protease inhibitors; Roche, Meylan, France) for 30 minutes at 4°C and centrifuged. A total of 800 μL buffer A (PBS containing sodium dodecyl sulfate [SDS] 0.1%, Triton X-100 0.5%, EDTA 5 mM, BSA 0.5%) was added to the supernatant. This was separated in 2 aliquots of 0.5 mL, one of which was incubated with 10 μL MPC8 rabbit anti-Rh antibody and the other with 100 μL LA18.18 anti-RhAG mMAb overnight at 4°C. The immune complexes extracted by Protein A–Sepharose (Amersham Pharmacia Biotech) were run on SDS–polyacrylamide gel electrophoresis (SDS-PAGE), and the dried gels were subjected to autoradiography.
Northern blot analysis
Total RNAs were extracted from K562 cells using the High Pure RNA Isolation Kit (Roche). RNA samples (10 μg) were resolved by electrophoresis on a 1.1% (vol/vol) formaldehyde, 1% (wt/vol) agarose gel and transferred to nylon filters (Zeta-probe GT; BioRad, Hercules, CA). Hybridization with [32P]-cDNA probes was performed in 7% SDS, 0.5 M NaHPO4 at 65°C for 16 hours. Final washes were performed in 0.1 × SSC, 0.1% SDS (w/v) at 65°C. The probes were as follows: RhAG and RhD cDNAs described above; Lu (1978 bp) and GPA (485 bp) as described37,38; CD44 (1087 bp), CD55 (1394 bp), and CD47 (IAP) (972 bp) obtained by reverse transcriptase (RT)–PCR of lymphocyte RNA using the Superscript One-Step RT-PCR System (Life Technologies); and primers based on published sequences.39-41
Results
HEK293 cells stably expressing the recombinant RhAG and/or RhD polypeptides
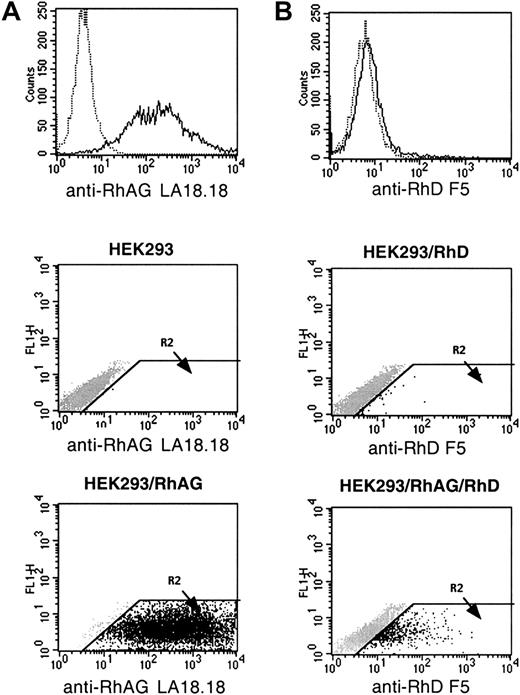
Cell-surface expression of RhAG polypeptide was investigated by flow cytometry analysis of HEK293 cells transfected by the pCEP4-RhAG plasmid. After 3 weeks of selection in hygromycin-containing medium, a high level (mean fluorescence intensity [MFI] 362) and stable expression of the recombinant protein was detected by the LA18.18 anti-RhAG MAb on pCEP4-RhAG–transfected cells (HEK293/RhAG)but not on untransfected cells (Figure1A). Using the F5 anti-D MAb, no expression of RhD was detected on both cells (not shown).
Flow cytometric analysis of HEK293 cells expressing recombinant RhAG and RhD antigen.
(A) The mouse MAb LA18.18 was used to compare RhAG antigen expression on hygromycin-resistant HEK293/RhAG cells (____) and untransfected HEK293 cells (….). (B) The human MAb anti-D F5 was used to compare RhD antigen expression on hygromycin- and G418-resistant HEK293/RhAG/RhD cells (____) and HEK293/RhD cells (….). In dot-plot representations, the R2 region was defined as a positive region in which no signal was detected with negative controls (mouse IgG1 and human anti-K1 MAb).
Flow cytometric analysis of HEK293 cells expressing recombinant RhAG and RhD antigen.
(A) The mouse MAb LA18.18 was used to compare RhAG antigen expression on hygromycin-resistant HEK293/RhAG cells (____) and untransfected HEK293 cells (….). (B) The human MAb anti-D F5 was used to compare RhD antigen expression on hygromycin- and G418-resistant HEK293/RhAG/RhD cells (____) and HEK293/RhD cells (….). In dot-plot representations, the R2 region was defined as a positive region in which no signal was detected with negative controls (mouse IgG1 and human anti-K1 MAb).
HEK293 and HEK293/RhAG cells were also transfected by the pREP9-RhD plasmid. Flow cytometric analysis using the F5 anti-D MAb revealed that a weak but significant transient expression of the RhD polypeptide could be detected on the HEK293/RhAG cells but not on the HEK293 cells 48 hours after transfection (Figure 1B).
Stable K562 clone expressing the recombinant RhD polypeptide
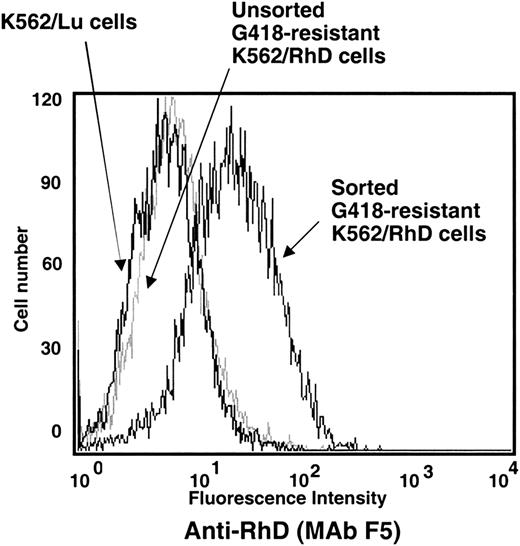
A G418-resistant K562/RhD cell pool was generated after standard plasmid transfection with the pcDNA3-RhD vector. Flow cytometry performed as soon as a pool of G418-resistant cells could be obtained indicated that the F5 anti-D MAb bound much more strongly to K562/RhD cells than to the control K562/Lu clone expressing the recombinant Lu glycoprotein,42 with a 2-fold increase in MFI (not shown). However, after 2 additional weeks of culture, K562/RhD cells exhibited only a weak increase in RhD antigen expression as compared with K562/Lu cells (Figure 2). Therefore, the cell fraction overexpressing RhD was enriched after the neomycin selection step by mixing magnetic beads coated with anti-human IgG Fc MAb with the K562/RhD cells preincubated with the anti-D MAb. Figure 2 shows that the F5 anti-D MAb bound much more strongly to the sorted than to the unsorted K562/RhD cells or K562/Lu cells. Flow cytometric analysis of a K562/RhD clone obtained showed that MAbs to all the RhD epitopes (9-epitope model) as well as BRIC69 (anti-Rh polypeptide), F5 and LOR-15C9 (anti-RhD), and H2G11 (anti-RhG) bound more strongly to K562/RhD than to K562/Lu cells (Table 1), with a 1.9- to 6.2-fold increase in MFI. Anti-CD47 and anti-LW MAbs showed a 2-fold increase in binding between K562/RhD and K562/Lu cells, whereas MAbs directed against epitopes specific for the RHCEencoded polypeptide (c, E, e) or the anti-RhAG MAb showed no difference between the recombinant clones.
Flow cytometric analysis of the enrichment of K562 cells expressing recombinant RhD antigen by magnetic-bead sorting.
The human MAb anti-D F5 was used to compare RhD antigen expression on G418-resistant K562/RhD cells before and after magnetic bead sorting, as described in “Materials and methods.” K562/Lu stably expressing the recombinant Lu blood group glycoprotein was used as negative control.
Flow cytometric analysis of the enrichment of K562 cells expressing recombinant RhD antigen by magnetic-bead sorting.
The human MAb anti-D F5 was used to compare RhD antigen expression on G418-resistant K562/RhD cells before and after magnetic bead sorting, as described in “Materials and methods.” K562/Lu stably expressing the recombinant Lu blood group glycoprotein was used as negative control.
Flow cytometric analysis of the binding of anti-Rh and anti-Rh-related MAbs to K562/RhD and K562/Lu stable clones
| Antibody . | Directed against . | K562/RhD MFI . | K562/Lu control MFI . | MFI ratio, K562/RhD over K562/Lu . |
|---|---|---|---|---|
| M. control | 2.9 | 2.3 | 1.2 | |
| BRIC69 | Rh polypeptides | 51.6 | 16.9 | 3.7 |
| BS56 | LW | 15.2 | 8.3 | 1.8 |
| 6H9 | CD47 | 146.4 | 81.8 | 1.8 |
| 2D10 | RhAG | 34.3 | 39.4 | 0.9 |
| H. control | 3.4 | 2.8 | 1.2 | |
| F5 | D | 38.9 | 7.3 | 5.3 |
| LOR-15C9 | D | 30.2 | 5.5 | 5.8 |
| RegA | epD1 | 6.2 | 2.2 | 2.7 |
| 5C8 | epD2 | 17.0 | 3.9 | 4.4 |
| 75 | epD3 | 27.5 | 8.0 | 3.4 |
| H27 | epD4 | 12.3 | 2.8 | 4.3 |
| Fom1 | epD5 | 3.6 | 1.9 | 1.9 |
| FogB | epD6 | 7.2 | 3.0 | 2.4 |
| RUM1 | epD7 | 38.1 | 9.8 | 3.9 |
| 105 | epD8 | 23.7 | 4.1 | 5.7 |
| MS26 | epD9 | 31.8 | 5.1 | 6.2 |
| RaE11 | c | 8.8 | 8.9 | 1.0 |
| Diagast IgM | C | 3.0 | 2.4 | 1.2 |
| MCG8 | E | 4.4 | 7.5 | 0.6 |
| H2G11 | G | 11.3 | 3.8 | 3.0 |
| Antibody . | Directed against . | K562/RhD MFI . | K562/Lu control MFI . | MFI ratio, K562/RhD over K562/Lu . |
|---|---|---|---|---|
| M. control | 2.9 | 2.3 | 1.2 | |
| BRIC69 | Rh polypeptides | 51.6 | 16.9 | 3.7 |
| BS56 | LW | 15.2 | 8.3 | 1.8 |
| 6H9 | CD47 | 146.4 | 81.8 | 1.8 |
| 2D10 | RhAG | 34.3 | 39.4 | 0.9 |
| H. control | 3.4 | 2.8 | 1.2 | |
| F5 | D | 38.9 | 7.3 | 5.3 |
| LOR-15C9 | D | 30.2 | 5.5 | 5.8 |
| RegA | epD1 | 6.2 | 2.2 | 2.7 |
| 5C8 | epD2 | 17.0 | 3.9 | 4.4 |
| 75 | epD3 | 27.5 | 8.0 | 3.4 |
| H27 | epD4 | 12.3 | 2.8 | 4.3 |
| Fom1 | epD5 | 3.6 | 1.9 | 1.9 |
| FogB | epD6 | 7.2 | 3.0 | 2.4 |
| RUM1 | epD7 | 38.1 | 9.8 | 3.9 |
| 105 | epD8 | 23.7 | 4.1 | 5.7 |
| MS26 | epD9 | 31.8 | 5.1 | 6.2 |
| RaE11 | c | 8.8 | 8.9 | 1.0 |
| Diagast IgM | C | 3.0 | 2.4 | 1.2 |
| MCG8 | E | 4.4 | 7.5 | 0.6 |
| H2G11 | G | 11.3 | 3.8 | 3.0 |
MFI indicates mean fluorescence intensity; M. control and H. control refer to murine and human isotype controls, respectively.
Stable K562 clone expressing the recombinant RhAG glycoprotein
Because of the substantial expression of endogenous RhAG (40 000-50 000 molecules/cell), recombinant RhAG could not be distinguished by comparative flow cytometric analysis between mock and transiently RhAG-transfected K562 cells. However, when these cells were treated by TPA, overexpression of RhAG antigen was detected on a small number of cells transfected by pcDNA3-hyg/RhAG. These cells most likely corresponded to the transfected cell subpopulation because, as discussed below, TPA treatment enhanced CMV promoter–based expression of transgenes and abolished expression of endogenous erythroid-specific markers. To derive a K562 clone stably expressing recombinant RhAG, we sorted TPA-treated cells that overexpressed RhAG and recultured them in hygromycin-containing medium without TPA. After limiting dilution cloning, a stable K562/RhAG clone was obtained. As estimated from the binding capacity of the 2D10 MAb, the K562/RhAG clone expressed 3- to 4-fold more RhAG glycoprotein (SABC 133 000 molecules/cell) than control clones stably transfected by Lu or RhD (52 000 and 40 000 molecules/cell, respectively) (Table 2).
Specific antibody-binding capacity (SABC) of TPA-treated and -untreated stably transfected K562 clones
| MAb . | Directed against . | K562/Lu (control) . | K562/RhD . | K562/RhAG . | K562/RhD/RhAG . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TPA . | TPA . | TPA . | TPA . | ||||||||||
| 0 h . | 24 h . | 48 h . | 0 . | 24 h . | 48 h . | 0 . | 24 h . | 48 h . | 0 . | 24 h . | 48 h . | ||
| 6H9 | CD47 | 41.9 | 32.8 | 30.0 | 45.8 | 26.2 | 26.6 | 81.7 | 69.7 | 88.9 | 47.4 | 36.2 | 37.9 |
| 2D10 | RhAG | 52.8 | 13.8 | 9.1 | 40.0 | 14.0 | 4.6 | 133.0 | 326.0 | 426.0 | 79.3 | 100.9 | 191.0 |
| BRIC69 | Rh | 1.9 | 0.5 | 0.0 | 7.8 | 1.2 | 0.0 | 4.8 | 1.8 | 1.5 | 25.2 | 16.8 | 23.8 |
| LM342 | Lub | 109.0 | 320.0 | 454.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| MAb . | Directed against . | K562/Lu (control) . | K562/RhD . | K562/RhAG . | K562/RhD/RhAG . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TPA . | TPA . | TPA . | TPA . | ||||||||||
| 0 h . | 24 h . | 48 h . | 0 . | 24 h . | 48 h . | 0 . | 24 h . | 48 h . | 0 . | 24 h . | 48 h . | ||
| 6H9 | CD47 | 41.9 | 32.8 | 30.0 | 45.8 | 26.2 | 26.6 | 81.7 | 69.7 | 88.9 | 47.4 | 36.2 | 37.9 |
| 2D10 | RhAG | 52.8 | 13.8 | 9.1 | 40.0 | 14.0 | 4.6 | 133.0 | 326.0 | 426.0 | 79.3 | 100.9 | 191.0 |
| BRIC69 | Rh | 1.9 | 0.5 | 0.0 | 7.8 | 1.2 | 0.0 | 4.8 | 1.8 | 1.5 | 25.2 | 16.8 | 23.8 |
| LM342 | Lub | 109.0 | 320.0 | 454.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
SABC values (× 10−3) were determined by indirect immunofluorescence using QIFIKIT calibrated beads (see “Materials and methods”). The values represent an estimate of the mean number of antibody molecules bound per cell. TPA indicates 12-O-tetradecanoyl phorbol 13-acetate.
Effect of TPA on the expression levels of endogenous and recombinant transcripts in K562 cells
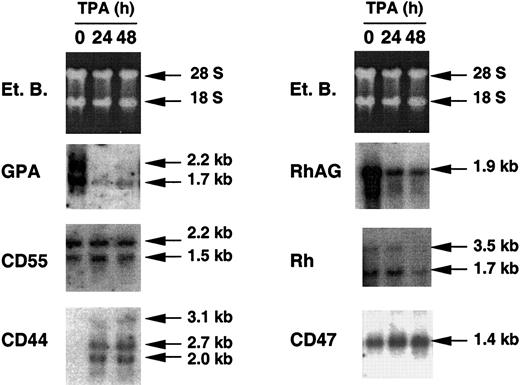
TPA treatment of K562 cells has been shown to down-regulate the transcription of erythroid-specific markers such as GPA and PBGD43,44 and to up-regulate transcription of the megakaryocytic differentiation marker CD44.45 The effect of TPA on the endogenous expression of genes encoding the major proteins of the Rh complex (RhAG, Rh, and CD47) was estimated by Northern blot analysis of K562/Lu cells. Figure3 shows that the endogenous expression of RhAG and Rh transcripts was down-regulated, as expected for erythroid-specific genes, whereas expression of the ubiquitous CD47 gene was not affected. As controls, hybridizations with GPA, CD55, and CD44 probes showed a negative effect, no effect, and a positive effect of TPA, respectively.
Effect of TPA on the levels of erythroid, megakaryocytic, and ubiquitous endogenous transcripts of K562 cells.
Northern blot analyses were performed with RNA samples from wild-type K562 cells. Total RNAs were extracted from TPA-untreated cells (0) and from cells preliminarily treated with TPA for 24 and 48 hours (24 and 48). Ethidium bromide (Et. B.) staining revealed 2 bands, which corresponded to the ribosomal RNAs 28S and 18S and the intensity of which correlated with the amount of total RNA loaded on the agarose gel (10 μg). GPA, CD55, CD44, RhAG, Rh, and CD47 probes were prepared and labeled as described in “Materials and methods.” Blots were exposed overnight to Kodak BioMax MS film. With the Rh probe, exposure was performed for 48 hours. Several endogenous transcripts were found for GPA, CD55, CD44, and Rh.
Effect of TPA on the levels of erythroid, megakaryocytic, and ubiquitous endogenous transcripts of K562 cells.
Northern blot analyses were performed with RNA samples from wild-type K562 cells. Total RNAs were extracted from TPA-untreated cells (0) and from cells preliminarily treated with TPA for 24 and 48 hours (24 and 48). Ethidium bromide (Et. B.) staining revealed 2 bands, which corresponded to the ribosomal RNAs 28S and 18S and the intensity of which correlated with the amount of total RNA loaded on the agarose gel (10 μg). GPA, CD55, CD44, RhAG, Rh, and CD47 probes were prepared and labeled as described in “Materials and methods.” Blots were exposed overnight to Kodak BioMax MS film. With the Rh probe, exposure was performed for 48 hours. Several endogenous transcripts were found for GPA, CD55, CD44, and Rh.
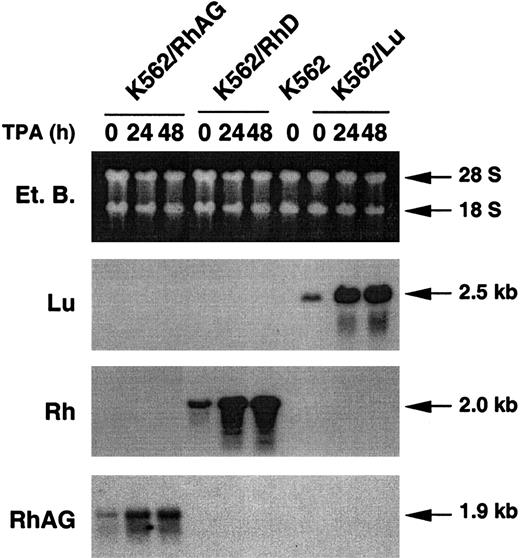
TPA treatment was shown to activate the transcriptional activity of the HCMV MIE enhancer/promoter region.46 In the pcDNA3 vector, this CMV promoter region regulates expression of recombinant genes. Therefore, in an attempt to increase expression of the recombinant RhD and RhAG cDNAs, the K562/RhD and K562/RhAG clones were maintained for 24 or 48 hours in culture medium containing TPA. As a control, K562/Lu cells were similarly treated. Northern blot analysis showed that the levels of recombinant RhAG, RhD, and Lu transcripts were substantially increased by TPA treatment of K562/RhAG, K562/RhD, and K562/Lu cells, respectively (Figure4).
Expression of recombinant Rh and RhAG transcripts under the control of CMV promoter is enhanced by TPA treatment of transfected K562 cells.
Northern blot analyses were performed with RNA samples from K562 cells and K562/RhAG, K562/RhD, and K562/Lu cells stably expressing RhAG, RhD, and Lu proteins, respectively. Total RNAs were extracted from TPA-untreated cells (0) and from transfected cells preliminarily treated with TPA for 24 and 48 hours (24 and 48). Lu, Rh, and RhAG probes were prepared and labeled as described in “Materials and methods.” Blots were exposed to Kodak BioMax MS film for 4 hours. On longer exposure, the endogenous Rh and RhAG transcripts were detected from K562 untransfected and transfected cells (not shown).
Expression of recombinant Rh and RhAG transcripts under the control of CMV promoter is enhanced by TPA treatment of transfected K562 cells.
Northern blot analyses were performed with RNA samples from K562 cells and K562/RhAG, K562/RhD, and K562/Lu cells stably expressing RhAG, RhD, and Lu proteins, respectively. Total RNAs were extracted from TPA-untreated cells (0) and from transfected cells preliminarily treated with TPA for 24 and 48 hours (24 and 48). Lu, Rh, and RhAG probes were prepared and labeled as described in “Materials and methods.” Blots were exposed to Kodak BioMax MS film for 4 hours. On longer exposure, the endogenous Rh and RhAG transcripts were detected from K562 untransfected and transfected cells (not shown).
Effect of TPA on the expression levels of endogenous and recombinant antigens in K562 cells
Flow cytometric analysis of cell-surface antigen expression using specific MAbs fully correlated with Northern blot analysis as regards the effect of TPA on endogenous markers of recombinant K562 clones. TPA treatment resulted in up-regulation and down-regulation of the cell-surface expression of CD44 and GPA, respectively, whereas no effect was detected on CD55 and CD47 expression (not shown).
The down-regulation of endogenous RhAG expression was confirmed in K562/Lu and K562/RhD cells. Endogenous expression of Rh in K562/Lu and K562/RhAG cells was poorly detectable, but a TPA-mediated down-regulation could be observed (Table 2). Flow cytometric analysis showed that the TPA-mediated enhancement of recombinant transcripts resulted in a 4-fold and a 3-fold increase in the surface expression of the recombinant Lu and RhAG antigens in K562/Lu and K562/RhAG cells, respectively (Figure 5; Table 2). In contrast, the surface expression of the recombinant RhD antigen in K562/RhD cells, as detected by LOR-15C9 and F5 anti-D hMAbs (Figure 5) or by BRIC69 anti-Rh mMAb (Table 2), exhibited a 5-fold decrease after 24 hours of TPA treatment and was not detectable after 48 hours.
Effect of TPA treatment on the cell-surface expression of RhD, RhAG, and Lu antigens in K562/RhD, K562/RhAG, and K562/Lu transfectants.
Cells were analyzed by flow cytometry before and after TPA treatment for 48 hours. Antibodies included (A) anti-RhD, LOR-15C9 hMAb; (B) anti-RhAG, LA18.18 mMAb; and (C) anti-Lu, LM342 mMAb. Similar results were obtained when RhD expression was analyzed with F5 and BRIC69 MAbs (not shown).
Effect of TPA treatment on the cell-surface expression of RhD, RhAG, and Lu antigens in K562/RhD, K562/RhAG, and K562/Lu transfectants.
Cells were analyzed by flow cytometry before and after TPA treatment for 48 hours. Antibodies included (A) anti-RhD, LOR-15C9 hMAb; (B) anti-RhAG, LA18.18 mMAb; and (C) anti-Lu, LM342 mMAb. Similar results were obtained when RhD expression was analyzed with F5 and BRIC69 MAbs (not shown).
Flow cytometric analysis of K562 and K562/RhD clones transiently transfected with RhAG cDNA
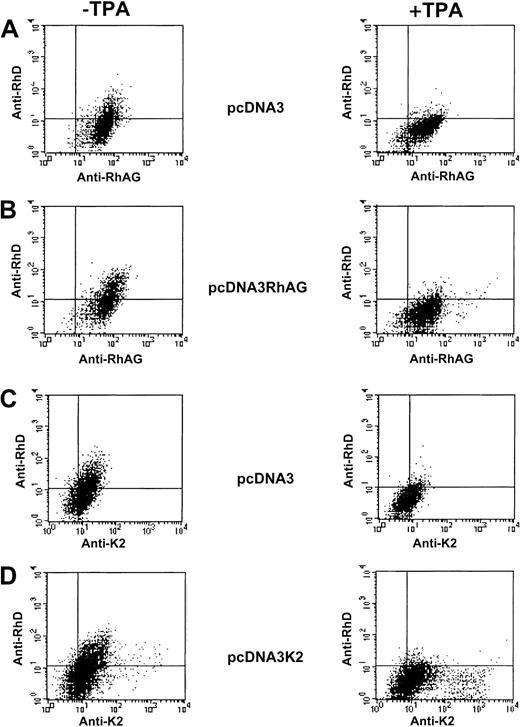
To evaluate the postulated role of RhAG in membrane expression of RhD, we transiently transfected K562 and K562/RhD clones with the pcDNA3-RhAG vector. TPA treatment was particularly convenient for extinguishing endogenous expression of RhAG and thus focusing only on the effect of the overexpression of the recombinant RhAG protein. Moreover, transient transfections allowed us to analyze at once several transfected and untransfected K562/RhD cells exhibiting heterogeneous levels of RhAG membrane expression. As controls, these cells were transfected with the empty pcDNA3 vector (mock control) and with a pcDNA3-K2 plasmid. K2 antigen was chosen as irrelevant control because, like RhAG, it is expressed endogenously in K562 cells,47and therefore, we expected TPA-mediated regulation of endogenous and recombinant K2 antigen expression similar to that described above for RhAG. Simultaneous analysis of RhD and RhAG or RhD and Kell expression was performed by flow cytometry after double immunostaining.
Analysis of mock-transfected K562 cells showed that the endogenous expression of RhAG and K2 antigens was down-regulated by TPA treatment (Figure 6A,C). Analysis of the TPA effect on cells transiently transfected by the RhAG or Kell cDNAs revealed 2 cell subpopulations (Figure 6B,D). A major subpopulation, most likely corresponding to nontransfected cells, exhibited a decreased expression of RhAG and Kell upon TPA treatment. However, a TPA-mediated overexpression of RhAG and Kell antigens was clearly observed in a small subpopulation of K562 cells transiently transfected by RhAG and K2 cDNAs, respectively, as compared with mock transfectants. Regarding RhD expression in TPA-untreated cells, we could not detect a positive effect of the transient expression of RhAG or, as expected, of Kell proteins (Figure 6B,D). Furthermore, in contrast to recombinant RhAG and Kell antigens, the weak endogenous expression of RhD was repressed, as expected, under TPA treatment of mock-, RhAG-, and Kell-transfected cells (Figure 6).
Flow cytometric analysis of K562 cells transiently transfected with pcDNA3, pcDNA3/RhAG, and pcDNA3/K2 vectors.
Following double immunostaining experiments (as described in “Materials and methods”), simultaneous analysis of the RhD and RhAG antigen expressions (A,B) and of RhD and K2 antigen expressions (C,D) were performed on TPA-untreated cells (left panels) and TPA-treated cells (right panels).
Flow cytometric analysis of K562 cells transiently transfected with pcDNA3, pcDNA3/RhAG, and pcDNA3/K2 vectors.
Following double immunostaining experiments (as described in “Materials and methods”), simultaneous analysis of the RhD and RhAG antigen expressions (A,B) and of RhD and K2 antigen expressions (C,D) were performed on TPA-untreated cells (left panels) and TPA-treated cells (right panels).
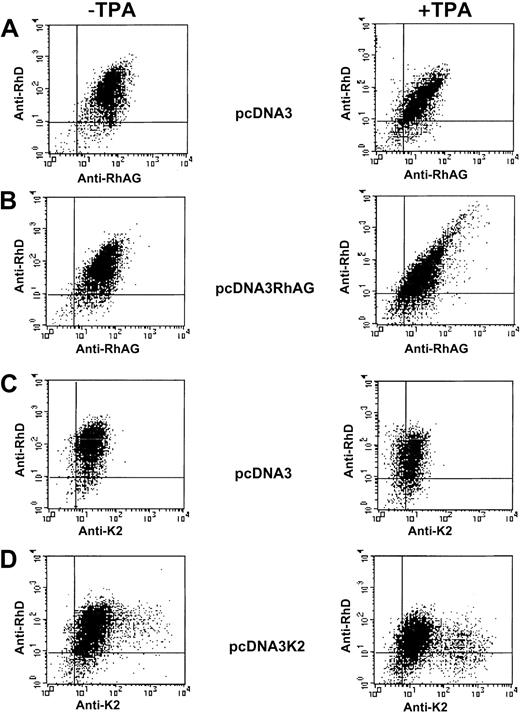
K562/RhD cells transiently transfected by empty, RhAG, and K2 expression vectors (Figure 7) exhibited TPA responses similar to those discussed above for K562 cells when endogenous and recombinant RhAG and Kell antigens were analyzed. However, different results were obtained when RhD expression was analyzed. First, the LOR-15C9 anti-D MAb bound more strongly to K562/RhD than to K562 cells (Figures 6A and 7A), as discussed above. Second, a cell subpopulation exhibiting a substantial increase of RhD and RhAG surface expression could be detected after TPA treatment of K562/RhD cells transfected by the RhAG cDNA (Figure 7B), but not by empty vector (Figure 7A,C). Moreover, the typical tapering shape of the diagram observed with TPA-treated RhAG-transfected K562/RhD cells indicates a good correlation between RhAG and RhD antigen expression levels. As a control, overexpression of the Kell antigen in TPA-treated K2-transfected cells did not lead to overexpression of RhD (Figure7D).
Flow cytometric analysis of K562/RhD cells transiently transfected with empty pcDNA3, pcDNA3/RhAG, and pcDNA3/K2 vectors.
Following double immunostaining experiments, simultaneous analysis of the RhD and RhAG antigen expressions (A,B) and of RhD and K2 antigen expressions (C,D) were performed on TPA-untreated cells (left panels) and TPA-treated cells (right panels).
Flow cytometric analysis of K562/RhD cells transiently transfected with empty pcDNA3, pcDNA3/RhAG, and pcDNA3/K2 vectors.
Following double immunostaining experiments, simultaneous analysis of the RhD and RhAG antigen expressions (A,B) and of RhD and K2 antigen expressions (C,D) were performed on TPA-untreated cells (left panels) and TPA-treated cells (right panels).
Stable K562/RhD/RhAG clone expressing both RhD and RhAG recombinant polypeptides
The transient transfection experiments discussed above strongly suggested that overexpression of RhD upon TPA treatment of K562/RhD cells was achieved only in the RhAG-transfected cell subpopulation. To generate K562 cells stably expressing both RhD and RhAG recombinant polypeptides, K562/RhD cells were transfected by the RhAG cDNA cloned in a pcDNA-hyg vector, treated by TPA, and stained with the BRIC69 anti-Rh polypeptide MAb. Cells with the highest expression of RhD were sorted and cultured in medium containing both neomycin and hygromycin. After limiting dilution cloning, a K562/RhD/RhAG stable clone was obtained. Flow cytometric analysis indicated that K562/RhD/RhAG cells, as did the K562/RhAG clone, expressed more RhAG antigen (79 000 copies/cell) than the K562/Lu (52 800 copies/cell) or K562/RhD (40 000 copies/cell) clones (Table 2). As expected from the analysis of the K562/RhAG clone, the K562/RhD/RhAG clone also exhibited a TPA-mediated enhancement (>2-fold) in cell-surface expression of RhAG. The K562/RhD/RhAG cells also bound more strongly the BRIC69 anti-Rh MAb than all other clones, and, more important, did not exhibit a TPA-mediated down-regulation of cell-surface expression of RhD, in contrast to all other clones, including K562/RhD. However, it should be noted that in contrast to RhAG, expression of RhD could not be enhanced by TPA treatment of these cells.
Expression of the RhD recombinant polypeptide in the K562/RhD/RhAG cells was demonstrated by immunoprecipitating the [35S]-methionine–labeled proteins of the cell lysates with the MPC8 anti-Rh polypeptide antibody and by analyzing the immunoprecipitates on SDS-PAGE. As shown in Figure8A, a 32-kd band corresponding to the Rh polypeptide was clearly identified (with the same intensity) from TPA-treated or -untreated K562/RhD/RhAG cell samples, but poorly or not at all from parental K562 cell samples. Using the same immunoprecipitation procedure on K562 cells and the LA18.18 monoclonal anti-RhAG antibody, we found a 32-kd band (Figure 8B) corresponding to endogenous expression of the partially glycosylated RhAG protein.48 In agreement with the flow cytometric analysis showing a TPA-mediated down-regulation of endogenous RhAG, the 32-kd signal was fainter in TPA-treated than in TPA-untreated K562 cell samples. In contrast, the 32-kd signal detected in the K562/RhD/RhAG cell sample was clearly enhanced when the cells were treated with TPA (Figure 8B). These results suggest that this strong signal corresponded to the expression of recombinant RhAG in TPA-treated K562/RhD/RhAG cells and indicate that TPA did not affect the glycosylation state of the RhAG protein in K562 cells.
Immunoprecipitation of RhD and RhAG from K562 and K562/RhD/RhAG cells.
TPA-treated (+) and untreated (−) cells were incubated for 3 hours with [35S]-methionine. Cell lysates from K562 (W.T.) and K562/RhD/RhAG cells were used for immunoprecipitation with the MPC8 anti-Rh polyclonal antibody (PAb) (A) or the LA18.18 anti-RhAG mMAb (B) and Protein A–Sepharose. The isolated proteins were separated by 10% SDS-PAGE under reducing conditions. Low molecular weight proteins from Biorad were used as standards. Arrows indicate the 32-kd Rh and RhAG proteins.
Immunoprecipitation of RhD and RhAG from K562 and K562/RhD/RhAG cells.
TPA-treated (+) and untreated (−) cells were incubated for 3 hours with [35S]-methionine. Cell lysates from K562 (W.T.) and K562/RhD/RhAG cells were used for immunoprecipitation with the MPC8 anti-Rh polyclonal antibody (PAb) (A) or the LA18.18 anti-RhAG mMAb (B) and Protein A–Sepharose. The isolated proteins were separated by 10% SDS-PAGE under reducing conditions. Low molecular weight proteins from Biorad were used as standards. Arrows indicate the 32-kd Rh and RhAG proteins.
Immunolocalization of Rh and RhAG in K562 cells
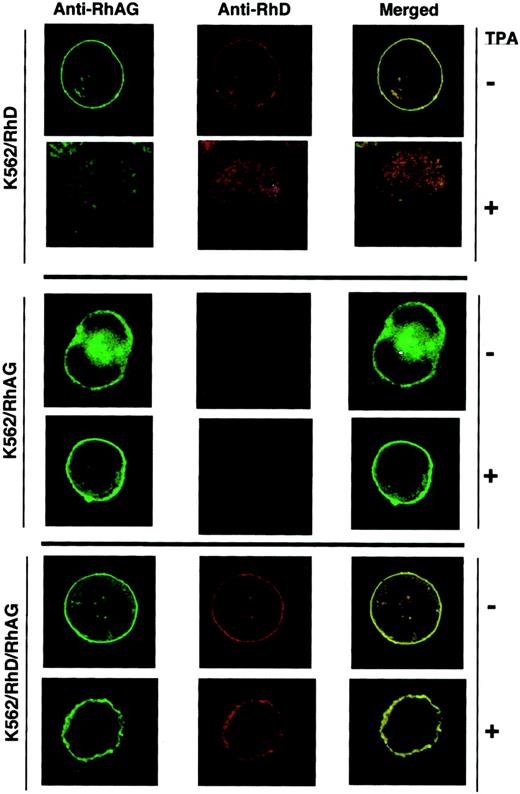
To further illustrate the results obtained by flow cytometry and immunoprecipitation analysis, we performed fluorescence microscopy analysis of transfected K562 cells after labeling with the LOR-15C9 anti-RhD and the LA18.18 anti-RhAG antibodies. As shown in Figure9, the membrane of K562/RhD cells was clearly stained by anti-RhAG, and a faint staining by anti-RhD could be detected despite the relatively low surface expression of RhD in these cells (7800 molecules/cell; Table 2). As a control, no membrane staining was detectable when the anti-RhD MAb was replaced by an anti-K1 human antibody (not shown), the K1 antigen not being expressed in K562 cells. The plasma membrane of K562/RhD cells was not stained anymore by anti-RhAG and anti-RhD MAbs after TPA treatment. The diffuse intracellular staining observed in TPA-treated cells should not be attributed to an intracellular accumulation of the RhAG and RhD polypeptides because of the high cytoplasmic background also observed in these cells with the anti-K1 negative control (not shown). The cell membrane of untreated or TPA-treated K562/RhAG cells was stained only by anti-RhAG MAb. Finally, the plasma membrane of K562/RhD/RhAG cells was stained by both the anti-RhAG (green fluorescence) and anti-RhD (red fluorescence) antibodies, and in contrast to K562/RhD cells, the same image was obtained after TPA treatment of these cells. Because the LOR-15C9 anti-RhD antibody has been previously shown to recognize the denatured RhD polypeptide in SDS gels32 and the purified RhD polypeptide (without RhAG),49 these results indicate that even when a large amount of RhD transcript is produced, as in TPA-treated K562/RhD cells, the lack of Rh antigens in the absence of RhAG is the result of nonexpression of the Rh polypeptide per se at the cell surface and not of a conformational change preventing accessibility of antibody epitopes.
Immunolocalization of Rh and RhAG in K562 transfectants.
TPA-treated and untreated cells were incubated with mMAb LA18.18 and hMAb LOR-15C9. MAbs bound to RhAG and RhD polypeptides were probed with Alexa-Fluor 488–conjugated anti-mouse IgG (green fluorescence) and Alexa-Fluor 568–conjugated anti-human IgG (red fluorescence), respectively. Fluorescence images were visualized using a confocal laser microscope. Coexpression of Rh and RhAG resulted in yellow fluorescence on merged images. Because of the small size and high nucleus/cytoplasmic ratio of K562 cells, discriminative fluorescence staining patterns can be interpreted with confidence only for expression or nonexpression of the relevant antigens at the cell membrane.
Immunolocalization of Rh and RhAG in K562 transfectants.
TPA-treated and untreated cells were incubated with mMAb LA18.18 and hMAb LOR-15C9. MAbs bound to RhAG and RhD polypeptides were probed with Alexa-Fluor 488–conjugated anti-mouse IgG (green fluorescence) and Alexa-Fluor 568–conjugated anti-human IgG (red fluorescence), respectively. Fluorescence images were visualized using a confocal laser microscope. Coexpression of Rh and RhAG resulted in yellow fluorescence on merged images. Because of the small size and high nucleus/cytoplasmic ratio of K562 cells, discriminative fluorescence staining patterns can be interpreted with confidence only for expression or nonexpression of the relevant antigens at the cell membrane.
Discussion
In this paper, we report the expression of recombinant RhD and RhAG polypeptides following a standard plasmid transfection of HEK293 and K562 cells. This approach was used to demonstrate that surface expression of Rh depends on coexpression of RhAG, as previously suggested by molecular genetic analysis of Rhnullvariants.
First, HEK293 and HEK293/RhAG cells were transiently transfected with the RhD cDNA. As previously shown in the green monkey kidney COS-1 cells,50 our present study confirmed that cell-surface expression of recombinant RhAG glycoprotein could be achieved in nonerythroid cells. Furthermore, whereas surface expression of RhD was not recognized in stable COS-1 cells carrying both RhD and RhAG cDNAs,50 we were able to detect cell-surface expression of RhD in HEK293/RhAG cells but not in HEK293 cells, both transfected with the RhD cDNA. The very high expression level of recombinant RhAG in HEK293 cells as compared with COS-1 cells (not shown) might account for this apparent discrepancy. This result showed for the first time that transfected RhAG is able to influence the cell-surface expression of RhD in nonerythroid cells. However, because of the very different expression levels of recombinant RhAG and RhD in HEK293/RhAG/RhD cells, we assumed that this nonerythroid expression system is currently not suitable for investigation of the assembly of the Rh-RhAG complex, in which 2 RhAG polypeptides associate with 2 Rh polypeptides in the native RBC membrane.3
Erythroid K562 cells exhibiting endogenous expression of RhAG have recently proved to be particularly useful for demonstrating the genetic basis of the major Rh antigens using a retroviral expression system.27-30 In the present study, using a standard plasmid cell transfection, we were able to detect a significant surface expression of recombinant RhD despite a relatively low expression of endogenous RhAG in these K562 cells (MFI 35-40) as compared with the high expression level of recombinant RhAG in HEK293/RhAG cells (MFI 362). Therefore, to investigate the role of erythroid-specific proteins involved in the Rh complex assembly, such an erythroid system was found to be more suitable than the HEK293 model described above. Because of the reduced growth rates of G418-resistant/Rh-expressing cells compared with that of G418-resistant/non–Rh-expressing cells (doubling time, 27 hours versus 18 hours; not shown), a magnetic bead–based enrichment step was necessary for the isolation of a stable K562/RhD clone. Because of the rather low expression level of RhD antigen in K562/RhD cells (<10 000 copies/cell), these cells were treated by TPA, a phorbol ester described as an inducer of the CMV-based promoters,46 like those present in the vectors used in this study. However, whereas the amount of RhD transcripts was found to be substantially increased by TPA cell treatment, the amount of RhD antigen detected at the surface of K562/RhD cells was dramatically reduced. Because TPA has also been described as a negative effector of erythroid-specific promoters,43,44 we assumed that this unexpected result could account for a modulation of Rh membrane expression by an erythroid-specific protein whose expression was down-regulated by TPA. Among such proteins, the RhAG glycoprotein, whose expression is under the control of an erythroid-specific promoter,51 52 was the best candidate.
To analyze the effect of RhAG expression levels on RhD surface expression, we transiently or stably transfected K562 and K562/RhD cells with RhAG cDNA. We found that cell-surface expression of RhAG in K562/RhAG cells could be dramatically increased by enhancing the recombinant RhAG transcript level by TPA treatment. This result indicated that, at least in K562 cells, RhAG antigen level is not limited by the absence of Rh, as previously postulated,48but is rather regulated at the transcriptional level. However, mutations of the RH genes identified in Rhnullindividuals of the amorph type are associated with a severely reduced amount of RhAG polypeptide on RBCs.1 These apparently contradictory results obtained with nucleated cells and mature RBCs suggest that Rh might be involved in the stability of RhAG once expressed at the cell membrane, rather than in the dynamic translocation process of RhAG toward the membrane. According to this hypothesis, the membrane instability of RhAG in the absence of Rh is not observed in nucleated cells, presumably because of the constant neosynthesis of RhAG polypeptide. Although the underglycosylation of RhAG in Rhnull cells suggested that the transit time of RhAG in the Golgi might be regulated by Rh,53 our present immunoprecipitation studies failed to reveal any modification of RhAG glycosylation between wild-type and RhD-transfected K562 cells (Figure8). This discrepancy may be explained by a defect of K562 cells in some glycosylation branching enzymes, as previously illustrated by the incomplete glycosylation of glycophorins A and C in K562 cells as compared with late erythroid precursors.54
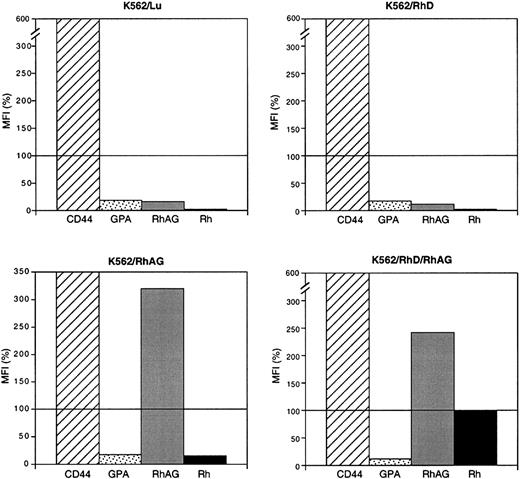
Flow cytometric analysis of TPA-treated and TPA-untreated K562/RhD cells transiently transfected by RhAG revealed that only the subpopulation of cells that overexpressed recombinant RhAG also overexpressed the RhD antigen. Moreover, as recently described for band 3 and GPA using a similar expression system,55 a linear correlation between RhAG and RhD antigen expression levels was observed. The regulation of RhD and RhAG antigen coexpression was further investigated in K562 cells stably expressing both recombinant RhD and RhAG proteins (clone K562/RhD/RhAG) by analyzing the effect of different times of TPA treatment. At first, the 2-fold overexpression of RhAG obtained after stable transfection of RhAG cDNA in K562/RhD cells was found to be associated with a 3-fold enhancement of RhD expression. Most important, TPA treatment of these cells (K562/RhD/RhAG transfectants), in contrast to that of K562/RhD cells, did not lead to a down-regulation of RhD expression. We assumed that this different regulation of RhD by TPA treatment resulted from the up-regulation of recombinant RhAG in K562/RhD/RhAG cells as opposed to the down-regulation of endogenous RhAG in K562/RhD cells (Figure10).
Schematic representation of the effect of TPA treatment of K562 cells on the cell-surface expression of endogenous or exogenous Rh and RhAG proteins.
Expression levels of each marker in untreated cells are designated as 100%. Bars correspond to the expression in cells treated by TPA for 48 hours. Values are from Table 2. CD44 and GPA are endogenously expressed in K562 cells and represent controls of the positive and negative effects of TPA, respectively.
Schematic representation of the effect of TPA treatment of K562 cells on the cell-surface expression of endogenous or exogenous Rh and RhAG proteins.
Expression levels of each marker in untreated cells are designated as 100%. Bars correspond to the expression in cells treated by TPA for 48 hours. Values are from Table 2. CD44 and GPA are endogenously expressed in K562 cells and represent controls of the positive and negative effects of TPA, respectively.
TPA treatment of K562/RhD/RhAG cells, although not resulting in a down-regulation of RhD expression as in K562 or K562/RhAG cells, did not result in an up-regulation of RhD as observed for RhAG (Table 2; Figure 10). Furthermore, after 48 hours of TPA treatment, the RhAG surface expression in K562/RhD/RhAG cells was similar to that found in adult RBCs (180 000-200 000 copies/cell),1 whereas the level of recombinant RhD membrane expression was still largely lower (23 800 copies/cell) than that found in RBCs (100 000-200 000 copies/cell). This observation suggests that, beside the clear role of RhAG as a strictly required factor, the potential role of other protein(s), missing or weakly expressed in K562 cells, for optimal membrane expression of the RhD antigen should be investigated. It has been reported that the cell-surface reactivity of Rh antigens in K562 cells could be enhanced by expression of recombinant band 3,56 but it was subsequently shown that band 3 exhibits a lower affinity for RhD-RhAG than for RhCcEe-RhAG complexes.57 Furthermore, the data presented in these 2 papers showed that recombinant band-3 expression resulted in a more drastic increase in RhAG expression as compared with Rh. Together with our present results, these studies also suggest that RhD expression in K562 cells could not be enhanced beyond a certain level by increasing RhAG expression, either by TPA treatment or by band-3 coexpression. Therefore, it is unlikely that band 3 is the only missing factor in K562 cells required to reach an RhD expression level similar to that observed in mature RBCs and to that of RhAG. Given the recent studies showing that Rh and RhAG proteins are linked to the membrane skeleton,47 58 the putative role of erythroid-specific cytoskeletal protein(s) that would stabilize RhD cell-surface expression and that might be down-regulated by TPA treatment should also be evoked. Characterization of the cytoskeletal and cytoplasmic partners of the Rh and Rh-related polypeptides and of their role in the membrane expression of Rh antigens should certainly represent one of the next important steps in the analysis of Rh complex biosynthesis.
We thank Drs C. Lopez and C. Rahuel from our institute for the gift of CD55 and GPA probes, E. Collec from our institute for the pcDNA3/K2 expression vector, and Dr P. Rameau (INSERM U362, Villejuif, France) for cell-sorting experiments.
Supported in part by the Institut National de la Santé et de la Recherche Médicale.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Yves Colin, INSERM U76, INTS, 6 Rue Alexandre Cabanel, 75015 Paris, France; e-mail: colin@idf.inserm.fr.


![Fig. 8. Immunoprecipitation of RhD and RhAG from K562 and K562/RhD/RhAG cells. / TPA-treated (+) and untreated (−) cells were incubated for 3 hours with [35S]-methionine. Cell lysates from K562 (W.T.) and K562/RhD/RhAG cells were used for immunoprecipitation with the MPC8 anti-Rh polyclonal antibody (PAb) (A) or the LA18.18 anti-RhAG mMAb (B) and Protein A–Sepharose. The isolated proteins were separated by 10% SDS-PAGE under reducing conditions. Low molecular weight proteins from Biorad were used as standards. Arrows indicate the 32-kd Rh and RhAG proteins.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/3/10.1182_blood.v100.3.1038/4/m_h81522885008.jpeg?Expires=1769097825&Signature=ER44ETzSE3B2d0aOMOByCjBRryBgYAvC0IY8Ig3-VFPRAriboUBepHiCa~iLGI6vD31rcEQIoWWzZWKoyGmAVYQIFPUT8tEICX-QAUkNUyUvUtc4PKd26hr8ChEEZGD9etf9FErqC9jqFzzEmlVcUWdUNZoAyj5-tzPb~9NfJ0wokJCF0wRn3B3bvF94dOhbXD5hA8lryUoY3GEQ0oWMs2DWUoVvduT~VwPkm1ByRegEx--VbmViM4~7dMt4VZuXJ1vehAAJcFP4TvG3XtiF3HRllgia9SBvXtsQphnDonLrs~jGH7MfemZcOqCkIJgw3uoC6I1C2D-xfuE0mi3zFQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal