Abstract
We describe a family with autosomal dominant inheritance of increased body iron stores characterized by raised serum ferritin concentration and normal transferrin saturation. Liver biopsy showed iron deposition in Kupffer cells without fibrosis. The clinical features of HFE-related hemochromatosis were absent, as were the Cys282Tyr and His63Asp mutations. Venesection therapy was poorly tolerated, suggesting a defect in iron release from reticuloendothelial stores. A 3–base pair deletion in exon 5 of the ferroportin 1 gene (SLC11A3) predicting Val162 deletion was found in affected members, but not in unaffected individuals or in 100 control subjects. Consensus structural predictions of the transmembrane helices showed that the deletion is in the extracellular loop between the third and fourth predicted transmembrane helices and lies within a spatial cluster of other known ferroportin 1 mutations. These results indicate that this extracellular cluster is functionally important for iron transport, and its disruption leads to iron overload.
Introduction
Genetic hemochromatosis is usually an autosomal recessive condition in which excessive iron absorption causes iron overload, primarily in parenchymal cells. It is associated withHFE missense mutations (Cys282Tyr, His63Asp). In the United Kingdom, more than 90% of cases of genetic hemochromatosis areHFE related.1 We describe a family with autosomal dominant, reticuloendothelial iron overload due to a mutation in ferroportin 1 (IREG1, MTP1, official name: solute carrier family 11, member A3, or SLC11A3), a newly discovered gene encoding a transmembrane protein involved in iron release from cells.2-4
Study design
Clinical evaluation
The proband (38 years) presented with fatigue. Her serum ferritin concentration was 2855 μg/L (normal range up to 200 μg/L) and transferrin saturation was 40% (normal range up to 50%). There was no intercurrent disease to explain the high ferritin level, and a liver biopsy revealed heavy iron deposition in both hepatocytes and Kupffer cells. After 5 venesections (450 mL blood per week), the hemoglobin dropped to 9.0 g/dL. A bone marrow aspiration indicated “mild dyserythropoiesis with defective hemoglobinization.”
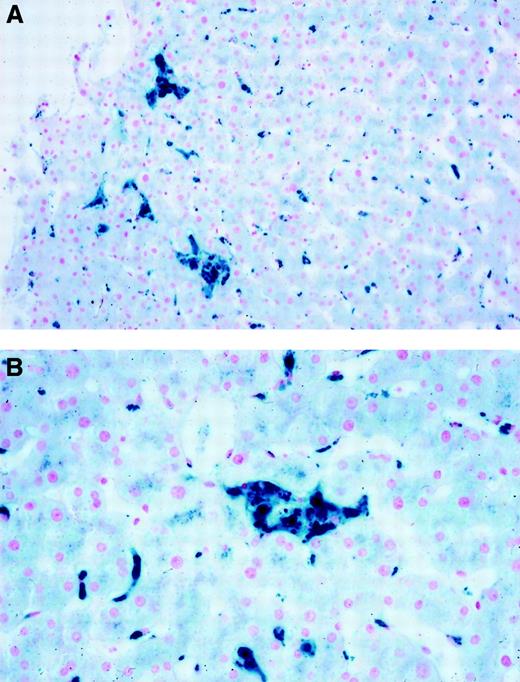
The proband's sister (34 years) had a high serum ferritin concentration (1150 μg/L) and a transferrin saturation of 31%. A magnetic resonance imaging (MRI) scan suggested iron accumulation in both the liver and spleen. Liver biopsy showed no fibrosis and marked iron accumulation in Kupffer cells (Figure1). Bone marrow aspiration showed “mild dyserythropoiesis.” Her hemoglobin concentration fell to 9.9 g/dL after 6 phlebotomies. The proband's father (61 years) had a serum ferritin concentration of 4850 μg/L and transferrin saturation of 37%. MRI showed evidence of iron deposition in the liver and spleen and some hepatic steatosis. He declined liver biopsy or venesection.
Liver histology (Perls stain).
Liver biopsy for the proband's sister showing iron staining predominantly in Kupffer cells. (A) Low power; (B) high power.
Liver histology (Perls stain).
Liver biopsy for the proband's sister showing iron staining predominantly in Kupffer cells. (A) Low power; (B) high power.
The 2 sisters received erythropoietin (4000 units twice a week) during phlebotomy to prevent anemia. The proband underwent venesection every 2 weeks for 15 months, and about 6 g Fe was removed. About 8 g Fe was removed from her sister over 18 months, when the serum ferritin had fallen to 230 μg/L. Erythropoietin was discontinued, but she became anemic and required further treatment with erythropoietin before the hemoglobin concentration returned to normal.
Elevated serum ferritin associated with normal transferrin saturation was found in other family members investigated, consistent with an autosomal dominant mode of inheritance. Some members of the family initially requested investigation but did not wish to be included in this paper.
Genetic studies
Genomic DNA isolation5 and Cys282Tyr and His63Asp mutation analysis of HFE were performed as previously described.1 The genomic structure of the ferroportin 1 gene was determined from a BLAST search using the cDNA (AF231121). Eight exons were mapped on the GenBank genomic contig sequence NT_022197.2. Direct sequencing of the exons and flanking intron–exon boundaries was carried out using the ABI Prism Terminator Cycle Sequencing Ready Reaction kit (PE Applied Biosystems, Foster City, CA) and ABI Prism 377 DNA sequencer. All products were sequenced in both directions (primers and conditions are available from the corresponding author).
Transmembrane helix prediction
Hydrophobicity plots were derived from 8 software packages (Figure 2) for each of the human, rat, mouse, and zebrafish ferroportin 1 sequences. The consensus of the 9 helix predictions was determined by a majority vote in all 32 calculations. The helix topology with respect to the orientation of the lipid bilayer was generally predicted6 by the “positive-inside rule.” The prediction of transmembrane helices is accurate to about 96%, and the accuracy of the determination of the transmembrane helical regions is ±3 residues. The expected accuracy of the topology prediction is greater than 86%, with higher than average accuracies for eukaryotic proteins.
Sequence analysis and topology of the predicted transmembrane helices in ferroportin 1.
The software packages used were: TM pred, TMAP, and DAS via the PIX program at www.hgmp.mrc.ac.uk/; SOSUI (www.tuat.ac.jp/∼mitaku/); TopPred2 (bioweb.pasteur.fr/seqanal/interfaces/toppred.html); TMHMM2.0 (www.cbs.dtu.dk/services/TMHMM-2.0/); HMMTOP2 (www.enzim.hu/hmmtop/); and PHDhtm/PHDtopology (www.embl-heidelberg.de/predictprotein/predictprotein.html). The sequence accession numbers were: human, NM_014585; mouse, NM_016917; rat, AF394785; and zebrafish, AF226612. In NM_016917, the unspecified amino acid Xaa365 was specified as Arg365 by comparison with AF215637. (A) The sequence is annotated in order to identify the consensus transmembrane helices predicted from 8 methods and the locations of the 4 mutations identified to date in human and zebrafish ferroportin 1 (bold and underlined). The zebrafish Leu167Phe mutation is responsible for the hypochromic anemia weissherbstphenotype.2 Leu167Phe in zebrafish is equivalent to Leu170Phe in the predicted human sequence. The sequence numbering is denoted by a vertical stroke at every fifth residue and the bracketed number at the end of each line. The locations of the 12 Cys residues (*) and 3 extracellular putative N-linked oligosaccharide sites (#) are in bold type and underlined (see text). (B) The 9 predicted transmembrane helices (1-9) are shown in relation to the lipid bilayer, with the positions of the 4 mutations (red circles), 12 Cys residues (yellow circles), and 3 putative exposed N-linked oligosaccharide sites (Y) marked as shown. The N- and C-termini are denoted by N and C, respectively. The residue length of each ferroportin 1 loop is denoted by the numbers adjacent to each one.
Sequence analysis and topology of the predicted transmembrane helices in ferroportin 1.
The software packages used were: TM pred, TMAP, and DAS via the PIX program at www.hgmp.mrc.ac.uk/; SOSUI (www.tuat.ac.jp/∼mitaku/); TopPred2 (bioweb.pasteur.fr/seqanal/interfaces/toppred.html); TMHMM2.0 (www.cbs.dtu.dk/services/TMHMM-2.0/); HMMTOP2 (www.enzim.hu/hmmtop/); and PHDhtm/PHDtopology (www.embl-heidelberg.de/predictprotein/predictprotein.html). The sequence accession numbers were: human, NM_014585; mouse, NM_016917; rat, AF394785; and zebrafish, AF226612. In NM_016917, the unspecified amino acid Xaa365 was specified as Arg365 by comparison with AF215637. (A) The sequence is annotated in order to identify the consensus transmembrane helices predicted from 8 methods and the locations of the 4 mutations identified to date in human and zebrafish ferroportin 1 (bold and underlined). The zebrafish Leu167Phe mutation is responsible for the hypochromic anemia weissherbstphenotype.2 Leu167Phe in zebrafish is equivalent to Leu170Phe in the predicted human sequence. The sequence numbering is denoted by a vertical stroke at every fifth residue and the bracketed number at the end of each line. The locations of the 12 Cys residues (*) and 3 extracellular putative N-linked oligosaccharide sites (#) are in bold type and underlined (see text). (B) The 9 predicted transmembrane helices (1-9) are shown in relation to the lipid bilayer, with the positions of the 4 mutations (red circles), 12 Cys residues (yellow circles), and 3 putative exposed N-linked oligosaccharide sites (Y) marked as shown. The N- and C-termini are denoted by N and C, respectively. The residue length of each ferroportin 1 loop is denoted by the numbers adjacent to each one.
Results and discussion
In this family, affected members had an elevated serum ferritin concentration and normal transferrin saturation without the clinical features of genetic hemochromatosis. Iron was deposited in Kupffer cells (Figure 1) with no evidence of fibrosis. No family members carried HFE Cys282Tyr or His63Asp. In the proband's sister, normal coding sequence and splice sites were demonstrated for bothHFE and β2-microglobulin.7
Analysis of the ferroportin 1 gene showed 4 changes from the genomic contig: (1) homozygosity for 8 rather than 7 CGG trinucleotides8 in the likely promoter region in one affected family member that was not family specific; (2) a G to C transversion within the first intron (IVSI-24; dbSNP entry 399162) that did not correlate with increased serum ferritin and that, in 124 control chromosomes, had a frequency of 0.17; (3) a T to C transversion at the third base of codon 221, homozygous in 3 family members and 3 control subjects and identical to the cDNA sequences AF231121 and AF226614; and (4) a 3–base pair deletion in exon 5; affected but not unaffected family members were heterozygous for this deletion. The deletion (delGTT/TTG/TGT) involves any 3 sequential bases from c779 to 790 (AF231121) and predicts the loss of 1 of 3 Val residues 160-162 conserved across several vertebrate species.2-4 The deletion was not found in 100 subjects without iron overload.
This Val162del mutation appears to lead to loss of function and deficiency in the release of iron from phagocytic cells, which becomes apparent on venesection. Serum ferritin concentrations reflect the increased level of storage iron in reticuloendothelial cells. In contrast, in HFE-related hemochromatosis, in which iron is initially confined to hepatic parenchymal cells, the transferrin saturation is elevated and the serum ferritin may be normal.9
Two other mutations in the human ferroportin 1 gene have been reported. Njajou et al10 described a Dutch pedigree with a missense mutation (Asn144His) in exon 5, resulting in autosomal dominant hemochromatosis with significant iron overload treatable by phlebotomy. Montosi et al11 reported a missense mutation (Ala77Asp) in exon 3 of the ferroportin 1 gene in an autosomal dominant, Italian hemochromatosis pedigree. The transferrin saturation was elevated in 8 of 15 family members with a raised serum ferritin concentration. There was a reduced tolerance of therapeutic venesection in 2 members. Njajou et al10 proposed that the effect of the Asn144His mutation was a gain of function causing enhanced iron absorption, but Montosi et al11 suggested a loss of function and haploinsufficiency resulting from the mutation Ala77Asp.
The structure shown in Figure 2 has 9 transmembrane helices with the expected lengths of 18 to 22 residues generally predicted consistently to within ±1 residue. Helix 4 was an exception and may start as early as Leu183 and finish as late as Trp219. Two methods predicted that helix 9 may start at Gly490 and be followed by a 10th helix, as previously reported.2 3 The distribution of 12 Cys residues in human, mouse, and rat ferroportin 1 within the lipid bilayer and on the 2 surfaces is consistent with the formation of disulfide bridges between pairs of Cys residues within each of these regions. Six prediction methods for the helix topology favored the location of the N-terminus of ferroportin 1 to be within the cell cytoplasm. This is supported by the location of 3 of the 4 putative N-glycosylation sites on the external loops of ferroportin 1, as expected for a membrane protein (the fourth is predicted to be inside transmembrane 2 and is assumed to be unglycosylated).
The mutations are all located at either the end of the transmembrane helices 1 and 3 or at the extracellular loop at the C-terminal end of helix 3. In common with other analyses of clusters of residue mutations,12,13 this suggests that these define a functional binding site for a protein, such as apotransferrin, ceruloplasmin, or hephaestin, that is important for the export of iron from the cell.14
The treatment of patients with this disorder remains unclear. Whether iron removal is absolutely necessary is questionable because the degree of overload was not great and the liver biopsies did not show organ damage.
This report highlights the importance of ferroportin 1 in regulating export of iron across the macrophage membrane. A study of the role of ferroportin 1 in other disorders characterized by elevated ferritin associated with anemia, namely the anemia of chronic disorders, may give further insights into the mechanism of iron release.
We thank the physicians who have investigated and treated the various family members: Dr Steve Flecknoe-Brown (New South Wales), Dr Luke Coyle (Sydney), Prof L. W. Powell (Brisbane), Dr J. Behrens (St Helier Hospital, Surrey), Prof A. V. Hoffbrand (Royal Free Hospital), and Dr D. P. Bentley (Llandough Hospital, Cardiff). We thank Dr Susan Davies for the histopathologic figures, Dr Derrick Bowen for advice, and Mr Julian T. Eaton for assistance with the protein structure predictions. We thank Joyce Hoy and Barrie Francis for their technical help with the ABI Prism 377 sequencer. We thank the family members who presented for investigation of iron overload and encouraged us to define the nature of their clinical condition.
Prepublished online as Blood First Edition Paper, April 17, 2002; DOI 10.1182/blood-2001-11-0132.
K.C. and research in A.P.W.'s laboratory were supported by the European Commission (QLK6-CT-1999-02237). Research in S.J.P.'s laboratory was supported by the Wellcome Trust. A.M. received support from the Leukaemia Research Appeal Wales.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Mark Worwood, Department of Haematology, University of Wales College of Medicine, Heath Park, Cardiff, CF14 4XN, United Kingdom; e-mail: worwood@cardiff.ac.uk.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal