Abstract
The protein C/protein S system is known to regulate thrombin generation in vivo by cleaving factors Va and VIIIa. We have examined the activity of activated protein C in several tissue factor–initiated models of coagulation. We used 4 models: monocytes as the tissue factor source with platelets as the thrombin-generating surface; endothelial cells as the tissue factor source with platelets as the thrombin-generating surface; endothelial cells as both the tissue factor source and the thrombin-generating surface; and relipidated tissue factor with lipid vesicles providing the surface for thrombin generation. With the lipid surface, activated protein C dose-dependently reduced thrombin generation. Similarly, when endothelial cells provided the only surface for thrombin generation, activated protein C dose-dependently decreased thrombin generation significantly. By contrast, whenever platelets were present, activated protein C only minimally affected the amount of thrombin generated. When endothelial cells were the tissue factor source with platelets providing the surface for thrombin generation, activated protein C did increase the time until the burst of thrombin generation but had minimal effects on the total amount of thrombin generated. Activated protein C had essentially no effect on thrombin generation when monocytes were the tissue factor source with platelets providing the surface for thrombin generation. From the studies reported here, we conclude that in vivo, despite the important role of the protein C system in regulating thrombosis, activated protein C does not serve as a primary regulator of platelet-dependent thrombin generation.
Introduction
The protein C/protein S system is known to regulate thrombin generation in vivo.1-5 Thrombin regulation results from inactivation of factors Va and VIIIa by activated protein C (APC) in the presence of its cofactor protein S.6-14 The role of different surfaces in inactivation of factors Va and VIIIa by the APC system has been the subject of several studies.7-9,15-22 Some of these studies have used assay systems incorporating lipid vesicles,7,8,13,19 whereas others have used monocytes,22 endothelial cells,9,18 or platelets15-17,20 21 as the surface for APC activity and the results have been somewhat different. Because the physiologic site of action of APC in inactivating factors Va and VIIIa is extremely important in terms of hemostasis and thrombosis, we have carried out experiments looking at the effect of APC on thrombin generation on a number of different surfaces. We have also compared the rate of inactivation of factor Va on platelet surfaces, endothelial cell surfaces, and lipid surfaces.
Materials and methods
Assays with monocytes as the source of tissue factor and platelets as the surface for thrombin generation
The proteins used in the assay are described in Table1 and the cells in Table2 (line 1). Monocytes were isolated as described below and incubated overnight in serum-free media. Lipopolysaccharide (500 ng/mL) was included in the serum-free media to induce expression of tissue factor.23 Zymogen protein factors were incubated overnight with 30× the plasma concentration of the inhibitors to ensure that no activated factors were present in the zymogens. After incubation, the levels of active proteases were undetectable (< 0.0001%) by chromogenic assays. Factor XI was incubated with C1-esterase inhibitor to remove any traces of the activated factor. Calcium chloride (3 mM) was included in all the reactions. Monocytes were washed 3 times in calcium-free Tyrode buffer before being pretreated with APC and protein S in the presence of calcium (3 mM) for 10 minutes at room temperature. Proteins and platelets were mixed and added to monocytes. The system was initiated by addition of factor VIIa. At timed intervals, samples were removed and thrombin generation measured by diluting the samples in 0.5 mM Chromozyme Th (Roche Diagnostics, Indianapolis, IN) with 50 μM factor Xa inhibitor (TenStop, American Diagnostica, Greenwich, CT), and 1 mM ethylenediaminetetraacetic acid (EDTA). Cleavage of the synthetic substrate proceeded for a timed interval before the reaction was stopped by adding an equal volume of 50% acetic acid. Cleaved substrate was measured at 405 nm and converted to a concentration of thrombin based on a standard curve.
Protein components of the assay systems
| Protein . | Concentration, μg/mL . | Concentration, molar . |
|---|---|---|
| Prothrombin | 100 μg/mL | 1400 nM |
| Factor V | 7 μg/mL | 20 nM |
| Factor VIII* | 0.1 μg/mL | 0.3 nM |
| Factor IX | 4 μg/mL | 70 nM |
| Factor X | 8 μg/mL | 135 nM |
| Factor XI | 5 μg/mL | 25 nM |
| Antithrombin III | 180 μg/mL | 3000 nM |
| Tissue factor pathway inhibitor | 0.1 μg/mL | 3 nM |
| Activated protein C | Varied | Varied |
| Protein S† | 8 μg/mL | 100 nM |
| Factor VIIa | 0.2 nM | |
| Tissue factor | ||
| From monocytes | — | 1 pM |
| From endothelial cells | — | < 1 pM |
| From lipid experiments | — | 100 pM or 10 pM |
| Protein . | Concentration, μg/mL . | Concentration, molar . |
|---|---|---|
| Prothrombin | 100 μg/mL | 1400 nM |
| Factor V | 7 μg/mL | 20 nM |
| Factor VIII* | 0.1 μg/mL | 0.3 nM |
| Factor IX | 4 μg/mL | 70 nM |
| Factor X | 8 μg/mL | 135 nM |
| Factor XI | 5 μg/mL | 25 nM |
| Antithrombin III | 180 μg/mL | 3000 nM |
| Tissue factor pathway inhibitor | 0.1 μg/mL | 3 nM |
| Activated protein C | Varied | Varied |
| Protein S† | 8 μg/mL | 100 nM |
| Factor VIIa | 0.2 nM | |
| Tissue factor | ||
| From monocytes | — | 1 pM |
| From endothelial cells | — | < 1 pM |
| From lipid experiments | — | 100 pM or 10 pM |
Factor VIII is associated with von Willebrand factor.
The protein S concentration approximates the plasma concentration that is free (ie, not bound to C4B binding protein). No C4B binding protein is added to the reactions.
Cells and lipids used in assay system
| . | Initiating surface . | Propagation surface . | Proteins . | Figure . |
|---|---|---|---|---|
| 1 | Monocytes | Platelets | Complete | 1A |
| 2 | Lipids* | Lipids | Complete | 1B-C |
| 3 | Endothelial cells | Platelets | Complete | 1D |
| 4 | Endothelial cells | Endothelial cells | II, X, V, S, APC VIIa | 2 |
| . | Initiating surface . | Propagation surface . | Proteins . | Figure . |
|---|---|---|---|---|
| 1 | Monocytes | Platelets | Complete | 1A |
| 2 | Lipids* | Lipids | Complete | 1B-C |
| 3 | Endothelial cells | Platelets | Complete | 1D |
| 4 | Endothelial cells | Endothelial cells | II, X, V, S, APC VIIa | 2 |
APC indicates activated protein C.
TF inserted into lipids.
Assays with endothelial cells as the source of tissue factor and platelets as the surface for thrombin generation
The cells used in this assay are described in Table 2 (line 3). Endothelial cells were prepared as described below. Washed endothelial cells were pretreated with APC and protein S in the presence of calcium (3 mM) for 10 minutes at room temperature. The proteins are described in Table 1 and were treated as described above. Proteins and platelets were mixed and the mixture added to endothelial cells. The system was initiated by addition of factor VIIa. At timed intervals, samples were removed and thrombin generation measured as described above.
Assays with lipids as both the surface for tissue factor and the surface for thrombin generation
The surface used in this assay is described in Table 2 (line 2). Lipids with a composition of 15:41:44 phosphatidylserine to phosphatidylcholine to phosphatidylethanolamine were prepared as described below. Apo tissue factor was added to lipids and allowed to insert for 30 minutes as previously described.24 The proteins are described in Table 1 and were treated as described above. Proteins were mixed with the lipids and the system was initiated by addition of factor VIIa (0.01 μg/mL, 0.2 nM). At timed intervals, samples were removed and thrombin generation measured as described above.
Assays with endothelial cells as the tissue factor source and the surface for thrombin generation
The cells used in this assay are described in Table 2 (line 4). Endothelial cells were prepared as described below. Washed endothelial cells were pretreated with APC and protein S in the presence of calcium (3 mM) for 10 minutes at room temperature. The proteins used in this assay were prothrombin, factor X, and factor V (Table 1; Table 2, line 4). Proteins were mixed and the mixture added to endothelial cells. APC and protein S were included in the protein mixture to maintain the concentrations of APC and protein S at the pretreatment levels. The system was initiated by addition of factor VIIa. At timed intervals, samples were removed and thrombin generation was measured as described above.
Analysis of factor Va inactivation
Factor Va inactivation was studied in 2 different ways: (1) by measuring the loss of factor Va activity and (2) by measuring cleavage of factor V(a).
Analysis of factor Va activity.
Conditions for inactivation of factor Va by APC were set up to be as identical as possible for lipids, platelets, and endothelial cells. In all cases, residual factor Va activity was measured as the ability to convert prothrombin to thrombin in the presence of factor Xa. Since the 3 surfaces have different abilities to promote thrombin generation, there were small differences in the conditions for assaying factor Va activity as detailed below.
For lipids, 6 nM factor Va with 40 μM lipid was incubated with 100 nM protein S. APC (13 nM) was added and the mixture incubated for timed intervals. To assay factor Va activity, the APC inactivation mixture was diluted 1:100 to give 0.06 nM factor Va. Factor Xa was added to give 4 nM and prothrombin 1400 nM. Thrombin generation was measured using the thrombin synthetic substrate Chromozyme Th.
For endothelial cells, 6 nM factor Va and 100 nM protein S were incubated with confluent human microvascular endothelial cells. As with lipids, 13 nM APC was added and the mixture incubated timed intervals. To assay factor Va activity, an equal volume of a solution of 8 nM factor Xa and 2800 nM prothrombin was added. This gave concentrations of 3 nM factor Va, 4 nM factor Xa, and 1400 nM prothrombin. Thrombin generation was measured using the thrombin synthetic substrate Chromozyme Th.
Platelets were activated with 10 nM thrombin for 20 minutes to ensure release of factor V(a) from the alpha granules and full activation to factor Va. The thrombin was then inactivated by addition of 10 nM hirudin (concentration determined by titration against the thrombin solution). Protein S (100 nM) was incubated with the platelets. APC was added to 13 nM and the mixture incubated for timed intervals. To assay factor Va activity, an equal volume of a solution of factor Xa and prothrombin was added to give final concentrations of 4 nM factor Xa and 1400 nM prothrombin. Thrombin generation was measured using the thrombin synthetic substrate Chromozyme Th.
Analysis of factor V(a) cleavage by Western blotting.
In addition to measuring factor Va activity, the pattern of factor V(a) proteolysis was assessed on platelets and endothelial cells by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting. Factor V(a) bands were detected using a rabbit polyclonal antibody directed against epitopes in the factor Va heavy chain.
For analysis of platelet factor V(a), platelet samples were layered over twice the sample volume of 10% sucrose with 3 mM calcium, then sedimented for one minute in a microcentrifuge. The supernatant was sampled for analysis, and the remaining volume discarded. The platelet pellet was resuspended in one third of the original volume of 1X SDS sample buffer (10 mM Tris-HCl, pH 8.0, 1 mM EDTA, 2.5% SDS, 0.01% bromphenol blue) and boiled for 5 minutes.
For analysis of endothelial cell factor V(a), reactions were performed on confluent endothelial cell monolayers grown in 24-well tissue culture plates. Cultures were preincubated with APC, protein S, and calcium for 10 minutes. At time 0, the remaining protein components were added (factors VIIa, X, V, prothrombin, antithrombin III, and tissue factor pathway inhibitor), along with sufficient APC, protein S, and calcium to maintain them at the indicated concentrations. The supernatants were sampled at timed intervals for thrombin assays. The cell monolayers were gently washed once with a calcium-containing buffer. The human microvascular endothelial cells were solubilized by the addition of 30 μL SDS sample buffer, sheared to fragment endothelial DNA, and boiled.
SDS-PAGE was performed with the PhastSystem (Pharmacia Biotech, Piscataway, NJ) on 4% to 15% gradient gels. Samples were transferred to Immobilon-P polyvinylidenefluoride (PVDF) membrane (Millipore, Bedford, MA) and blocked in 100 mg/mL powdered milk suspension. Blots were immunostained and developed using chemiluminescence detection as previously described.25 Films were scanned on a Microtek ScanMaker 35t (Microtek Lab, Torrance, CA) and analyzed using National Institutes of Health Image software. This approach allowed detection of the conversion of factor V to factor Va over time, and also detection of factor Va cleavage, as reflected by the disappearance of the factor Va heavy chain band.
Cell preparation
Monocytes were isolated from freshly drawn human blood as previously described using Accuprep lymphocyte separation media (Accurate Chemicals, Westbury, NY) and plating.23Monocytes were incubated overnight in serum-free media. Lipopolysaccharide (500 ng/mL) was included in the serum-free media to induce expression of tissue factor.23
Platelets were isolated as previously described using Accuprep lymphocyte separation media and gel filtration.23 Platelet activation was measured after fixing samples with 2% paraformaldehyde.23 Fixed samples were stained with phycoerythrin-conjugated anti–human CD62 antibody (Becton Dickinson, Mountain View, CA) and analyzed on a Becton Dickinson FACScan flow cytometer. Results are expressed as the percent of the platelet population which stained positive for the activation marker. Platelets for the studies described below were used within 4 hours of isolation.
Human microvascular endothelial cells were used as the model endothelial cell for our studies. They were purchased from Clonetics (Wakersville, MD) and maintained according to the manufacturer's instructions. They were used at no greater than passage 7. Tissue culture plastic was coated with 0.2% tissue culture–grade gelatin (Sigma, St Louis, MO) before seeding cells. Endothelial cells were cultured to be at least 80% confluent in the wells. To verify the health of the human microvascular endothelial cell (HMVEC) cultures, monolayers were viewed after fixation and staining with crystal violet or hematoxylin and eosin. The cultured cells were smooth, regular monolayers with a characteristic cobblestone appearance and no evidence of cell death. To verify that factor V binding was a feature of the entire population of HMVECs and not limited to a small number of possibly damaged or apoptotic cells, the monolayers were also evaluated by immunohistochemistry. Prior to use in assays, cultures were washed 3 times in 20 mM HEPES, 150 mM NaCl, pH 7.4, with 1 mg/mL bovine serum albumin (BSA) and 3 mM calcium. After washing away serum-containing medium, the cultures were washed briefly in warm 10 mM EDTA, 20 mM HEPES in Hanks balanced salt solution (HBSS) to remove residual calcium-dependent coagulation factors. The cells were then allowed to recover in the calcium-containing buffer.
Phospholipid vesicles
Large unilamellar phospholipid vesicles containing molar ratios of 15:41:44 phosphatidylserine to phosphatidylcholine to phosphatidylethanolamine were prepared by extrusion using a previously described procedure and stored at 4°C under argon.26 27Basically, lipids were dried from chloroform solution under nitrogen flow. The lipids were taken up in cyclohexane, shell-frozen in a dry ice/ethanol bath, and lyophilized. The dried lipids were taken up in buffer, sonicated for 5 minutes in an ice-cold sonicating bath, then extruded 10 times through an 0.2-μL polycarbonate filter.
Materials
Proteins were generally frozen at −80°C in small aliquots and were subjected to no more than 2 freeze/thaw cycles. Prothrombin was purified using barium citrate, diethylaminoethyl (DEAE)–cellulose, and a copper chelate column.28 Thrombin was prepared from prothrombin as described previously.29Factor IX was purified as described previously using ion exchange chromatography and heparin sepharose chromatography.30Factor X was purchased from Enzyme Research Labs (South Bend, IN). All zymogen coagulation factors were treated with an inhibitor mixture (1 μM each of tosyl-lysyl chloromethyl ketone, tosyl-phenylalanine chloromethyl ketone, phenylmethyl sulfonyl fluoride, Phe-Pro-Arg chloromethyl ketone, and dansyl Glu-Gly-Arg chloromethyl ketone) for 1 hour, then repurified on Q sepharose fast flow using calcium chloride elution. Factor XI, APC, and protein S were purchased from Haematologic Technologies (Essex Junction, VT). Factor V was purchased from Calbiochem (San Diego, CA). Factor VIII (with von Willebrand factor) was repurified from profilate by gel filtration on Sepharose CL-2B and the concentration determined by clotting assays compared with a standard curve. Antithrombin III was prepared as described previously using polyethyleneglycol (PEG) precipitation of plasma followed by heparin Sepharose chromatography,31 and ion exchange chromatography was used to remove traces of heparin. Antithrombin III activity was determined by thrombin inhibition assay and was 2.5 × 105 M-1min-1. Factor VIIa and full-length tissue factor pathway inhibitor were the generous gift of Dr Ulla Hedner (University of Lund, Sweden). Anti–factor V antibody was the generous gift of Dr William Kane, Duke University, NC. Antibody to the endothelial protein C receptor (EPCR) was the kind gift of Dr Charles Esmon and Dr Gary Ferrell, Oklahoma Medical Research Foundation. All other reagents were of the highest grade available.
Results
Effect of APC on a cell-based model of coagulation with monocytes as the source of tissue factor and platelets as the surface for thrombin generation
The cell-based model of coagulation contained plasma concentrations of zymogen procoagulant factors, unactivated coagulation cofactors, and coagulation inhibitors as described in “Materials and methods” and Table 1. Monocytes treated with lipopolysaccharide provided the tissue factor and unactivated platelets were added at 100 000/μL.
As shown in Figure 1A, when this system was initiated by addition of factor VIIa (0.2 nM), there was a lag phase followed by a burst of thrombin generation. Subsequently, the thrombin generation level decreased due to multiple regulatory mechanisms that included antithrombin III inhibition of formed thrombin. The total amount of thrombin produced in the system was determined by integrating the plot of thrombin as a function of time (data not shown). Very surprisingly, incubation of the monocytes with varied concentrations of APC up to 65 nM, even in the presence of protein S, had no effect on thrombin generation in this model system. These results were different than those that had been observed in studies using phospholipid vesicles to provide the surface for the reactions.19 So we substituted lipids for the cells in our model system assays.
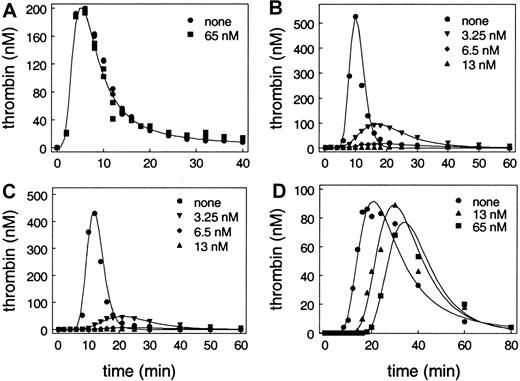
Effect of APC on thrombin generation in models of coagulation.
Each of the reactions contained plasma concentrations of zymogen procoagulant factors, unactivated coagulation cofactors, and coagulation inhibitors, including protein S as described in “Materials and methods” and shown in Table 1. APC was added to each reaction at none, 3.25 nM, 6.5 nM, 13 nM, and 65 nM, respectively. Note that the x-axis (thrombin generation) and y-axis (time) scales differ somewhat between experiments. The results in panel A and panel D are representative of experiments using platelets from at least 4 different individuals. (A) Tissue factor was derived from cultured monocytes treated with lipopolysaccharide. Unactivated platelets were added at 100 000/μL. Data for 3.25 nM, 6 nM, and 13 nM overlie the data for 0 and 65 nM APC and are not shown. (B) Tissue factor (100 pM) was incorporated into phospholipid vesicles (PC/PE/PS, 41:44:15). Results at 65 nM are identical to results at 13 nM and are not shown. (C) Tissue factor (10 pM) was incorporated into phospholipid vesicles (PC/PE/PS, 41:44:15). Results at 65 nM are not shown and are identical to results at 13 nM. (D) Tissue factor was derived from cultured HMVECs. Unactivated platelets were added at 100 000/μL. Results at 3.25 nM and 6.5 nM are intermediate between no APC and 13 nM APC and are not shown.
Effect of APC on thrombin generation in models of coagulation.
Each of the reactions contained plasma concentrations of zymogen procoagulant factors, unactivated coagulation cofactors, and coagulation inhibitors, including protein S as described in “Materials and methods” and shown in Table 1. APC was added to each reaction at none, 3.25 nM, 6.5 nM, 13 nM, and 65 nM, respectively. Note that the x-axis (thrombin generation) and y-axis (time) scales differ somewhat between experiments. The results in panel A and panel D are representative of experiments using platelets from at least 4 different individuals. (A) Tissue factor was derived from cultured monocytes treated with lipopolysaccharide. Unactivated platelets were added at 100 000/μL. Data for 3.25 nM, 6 nM, and 13 nM overlie the data for 0 and 65 nM APC and are not shown. (B) Tissue factor (100 pM) was incorporated into phospholipid vesicles (PC/PE/PS, 41:44:15). Results at 65 nM are identical to results at 13 nM and are not shown. (C) Tissue factor (10 pM) was incorporated into phospholipid vesicles (PC/PE/PS, 41:44:15). Results at 65 nM are not shown and are identical to results at 13 nM. (D) Tissue factor was derived from cultured HMVECs. Unactivated platelets were added at 100 000/μL. Results at 3.25 nM and 6.5 nM are intermediate between no APC and 13 nM APC and are not shown.
Effect of APC on a model of coagulation where apo tissue factor is incorporated into lipids and lipids provide the surface for thrombin generation
The surface was provided by 400 μM of 41% phosphatidylcholine, 44% phosphatidylethanolamine, and 15% phosphatidylserine vesicles. Tissue factor, rather than being supplied by monocytes, came from apo tissue factor that was incorporated into the lipid vesicles at 10 pM and 100 pM. As shown in Figure 1B-C, when this system was initiated by addition of factor VIIa (0.2 nM), as with the cell-based model, there was a lag phase followed by a burst of thrombin generation with subsequent inhibition of the formed thrombin. As previously described by van't Veer et al,32 the peak of thrombin generation was higher when 100 pM tissue factor was present than when 10 pM was present. In contrast to the cell-based system described above, addition of APC decreased the total amount of thrombin generated in the lipid-based system (Figure 1B-C). This decrease occurred in a dose-dependent fashion and at 13 nM APC there was no detectable thrombin generation. Furthermore, the inhibition of thrombin generation occurred regardless of the concentration of tissue factor used to initiate the system (Figure 1B-C).
Effect of APC on a cell-based model with endothelial cells as the source of tissue factor and platelets as the surface for thrombin generation
Endothelial cells are hypothesized to be the primary site of protein C activation in vivo.33-35 Therefore, we examined the effects of APC when the model system was initiated with tissue factor supplied by cultured endothelial cells and platelets were the surface for thrombin generation. The proteins used in the endothelial cell–initiated system were identical to the proteins used in the monocyte-initiated model system and the lipid-based system. Tissue factor was not added to the system but was instead supplied by cultured HMVECs. There was too little tissue factor activity to reliably assay factor X activation directly, which means that the concentration of tissue factor was less than 0.1 pM based on a standard curve made with relipidated tissue factor. However, this small amount of tissue factor was important, since initiation of coagulation in this model was dramatically prolonged by the addition of an inhibitory anti–tissue factor antibody. As with the monocyte-initiated model system, unactivated platelets were present at 100 000/μL.
As shown in Figure 1D, the pattern of thrombin generation was similar to that observed with the previous 2 assay systems. Despite the similarities of the systems in the absence of APC, the effect of APC on thrombin generation was markedly different between the lipid-based system (Figure 1B-C) and model systems using monocytes or endothelial cells, both of which use platelets as the surface for thrombin generation (Figure 1A,D). Both cell-based model systems that contain platelets are resistant to the effects of APC relative to the lipid-based system. In the cell-based models, APC has only a minor effect on total thrombin generation. Despite the fact that 13 nM APC completely abolished thrombin generation in the lipid-based system, it had almost no effect on total thrombin generation in the cell-based model. Even 5 times as much APC (equivalent to the plasma concentration of protein C) had only a modest effect on total thrombin generation.
The monocyte-initiated model is, however, different than the endothelial cell–initiated model (compare panels A and D in Figure 1). The lag phase in the monocyte-initiated system is consistently shorter than the lag phase in the endothelial cell–initiated system. This is probably a consequence of both higher tissue factor activity on monocytes than on endothelial cells, and the presence on monocytes of mechanisms for activating factor V36 and the significant ability of monocytes to support thrombin generation.37 As shown in Figure 1D, APC increases the lag phase observed before the burst of thrombin generation in the endothelial cell–initiated model (compare panels A and D in Figure 1). The increase in the lag phase is dose dependent.
Effect of APC on thrombin generation in a cell-based model in which endothelial cells provide both the source of tissue factor and the surface for thrombin generation
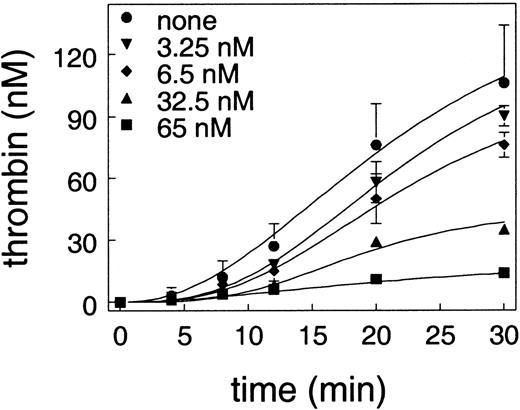
In the cell-based model system, a small amount of thrombin generation on the tissue factor–bearing cell is a trigger for platelet activation, which is a prerequisite for the subsequent burst of thrombin generation.37 The effect of APC on thrombin generation on endothelial cells in the absence of platelets was examined using cells that were preincubated with APC and protein S. To initiate thrombin generation in this system, it was necessary that prothrombin, factor X, factor V, and factor VIIa be added without any coagulation inhibitors. When coagulation inhibitors were present, no measurable thrombin could be detected. Without inhibitors, thrombin generation could be observed on cultured endothelial cells as shown in Figure 2. This thrombin generation was a property of the total cell population and not attributable to a few dead cells, since the cultured endothelial cells stained uniformly for factor V antigen (data not shown). As can be seen in Figure 2, APC inhibited thrombin generation on endothelial cells in a dose-dependent fashion and completely abolished thrombin generation at 65 nM APC.
Effect of APC on thrombin generation on endothelial cells.
Endothelial cells were preincubated with the indicated concentration of APC and 150 nM protein S. To initiate thrombin generation, plasma concentrations of prothrombin, factor X, and factor V, with factor VIIa but without any coagulation inhibitors, were added to the cells. The plot shows the amount of thrombin as a function of time.
Effect of APC on thrombin generation on endothelial cells.
Endothelial cells were preincubated with the indicated concentration of APC and 150 nM protein S. To initiate thrombin generation, plasma concentrations of prothrombin, factor X, and factor V, with factor VIIa but without any coagulation inhibitors, were added to the cells. The plot shows the amount of thrombin as a function of time.
Effect of lipids, platelets, and endothelial cells on APC cleavage of factor Va as measured by activity assays
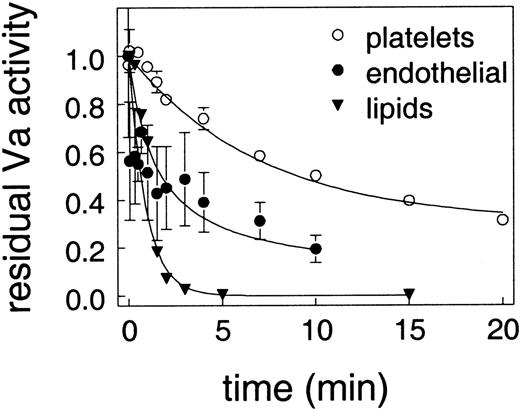
APC acts by cleaving and inactivating factors VIIIa and Va, so we examined the effect of surface on APC cleavage of factor Va. In these experiments, factor Va inactivation was set up so that the conditions would be identical regardless of the surface used. Factor Va activity was normalized for each surface to account for small differences in assay conditions as detailed in “Materials and methods”; therefore, the data shown in Figure 3 are expressed as residual factor Va activity. As shown in Figure 3, factor Va inactivation by APC was most rapid on lipids, somewhat slower on endothelial cells, and dramatically slower on platelets. These differences are highly significant and consistent with the observations of Camire et al15 20 that factor Va on platelets is relatively protected from inactivation by APC.
Effect of surface on the loss of factor Va activity.
Protein S (100 nM) was incubated with 6 nM factor Va and the indicated surface before the addition of 13 nM APC. At timed intervals, samples were removed and assayed for residual factor Va activity. The amount of prothrombinase activity without APC was defined as one for each surface. The surfaces were lipid vesicles (▴), endothelial cells (○), and platelets (●).
Effect of surface on the loss of factor Va activity.
Protein S (100 nM) was incubated with 6 nM factor Va and the indicated surface before the addition of 13 nM APC. At timed intervals, samples were removed and assayed for residual factor Va activity. The amount of prothrombinase activity without APC was defined as one for each surface. The surfaces were lipid vesicles (▴), endothelial cells (○), and platelets (●).
To determine whether the EPCR might regulate cleavage of factor Va by APC, we repeated the studies on endothelial cells in the presence of saturating concentrations of an inhibitory antibody to EPCR. We found that the antibody did not decrease the rate of factor Va inactivation by APC.
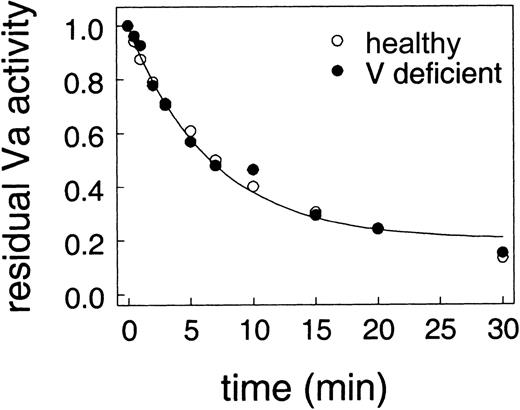
We next addressed the question of whether the relative protection of factor Va on platelets was due to some component of the platelet surface or was due to platelet-released factor Va being different from plasma-derived factor Va. We examined inactivation of factor Va on platelets from a healthy donor (which released internal stores of factor Va) compared with inactivation of plasma factor Va added to factor V–deficient platelets from a factor V–deficient donor. Inactivation of factor Va was assessed as described for Figure 3 except that plasma levels of factor V were included when platelets were incubated with thrombin to activate the platelets and factor V. If plasma factor V was not included with platelets from the factor V–deficient donor, prothrombinase activity was less than 1% of the value in the presence of added factor V. As shown in Figure4, there is remarkable agreement between the rates of factor Va inactivation on platelets from a healthy donor and platelets from a factor V–deficient donor. Thus, platelet-derived and plasma-derived factor Va are inactivated identically by APC.
Comparison of APC inactivation of factor Va on normal platelets and platelets from a factor V–deficient donor.
The assays are the same as Figure 3. Thrombin-activated platelets from either a healthy donor (○) or a factor V–deficient donor with 6 nM factor Va (●) were incubated with 100 nM protein S before addition of 13 nM APC.
Comparison of APC inactivation of factor Va on normal platelets and platelets from a factor V–deficient donor.
The assays are the same as Figure 3. Thrombin-activated platelets from either a healthy donor (○) or a factor V–deficient donor with 6 nM factor Va (●) were incubated with 100 nM protein S before addition of 13 nM APC.
Effect of platelets and endothelial cells on APC cleavage of factor Va measured by Western blotting
In addition to the activity measurements, factor Va antigen can be extracted from platelets or endothelial cells and detected by Western blotting after gel electorphoresis. The relative amount of factor Va can be determined by quantitating the density of the band corresponding to the factor Va heavy chain. When platelet factor V from the cell-based model system with endothelial cells and platelets described above was analyzed by Western blotting, APC had little to no effect on factor Va cleavage (data not shown). This result is consistent with the relatively slow inactivation of factor Va observed above (Figure 3) and with the relative lack of effect of APC on the cell-based model systems that contained platelets (Figure 1A,D). By contrast, APC had a big effect on factor Va cleavage on endothelial cells. In conditions similar to those in the experiments above looking at thrombin generation on endothelial cells (Figure 2), when endothelial cells were incubated with synthetic plasma protein mixture (prothrombin, factor X, factor V, protein S, antithrombin III, and tissue factor pathway inhibitor [TFPI]), along with 20 nM activated factor V, factor Va accumulated on the endothelial cells (Figure5). When 13 nM APC was included in the reaction mixture, little to no factor Va heavy chain was detected on the endothelial cell surface (Figure 5).
APC cleavage of factor Va on endothelium.
Endothelial cells were incubated with prothrombin, factor X, protein S, antithrombin III, and TFPI, along with 20 nM activated factor V. Accumulation of factor Va on the endothelial cells was detected by western blotting. The plot shows the density of the heavy chain band (measured as described in “Materials and methods”). Results are shown for the absence (●) or presence (▴) of 13 nM APC.
APC cleavage of factor Va on endothelium.
Endothelial cells were incubated with prothrombin, factor X, protein S, antithrombin III, and TFPI, along with 20 nM activated factor V. Accumulation of factor Va on the endothelial cells was detected by western blotting. The plot shows the density of the heavy chain band (measured as described in “Materials and methods”). Results are shown for the absence (●) or presence (▴) of 13 nM APC.
Effect of endothelial cells on APC blocking activation of factor V as measured by Western blotting
In the experiments above looking at thrombin generation on the endothelial cells (Figure 2), unactivated factor V was used in the experiments. Since thrombin generation was observed, there must have been at least some conversion of this factor V to factor Va. In the absence of APC, when unactivated factor V is substituted for factor Va in the experiment shown in Figure 5, factor V, detected as full-length antigen, binds to the endothelial cells and is converted to factor Va (Figure 6A). In the presence of 13 nM APC, factor V also binds to endothelial cells but is not converted to factor Va. These results are consistent with the results seen with the endothelial cell–initiated model system where APC increased the lag phase before the burst of thrombin generation by decreasing the initial thrombin generation that leads to platelet activation (Figure 1D). In this experiment, blocking thrombin generation prevents the feedback activation of factor V to factor Va (Figure 6B). We do not see significant APC cleavage of zymogen factor V on endothelial cells in this time frame.
APC blocks conversion of factor V to factor Va on the endothelial cell surface.
Conditions are similar to those for Figure 5. Endothelial cells were incubated with prothrombin, factor X, factor V, protein S, antithrombin III, and TFPI. Unactivated factor V at plasma concentrations was added rather than activated factor V. Accumulation and activation of factor V on the endothelial cells was detected by Western blotting. The plot shows the density of the unactivated factor V (●) and the heavy chain band (○). (A) Results in the absence of APC. (B) Results when 13 nM APC was included in the reaction mixture.
APC blocks conversion of factor V to factor Va on the endothelial cell surface.
Conditions are similar to those for Figure 5. Endothelial cells were incubated with prothrombin, factor X, factor V, protein S, antithrombin III, and TFPI. Unactivated factor V at plasma concentrations was added rather than activated factor V. Accumulation and activation of factor V on the endothelial cells was detected by Western blotting. The plot shows the density of the unactivated factor V (●) and the heavy chain band (○). (A) Results in the absence of APC. (B) Results when 13 nM APC was included in the reaction mixture.
Discussion
In order to understand the mechanisms behind both thrombosis and normal hemostasis, we have to understand the physiologic site of activity of the APC system. Previous studies have examined the ability of APC to cleave factors Va and VIIIa on lipid surfaces,7,8,13,15,19,21 on endothelial cell surfaces,9,18,38 and on platelet surfaces.21Some studies showed that factor Va on the platelet surface was relatively resistant to cleavage by APC.15,16 20 The previous work has generally focused on factor Va inactivation as the endpoint of the study.
We wanted to examine the activity of the APC system in a model in which thrombin generation was the primary endpoint. This approach allowed us to determine the overall effect of factor Va inactivation by APC in terms of thrombin generation. One previous study by van't Veer et al looked at thrombin generation as an endpoint using plasma coagulation proteins at their physiologic concentrations with the surface provided by lipids.19 They showed that addition of 1 nM soluble thrombomodulin decreased thrombin generation, presumably by activating protein C with the resulting APC inactivating factors Va and VIIIa.19 In the study reported here, we also used a lipid-based model system similar to that described by van't Veer et al and found that, in agreement with their results, addition of APC resulted in a dose-dependent decrease in thrombin generation such that at 13 nM APC (equivalent to activation of 20% of plasma levels of protein C) no thrombin generation above background was observed. This result was consistent at both 10 pM and 100 pM tissue factor.
We have previously shown that coagulation reactions can be strikingly different in a cell-based model of coagulation (using cells as a source of tissue factor and platelets as a surface for thrombin generation) when compared with results obtained in models using a lipid surface.25 39 Similarly, we have now found that APC has a qualitatively different effect in the presence of cell surfaces than when phospholipid vesicles provide the surface to support the coagulation reactions. When the cell-based model system was initiated with tissue factor–bearing monocytes, APC had no effect on thrombin generation. When the cell-based model was initiated with endothelial cells as the source of tissue factor, APC caused a dose-dependent prolongation of the lag phase before the burst of thrombin generation but had relatively little effect on the total thrombin produced. The increased lag time seen in the endothelial cell–initiated model is a consequence of decreased endothelial surface thrombin generation during the initiation phase leading to delayed activation of platelets. Thus, levels of APC that completely abolished thrombin generation in a lipid-based model had a minimal effect on the total amount of thrombin generated when platelets provided the surface for thrombin generation.
When we directly compared factor Va inactivation on lipids, platelets, and endothelial cells, we observed that factor Va inactivation was very rapid on phospholipid vesicles and endothelial cells but significantly slower and less complete on platelets (Figure 3). These results are consistent with the observations of Hockin et al18 and Colucci et al22 who showed that APC inactivation of factor Va on endothelial cells was similar to inactivation on lipids. These results differ from Briede et al,21 but are consistent with the observations of Camire et al15,20 who showed that on platelets, APC does not completely inactivate factor Va. However, while Camire et al concluded that platelet factor Va and plasma-derived factor Va differ in their resistance to cleavage by APC,15,20 our studies using factor V–deficient platelets showed that platelet-derived and plasma-derived factor Va are inactivated identically (Figure 4). Thus, our data suggest that the platelet surface provides some mechanism that protects factor Va from inactivation by APC. This result is in agreement with Luddington et al.17
Our studies show a relatively rapid inactivation of factor Va by APC when measured in isolation (Figure 3), yet we see relatively high levels of factor Va–dependent thrombin generation when the other coagulation factors are present in the various cell-based models (Figure 1A,D and Figure 2). The likely explanation for these results is that components of the prothrombinase complex protect factor Va from inactivation. This conclusion is supported by a number of studies showing that factor Xa and protein S compete for a binding site on factor Va40 and that the presence of factor Xa can reduce APC inactivation of factor V.41 However, the protective effect of the other coagulation factors cannot explain the difference in factor Va inactivation on the different cell and lipid surfaces. Thus, these differences must be due to attributes of the different cell types studied.
Possible mechanisms for the poor inactivation of factor Va on the platelet surface include the release of protein C inhibitor by platelets, poor binding of APC/protein S to platelet surfaces, and protection of factor Va by binding to platelet surface receptors. At this point we do not have any data to allow us to distinguish among these possibilities.
Based on our finding that APC is much more efficient in inactivating factor Va on endothelial cells than on platelets, we conclude that protein C plays its vital role in preventing thrombosis by down-regulating thrombin generation on endothelial cells. As previously described by Stern et al, factor Xa produced on endothelial cells can generate small amounts of thrombin42 and initiate a positive feedback loop in which endothelial surface factor V is activated to factor Va. In our view, thrombin generation on intact endothelium is normally subject to a down-regulatory process in which any small amount of thrombin formed on or near healthy endothelium binds to thrombomodulin and activates protein C. The presence of APC alters the balance of the reactions so that the factor Xa produced on these cells cannot generate enough thrombin to initiate a positive feedback loop. Thus, factor V binds to the endothelial cells but is not converted to factor Va. Our view is consistent with studies showing that factor V antigen is bound to the endothelial surface of normal blood vessels,43,44 but it is not present in the activated form45 and thrombin generation sufficient to promote fibrin formation is not generally observed on healthy endothelium.46 Activated factor V is found on damaged endothelium and may reflect disruption of the normal action of the protein C system on these cells.47 A testable hypothesis suggested by our work is that damaged or dysfunctional endothelium, in addition to expressing less thrombomodulin, may also have an impaired ability to support the inactivation of factor Va by APC. Protein C deficiency or dysfunction, whether at the level of protein or at the level of the cell, would potentially allow thrombin generation within the intact vasculature47 and, thereby, enhance the risk of thrombosis.
We further conclude that in vivo protein C does not serve as a primary regulator of platelet-dependent thrombin generation. In considering the anatomy of a wound site, a break in the endothelium is initially plugged by sticky platelets with intermingled plasma proteins. Since protein C can only be activated with any efficiency by thrombin in complex with thrombomodulin, APC is likely to be generated on endothelium at the margins of the platelet plug. Nemerson demonstrated that diffusion of any protein through a platelet plug is much slower than the time required for thrombin generation and formation of a fibrin mesh.48 Therefore, significant amounts of APC are not likely to diffuse into the interstices of the platelet plug. In addition, APC that does reach the platelet surface will not be effective in decreasing platelet-surface thrombin generation.
Our view that the protein C system does not serve as a primary regulator of normal thrombin generation in a platelet plug does not preclude a role for pharmacologic effect of infused preactivated protein C in treating thrombotic complications, including preventing platelet-dependent thrombus formation. If APC were present before the initial platelet plug formed, it could be incorporated into the growing clot. The resulting decrease in thrombin generation might well be sufficient to have a beneficial effect, as was observed in a platelet-dependent ex vivo model of thrombus formation.49APC would clearly be beneficial in cases in which there is a defect in generation of APC either at the protein level or because the local cells are not rich in thrombomodulin. In addition, since the results seen in Figure 1B-D and Figure 2 all showed a dose dependence for decreased thrombin generation, additional APC should always decrease local thrombin generation in vivo regardless of the surface presented.
Finally, the results of our current studies add to our previous observations showing that studying coagulation reactions on cells may give qualitatively different results than similar studies on lipid surfaces. The nature of the cell surface can significantly alter the ability of APC to inactivate factor Va. It is clear that the lipid composition of vesicles can alter the activity of APC toward factor Va.8,50 51 However, it is not clear whether differences in membrane lipid composition are sufficient to account for the differences seen on the various cell types. Studies to understand the mechanisms behind the differential activity of APC on different cells may aid in our understanding of thrombosis and in design of antithrombotic agents.
Supported by National Institutes of Health grants HL48320 and HL06350 and a grant-in-aid from American Heart Association.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Dougald M. Monroe, Department of Hematology/Oncology, 932 Mary Ellen Jones Bldg, Chapel Hill, NC 27599-7035; e-mail: dmonroe@med.unc.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal