Abstract
Magnetic resonance imaging (MRI) can be used to distinguish bone marrow (BM) from cartilage and may therefore be used to measure BM volume in intact bones. We used MRI to measure the total human fetal BM volume in intact fetuses during the second trimester of pregnancy and determined the contribution of the individual bones to the total compartment. The total BM volume ranged from 934 μL at 17 to 18 weeks to 4563 μL at 22 to 23 weeks of gestation. The largest contributor to the total BM volume was the spine, constituting 26.4% ± 2.7% of the total volume. By analyzing leukocyte content and percentages of CD34+ cells, lymphocytes, granulocytes, and monocytes of determined volumes, absolute numbers of these cell populations in BM could be measured. The cellular composition of the BM compartment did not significantly change throughout the second trimester of gestation. Absolute white blood cell counts per fetus increased from 111 × 106 at 16 to 17 weeks to 1229 × 106 at 21 to 22 weeks. The absolute numbers of CD34+ cells increased from 25 × 106 at 16 to 17 weeks to 256 × 106 at 21 to 22 weeks. Similar analysis of liver and spleen revealed comparable absolute numbers of CD34+ cells in BM and liver throughout the second trimester of gestation. In fetal liver, CD34+ cells differentiate into red cells, myeloid cells, and platelets, while lymphopoiesis mainly occurs in BM or spleen. Combining MRI and cell counts provides a method to quantify specific cell populations in fetal compartments. This study may enable better evaluation of fetal diagnostics and therapies.
Introduction
After birth, the major site of hematopoiesis is the bone marrow (BM) compartment. The human BM compartment consists of cavities in the bones of the extremities, skull, spine, scapulae, claviculae, costae, and pelvis. During ontogeny, fetal hematopoiesis develops at different sites depending on gestational age. The first intraembryonic hematopoietic site is found in the ventral wall of the dorsal aorta.1 At week 6 of gestation hematopoiesis is found in the fetal liver and at week 7 of gestation in the fetal spleen as well.2-4 Also in the first trimester of pregnancy are multipotent progenitor cells found in the fetal circulation.5 Around 14 weeks of gestation, the fetal BM acquires its hematopoietic function.6 Initially, lymphopoiesis is the main cell lineage developing in the BM. Thereafter, the BM compartment is developing rapidly, and an exponential expansion of hematopoiesis in the BM occurs.7
Recent developments with respect to in utero gene therapy and stem cell transplantation for congenital diseases have generated a need for more information regarding the fetal BM and hematopoietic stem cell compartment. Because pretransplantation conditioning with radiation or chemotherapy cannot be performed for in utero transplantation, the hematopoietic stem cells (HSCs) from the transplantation have to compete for engraftment with the endogenous HSCs present in the various hematopoietic organs of the fetus. Therefore, determination of the absolute number of HSCs in the various organs of a fetus is essential. Quantitative and qualitative analyses of the cellular composition of liver8,9 and spleen10 have been performed by measuring weight and cell counts in livers and spleens from aborted fetuses.11-13 In contrast, only qualitative measurements of the BM compartment have been performed.14 15Quantitatively, the BM compartment could not be analyzed because methods have not been available to date to measure the total BM volume of a fetus.
To analyze the total fetal BM volume, in this study we performed a quantitative analysis of the fetal BM compartment using magnetic resonance imaging (MRI). By combining BM volume measured by MRI with cellular analysis and measurement of the other hematopoietic tissues, we defined the hematopoietic compartments in BM, liver, and spleen during the second trimester of gestation.
Patients, materials, and methods
Patient population
To analyze the volume of the BM compartment in fetuses throughout gestation, MRI was performed in 11 newborns. These newborns, further to be called fetuses, were born between 18 to 22 weeks of gestation after immature induction of labor with a prostaglandin E2 analog (sulprostone) due to congenital abnormalities or spontaneous immature labor without signs of life at birth. Gestational age was determined by dates and by ultrasound examination. All fetuses were alive at the start of labor or induction. After onset of labor or induction, the fetuses were born within 52 hours (median 29 ± 15, range 1-52). Six female and 5 male fetuses were included in the study. There were 4 cases of immature labor without fetal abnormalities. Induction was performed in 4 cases of trisomy 21, 1 case of spina bifida, 1 case of Turner syndrome, and 1 with Potter syndrome (Table 1). Imaging was performed at a time suitable for the parents in order to avoid interference with the mourning process. No additional tests (ie, pathological examination, collection of cells) were performed in these fetuses. The Medical Ethics Review Board of the Leiden University Medical Center approved the investigation protocol, and informed consent was obtained from all parents. Additional data on fetal cells were obtained from tissues of elective abortions performed at the Center for Human Reproduction in Leiden after informed consent and not from the fetuses analyzed by MRI, for ethical reasons (avoiding interference with the mourning process).
Characteristics of fetuses included for MRI
| Fetus no. . | GA, w + d . | Weight, g . | Length, cm . | Sex . | Anomaly . | Total BM volume, μL . | Liver volume, μL . | Spleen volume, μL . | Thymus volume, μL . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 17 + 4 | 175 | 18 | Male | Potter | 982 | 6 868 | 120 | 61 |
| 2 | 18 + 2 | 140 | 19 | Female | Turner, hygroma colli | 934 | 5 819 | 47 | 34 |
| 3 | 18 + 3 | 220 | 23.3 | Male | Trisomy 21 | 1 270 | 11 836 | 109 | 247 |
| 4 | 18 + 6 | 190 | 22.8 | Female | Spina bifida | 1 255 | 11 030 | 213 | 117 |
| 5 | 19 + 6 | 265 | 23.4 | Female | None | 2 390 | 19 502 | 269 | 82 |
| 6 | 20 + 0 | 270 | 23.3 | Male | None | 2 232 | 15 583 | 135 | 250 |
| 7 | 20 + 3 | 300 | 24.5 | Female | Trisomy 21 | 2 470 | 17 888 | 107 | 182 |
| 8 | 21 + 1 | 190 | 28.4 | Female | Trisomy 21 | 2 817 | 19 011 | 207 | 603 |
| 9 | 21 + 4 | 430 | 28.2 | Female | Trisomy 21 | 3 014 | 23 219 | 282 | 371 |
| 10 | 22 + 1 | 420 | 29 | Male | None | 3 301 | 19 258 | 890 | 127 |
| 11 | 22 + 3 | 500 | 32 | Male | None | 4 563 | 21 672 | 342 | 392 |
| Correlation | — | — | — | 0.95 | 0.91 | 0.65 | 0.59 |
| Fetus no. . | GA, w + d . | Weight, g . | Length, cm . | Sex . | Anomaly . | Total BM volume, μL . | Liver volume, μL . | Spleen volume, μL . | Thymus volume, μL . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 17 + 4 | 175 | 18 | Male | Potter | 982 | 6 868 | 120 | 61 |
| 2 | 18 + 2 | 140 | 19 | Female | Turner, hygroma colli | 934 | 5 819 | 47 | 34 |
| 3 | 18 + 3 | 220 | 23.3 | Male | Trisomy 21 | 1 270 | 11 836 | 109 | 247 |
| 4 | 18 + 6 | 190 | 22.8 | Female | Spina bifida | 1 255 | 11 030 | 213 | 117 |
| 5 | 19 + 6 | 265 | 23.4 | Female | None | 2 390 | 19 502 | 269 | 82 |
| 6 | 20 + 0 | 270 | 23.3 | Male | None | 2 232 | 15 583 | 135 | 250 |
| 7 | 20 + 3 | 300 | 24.5 | Female | Trisomy 21 | 2 470 | 17 888 | 107 | 182 |
| 8 | 21 + 1 | 190 | 28.4 | Female | Trisomy 21 | 2 817 | 19 011 | 207 | 603 |
| 9 | 21 + 4 | 430 | 28.2 | Female | Trisomy 21 | 3 014 | 23 219 | 282 | 371 |
| 10 | 22 + 1 | 420 | 29 | Male | None | 3 301 | 19 258 | 890 | 127 |
| 11 | 22 + 3 | 500 | 32 | Male | None | 4 563 | 21 672 | 342 | 392 |
| Correlation | — | — | — | 0.95 | 0.91 | 0.65 | 0.59 |
GA indicates gestational age.
Identification of the BM compartment
The use of MRI to determine fetal anatomy has been described previously.16 17 To identify areas of BM in the skeleton, total body radiography of fetuses of different gestational ages was performed. Subsequently, sagittal MRIs were obtained of all bones to visualize the sites corresponding with the ossification centers detected by total body radiography. Differences in signal intensities between cartilage (white, higher intensity) and BM (gray, lower intensity) provide the possibility to accurately locate these structures in the fetus. To verify bone areas containing hematopoiesis, microscopic and macroscopic sections of various bones acquired after consented elective abortion were cut by the pathology department. Sections were made at specific points marked during MRI so that sections could be matched with the images. After fixation in formalin and short decalcification, sections of 4 μm were cut. Hematoxylin and eosin staining was performed according to conventional pathology standards. Furthermore, CD34 immunostaining of bone sections was performed. For CD34 staining, we used 4-μm sections cut from all tissue blocks. The sections were mounted on APES-coated slides (amino-propyl-ethoxy-silan) (SIGMA, Zwijndrecht, The Netherlands), deparaffined, rehydrated to 96% alcohol, and air dried. The slides were automatically incubated in the Lab Vision Autostainer, with CD34, clone QueBEnd/10 (1:800) (NeoMarkers, Fremont, CA). Two-step immunostaining was performed according to the manufacturer's procedure of the UltraVision detection kit, containing antigoat biotin and streptavidin peroxidase. Sections were counterstained with hematoxylin and mounted with medium. As a negative control, NHS (AB serum pooled) was used instead of CD34.
Image acquisition
MR studies were performed on an NT15 Gyroscan 1.5-T system (Philips Medical Systems, Best, The Netherlands) within 58 hours (range, 4-58 hours) after birth. The fetuses were kept at 4°C until 4 hours before scanning, which was performed at room temperature. A knee coil or phased array coil was used. The fetus was positioned supine with the arms parallel to the body and the legs extended. Scout images were obtained in sagittal and coronal planes using a T1-weighted turbo spin-echo sequence. A total body T2-weighted turbo spin-echo sequence was subsequently acquired in the axial plane (TR/effective TE, 9500-11 000/100 ms; flip angle, 90 degrees; turbofactor, 13; section thickness, 2 or 3 mm without interslice gap; field of view, 120 × 84 mm; acquisition matrix, 256 × 203; scan time, 11 minutes).
Image analysis
All axial images were quantitatively analyzed at an IPC computer workstation (SUN Microsystems, Mountain View, CA) using a modification of the MASS software package developed at our institution.18 This package allows manual tracing of anatomic contours in the images. The contours of liver, spleen, thymus, and BM were all traced by the same person and reviewed by the same radiologist. Consensus was reached for every traced contour. Following the tracing of contours in all images, volumes were calculated for each of the compartments by multiplying the area of the contours by the section thickness used. The total BM volume was measured by adding the volumes of all the different bones. Total body length was measured in the sagittal scout view.
Identification of cellular compartments
Human fetal tissue from 10 fetuses was obtained following informed consent from women undergoing elective termination of pregnancy between 16 and 22 weeks of gestational age. All terminations were performed at the Center for Human Reproduction. The Medical Ethics Review Board of the Leiden University Medical Center approved the protocol of this study. Liver, spleen, and long bones were collected in RPMI containing 10 IU heparin per milliliter. Cell suspensions of liver and spleen were made within 2 hours of collection by mincing these tissues thoroughly. Deoxyribonuclease was added to prevent clotting of the cells. The different long bones, 23 in total, were first scanned and their volume measured. Within 4 hours after MRI the bones were flushed with RPMI containing 2% fetal bovine serum (Bio Whittaker, Verviers, Belgium), 1% penicillin (10 000 U/mL)/streptomycin (10 000 μg/mL) (Bio Whittaker), and deoxyribonuclease. Cell suspensions were pelleted by centrifugation, resuspended, and total white blood cells (WBCs) per volume enumerated. Red blood cells were lysed by incubating the cell suspensions for 10 minutes with NH4Cl at 0°C, and then cells were washed 3 times in RPMI plus fetal calf serum and penicillin/streptomycin. Cells were stained with CD14, CD34, CD45, CD66e, and glycophorin A according to the method of the SIHON workshop19 for phenotypic characterization. After staining with monoclonal antibodies, the cells were washed with phosphate-buffered saline containing 1 mg/1 mL GPO (pasteurized serum protein). The phenotypic analysis of the cells was performed using a Becton Dickinson FACScan flow cytometer. A total of 10 000 events were collected for each sample and analyzed using the CellQuest software (Becton Dickinson). CD45+CD14+ cells were defined as monocytes, CD45+CD66e+CD14− cells were defined as granulocytes, and CD45+CD66e−CD14− cells were defined as lymphocytes.
The absolute number of CD34+ cells in BM was measured by multiplying the absolute WBC count by the percentage of CD34+ cells obtained by flow analysis. Knowing the percentage the obtained bones contributed to the total BM compartment as measured by MRI, absolute cell numbers for the whole BM compartment could be calculated. The absolute cell numbers in liver and spleen were calculated by multiplying total cell numbers per gram with standard weight values as described for different gestational ages.12 20
Results
Eleven fetuses were included, and their gestational ages varied from 18 to 22 weeks (Table 1). Four fetuses were without anomalies, and 7 fetuses had genetic abnormalities. The weight of the fetuses ranged from 140 to 500 g, and the lengths ranged from 18 to 32 cm, which is within normal limits for this age group.12 The correlation between gestational age and fetal weight was 0.85 and between gestational age and fetal length 0.95.
Identification of the BM compartment
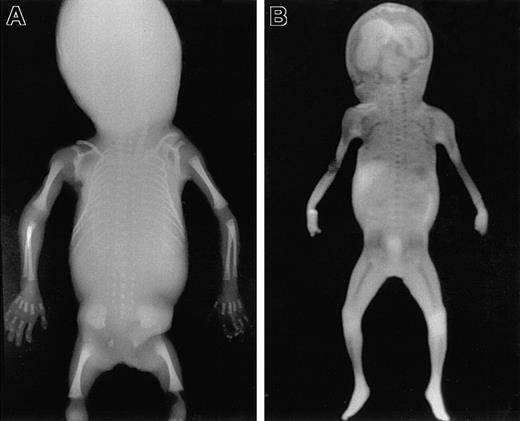
To identify areas of BM in the fetal skeleton, total body radiography of fetuses of different gestational ages was performed (Figure 1A) showing ossification centers in the skull, spine, claviculae, scapulae, costae, humeri, ulnae, radius, pelvis, femora, tibiae, fibula, and the bones of the hands and feet. Corresponding sites could be visualized using MRI (Figure 1B). All sites except the skull were confirmed to contain hematopoietic cells by microscopic histologic analysis (Figure2). In the skull, only the mandibula, maxilla, clivus (a bony surface in the posterior fossa), and occipital bones contained hematopoietic cells during the gestational ages studied. The other skull bones showed bone formation but no marrow containing hematopoietic cells and were therefore excluded from calculation of the BM compartment. The hematopoietic sites confirmed by histologic analysis correlated well with the BM areas found on the MRI (Figure 3), and thus the volume of these hematopoietic sites could be determined from the MRIs.
Total body radiography and coronal MRI.
Total body anteroposterior radiograph image (A) and T1-weighted coronal scout MRI (B) of a fetus 21 weeks of gestational age. Areas of ossification containing BM are clearly recognizable as radiodense structures in the radiograph. Corresponding areas of low signal intensity (dark) are visible in the MRI. These areas are well delineated from the surrounding cartilage, which shows much higher signal intensity (light).
Total body radiography and coronal MRI.
Total body anteroposterior radiograph image (A) and T1-weighted coronal scout MRI (B) of a fetus 21 weeks of gestational age. Areas of ossification containing BM are clearly recognizable as radiodense structures in the radiograph. Corresponding areas of low signal intensity (dark) are visible in the MRI. These areas are well delineated from the surrounding cartilage, which shows much higher signal intensity (light).
Microscopic histologic sections confirming hematopoietic sites.
(A) Microscopic longitudinal section (4 μm) of the proximal ulna showing the transition between BM and cartilage. Hematopoietic cells were found in the BM area. (B) Microscopic section (4 μm) of a 21-week-old fetal temporal skull showing absence of hematopoiesis. Although desmal and enchondral osteosynthesis has taken place in the temporal skull, no hematopoietic cells are found. Also, the other skull bones did not show hematopoietic cells at these gestational ages. Hematoxylin-eosin staining was used.
Microscopic histologic sections confirming hematopoietic sites.
(A) Microscopic longitudinal section (4 μm) of the proximal ulna showing the transition between BM and cartilage. Hematopoietic cells were found in the BM area. (B) Microscopic section (4 μm) of a 21-week-old fetal temporal skull showing absence of hematopoiesis. Although desmal and enchondral osteosynthesis has taken place in the temporal skull, no hematopoietic cells are found. Also, the other skull bones did not show hematopoietic cells at these gestational ages. Hematoxylin-eosin staining was used.
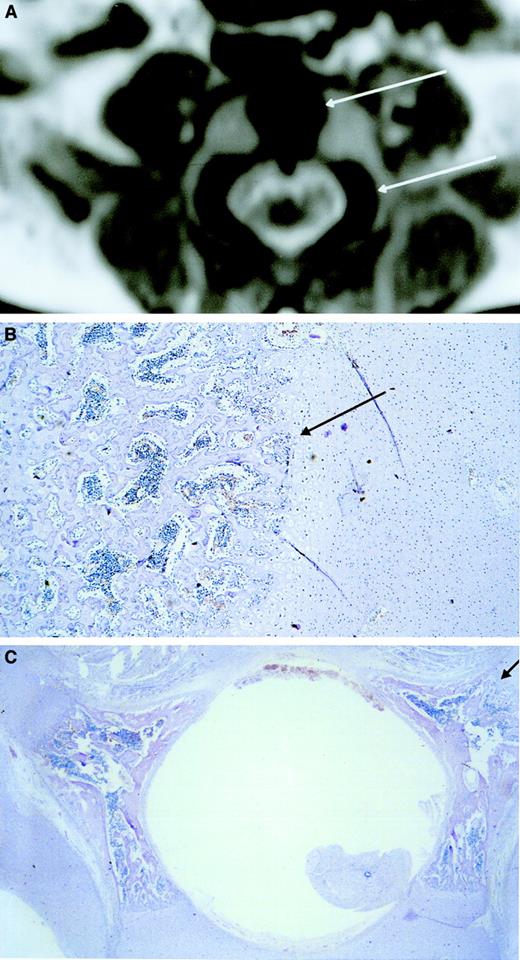
Ossification center.
MRI shows low signal intensity (black) in the central area of the vertebral body corresponding to an ossification center (upper arrow). Low signal intensity corresponding to an ossification center is also visible in the lateral area of the vertebral pedicles (lower arrow) (A). Microscopic transversal sections (4 μm) stained with hematoxylin-eosin of the central area of the vertebral body (B) and the lateral areas of the vertebral pedicles (C) show hematopoietic cells between the spiculae, indicating that the low-intensity areas visualized by MRI are hematopoietic areas. The upper and lower arrows in panel A correspond with the arrows in panels B and C, respectively. Original magnification × 40.
Ossification center.
MRI shows low signal intensity (black) in the central area of the vertebral body corresponding to an ossification center (upper arrow). Low signal intensity corresponding to an ossification center is also visible in the lateral area of the vertebral pedicles (lower arrow) (A). Microscopic transversal sections (4 μm) stained with hematoxylin-eosin of the central area of the vertebral body (B) and the lateral areas of the vertebral pedicles (C) show hematopoietic cells between the spiculae, indicating that the low-intensity areas visualized by MRI are hematopoietic areas. The upper and lower arrows in panel A correspond with the arrows in panels B and C, respectively. Original magnification × 40.
BM volume measurements of all bones could be performed accurately by MRI (Figure 4) except for the volume of the costae because of their oblique orientation in the axial plane of scanning. Total BM volume was measured by adding the volumes of all the different bones determined from the MRIs, ranging from 934 to 4563 μL (Table 1 and Figure 5). The percentage each bone contributed to the total BM volume was constant during the gestational ages investigated.
Components of the BM compartment measured by MRI.
Mean percentage of the total BM volume of the different bones contributing to the BM compartment, including the 95% confidence interval (± 2 × SEM).
Components of the BM compartment measured by MRI.
Mean percentage of the total BM volume of the different bones contributing to the BM compartment, including the 95% confidence interval (± 2 × SEM).
BM volume.
Total BM volume measured by MRI in fetuses without anomaly (●) and with anomaly (■).
BM volume.
Total BM volume measured by MRI in fetuses without anomaly (●) and with anomaly (■).
To include the contributing part of the costae to the total BM compartment, an estimation of the volume of the BM compartment of the costae was made. This was based on the relationship between volume of the BM compartment measured by MRI and the total number of WBCs in bones of which both volume and cell numbers could be measured.
The costae of 3 fetuses were flushed, and absolute cell numbers of the costae were counted. The number of cells harvested from all 24 costae of each fetus was 5% ± 0.6% of the total number of cells present in all BM-containing bones of the fetus, and therefore the volume of the costae was estimated to be 5% of the total BM volume. The largest component of the total BM compartment was the spine, which constituted 26.4% ± 2.7% of the total volume. The femora contained 14.1% ± 2.9%, the pelvis 9.5% ± 2.0%, the fibulae and tibiae 9.4% ± 2.1%, the humeri 8.8% ± 1.9%, the scapulae 7.7% ± 2.2%, the skull 6.7% ± 1.8%, the ulnae/radii 5.4% ± 1.6%, the claviculae 4.6% ± 2.6%, the bones from both hands 2.3% ± 2.1%, and the bones from both feet 0.8% ± 0.4%.
Cellular analysis of the BM compartment
In addition to this quantitative analysis, we also performed a cellular analysis of the different parts of the BM compartment to establish the uniformity of this compartment during gestation. Twenty-three different bones of 10 fetuses of 16 to 22 weeks of gestation were analyzed after termination of pregnancy. Of these individual bones, MRIs were made and the BM volumes were measured. Next, these bones were flushed, and the harvested cells were counted. The volume of the different long bones ranged from 5 to 250 μL and the WBC number of the different bones from 0.7 × 106 to 31 × 106 cells. A significant correlation of 0.67 (P < .001) between the volume of the different bones and the total numbers of WBCs obtained from these bones was found. To determine the hematopoietic progenitor cell content of the BM compartment, CD34+ cells were determined using immunohistochemistry and flow cytometry. CD34 immunostaining showed that both CD34 bright and CD34 dull hematopoietic cells were equally spread throughout the BM compartment. This was independent of the time of gestation, the different bones, and the developing state of the BM examined (data not shown). No clustering of the CD34+ cells was found at the specific sites where new formation of BM sites was evident.
The percentage of CD34+ cells within the hematopoietic cell population was determined using flow cytometry. The mean percentage of CD34+ cells in the different bones was 23% ± 3% (range, 19%-31%). No statistically significant relation (P > .50) was found between the percentages of CD34+ cells and gestational age (Figure6A). Similarly, the percentage of CD34+ cells in the different bones within one fetus did not vary (data not shown). The mean percentage of lymphocytes in BM at various gestational ages was 39% ± 7% (range, 31%-50%). The mean percentages of granulocytes and monocytes were 29% ± 2% (range, 27%-34%) and 11% ± 3% (range, 8%-17%), respectively. No statistically significant relation was found in the percentages of differentiated cell types in the same bones throughout gestation or in various bones from the same fetus (data not shown). In conclusion, the cellular composition of different bones did not vary throughout the second trimester of gestation, illustrating that the BM contained a uniform compartment.
Definition of the absolute number of CD34+cells.
(A) The percentage CD34+ cells in BM (♦), fetal liver (▪), and spleen (▵) was independent of gestational age. Data plotted are derived only from fetuses of which all 3 tissues (BM, liver, and spleen) were available for analysis. (B) Absolute numbers of CD34+ cells in BM (♦), human fetal liver (▪), or spleen (▵). (C) Total absolute numbers of CD34+cells per fetus are given as measured in liver plus BM plus spleen.
Definition of the absolute number of CD34+cells.
(A) The percentage CD34+ cells in BM (♦), fetal liver (▪), and spleen (▵) was independent of gestational age. Data plotted are derived only from fetuses of which all 3 tissues (BM, liver, and spleen) were available for analysis. (B) Absolute numbers of CD34+ cells in BM (♦), human fetal liver (▪), or spleen (▵). (C) Total absolute numbers of CD34+cells per fetus are given as measured in liver plus BM plus spleen.
Absolute cell numbers in the BM compartment
After analyzing the number of cells per bone and the contribution of each bone to the total BM volume, absolute cell numbers could be calculated. In BM the absolute WBC number was found to increase from 111 × 106 at 16 to 17 weeks of gestation to 1229 × 106 at 21 to 22 weeks of gestation. The best-fitting regression line was y = 1.3561e0.23×. Because the mean percentage of CD34+ cells was 23% ± 3% (Figure 6A), the absolute number of CD34+cells increased from 25 × 106 at 16 to 17 weeks to 256 × 106 at 21 to 22 weeks (y = 0.5741e0.25×) (Figure 6B). The correlation between gestational age and absolute WBC numbers was 0.72 (P < .05) and between gestational age and absolute numbers of CD34+ cells was 0.69 (P < .05).
Quantitative and qualitative analysis of liver and spleen
Using MRI, the volumes of liver, spleen, and thymus were determined (Table 1). The percentage of CD34+ cells in liver and spleen did not change at different weeks of gestation and were 5% ± 2.8% and 8% ± 6.3% for liver and spleen, respectively (Figure 6A). The volume of the thymus was small and, in combination with percentage of CD34+ cells under 2%, it was excluded from the analysis. In liver, the mean percentage of lymphocytes was 12% ± 3.7 % (range, 7%-19%), the mean percentage of monocytes was 5% ± 3.2% (range, 1%-11%), and the mean percentage of granulocytes was 1% ± 0.7% (range, 0.2%-3%), with the remaining cells being nucleated red cells. In the spleen, the mean percentage of lymphocytes was 53% ± 28% and showed a large range of 16% to 87%. The mean percentages of monocytes and granulocytes were 7% ± 5.9% (range, 3%-24%) and 3% ± 2.1% (range, 0.3%-8%), respectively.
Fetal liver contained more WBCs than the BM compartment and increased from 563 × 106 at 16 to 17 weeks to 3384 × 106 at 21 to 22 weeks (y = 7.5477e0.27×). The absolute number of CD34+ cells increased from 16 × 106 at 16 to 17 weeks to 117 × 106 CD34+ cells at 21 to 22 weeks (y = 0.2574e0.27×) (Figure 6B), which is not different from that observed in the BM compartment (Figure 6B).
The total number of WBCs in spleen increased from 14 × 106 at 16 to 17 weeks to 151 × 106at 21 to 22 weeks (y = 0.0654e0.34×). The absolute number of CD34+ cells increased from 1 × 106at 16 to 17 weeks to 11 × 106 at 21 to 22 weeks (y = 0.0344e0.23×) (Figure 6B). The correlation between gestational age and absolute WBC numbers was 0.90 (P < .01) for liver and 0.92 (P < .01) for spleen. The correlation between gestational age and absolute number of CD34+ cells was 0.92 (P < .01) for liver and 0.82 (P < .05) for spleen.
Total absolute numbers of CD34+ cells in the fetal compartment
By adding the CD34+ cells in BM, liver, and spleen, the absolute numbers of hematopoietic precursor cells in the fetus could be calculated. The total absolute number of CD34+cells in the BM, liver, and spleen in the fetus increased from 73 × 106 at 16 to 17 weeks to 362 × 106at 21 to 22 weeks (y = 1.09e0.26×) (Figure 6C).
Discussion
The development of new prenatal therapies including in utero gene therapy and stem cell transplantation requires insight into qualitative information of the human fetal BM compartment.6,15 21 The present study is the first to describe a quantitative analysis of the fetal BM compartment by measuring the volume of this expanding hematopoietic organ during ontogeny.
To identify the BM hematopoietic sites in the fetus at different gestational ages, total body radiography was performed identifying ossification centers. Corresponding sites were made visible using MRI. The BM compartment could be measured reliably with MRI because differences in signal intensities between cartilage and BM provided the possibility to accurately discriminate these structures in the fetus. To verify that these areas were indeed hematopoietic sites, corresponding histologic sections were made of all these structures. Up to 22 weeks of gestation, hematopoietic cells were detected in all bones except the calvarial bones (skull). Only after 22 weeks of gestation a few hematopoietic cells could be detected in histologic sections, suggesting the beginning of hematopoiesis in the calvarial bones. The absence of hematopoietic cells in the calvarial bones can be explained by the fact that in the frontal and parietal skull bones osteogenesis does not develop from cartilage but takes place through intramembranous ossification. This apparently occurs later in development than enchondral ossification. The connective tissue in which intramembranous ossification occurs and the ossification centers containing the BM compartment in other bones were shown to have the same intensity using MRI. This initially suggested that all these bones belonged to the hematopoietic BM compartment. Based on the histologic analysis, however, the calvarial bones were excluded from addition to the BM compartment. All other bones including the other skull bones have enchondral osteogenesis22 and do develop from cartilage. In these bones cartilage could be easily distinguished from BM using MRI because of the different intensity.
The volume of all bones contributing to the BM compartment could be measured accurately by MRI except for the costae because of their orientation in the axial plane of scanning. Therefore, a different calculation was performed based on the number of WBCs, resulting in a contributing volume of only 5% ± 0.6% for the costae. Variations in the volume of the costae will be of little influence because of the low contribution to the total volume.
To determine whether the appearance of CD34+ cells in the BM compartment differed during the development of new BM sites as compared with later stages of the BM compartment, we analyzed whether clustering of CD34+ cells could be observed at any stage during the development of the BM compartment. Using immunostaining of CD34+ cells, both CD34 bright and dull cells were equally spread throughout the marrow independent of the time of gestation and the development state of the BM examined, and no clustering was observed in newly formed BM sites. These results are compatible with a relatively random homing of the CD34+ cells to the BM cavities. In addition, no changes in the composition of other leukocyte subsets were found. These results indicate that by measuring the volumes of the BM cavities, an appropriate estimation can be made of its contents. Thus, following the analysis of the total BM compartment as described in this paper, the quantitative determination of the total hematopoietic compartment could be completed. Between 16 and 22 weeks of gestation the composition of the bones, liver, and spleen did not change. No hematopoietic shift from liver to BM was observed up to 23 weeks of gestation, which is in line with previous studies describing this shift after 24 weeks of gestation.7 Quantitatively the liver exceeded the BM in volume and absolute number of WBCs but not in absolute number of CD34+ cells. Thus, the main difference between these organs did not appear to be a quantitative difference in stem cell compartment but in the lineages of cell differentiation between tissues. Stem cells present in BM are mainly involved in B-cell lymphopoiesis, whereas the liver in the second trimester of gestation appears to be responsible for sustaining erythropoiesis, myelopoiesis, and thrombopoiesis.
As illustrated in previous reports, spleen and thymus are mainly involved in B-cell and T-cell lineage development. The similar and constant numbers of CD34+ cells during the second trimester of gestation do not support the concept of a stem cell compartment shifting from fetal liver to BM. It may be more likely that a changing microenvironment during development is responsible for different signals to the differentiating precursor cells resulting in lineage-specific development.
In conclusion, MRI has proven to be a feasible method for the quantitative analysis of the human fetal BM compartment. Liver and spleen volumes measured by MRI were in concordance with the organ weight previously published in the literature,12 20 which illustrates the reliability of MRI as a method to quantify fetal organ volumes. By combining MRI and cell enumeration we have established a method to quantify specific cell populations in fetal compartments throughout gestation. This study may be enable better understanding of fetal hematopoiesis and may be used for the evaluation of fetal diagnostics and therapies.
The authors gratefully acknowledge W. Beekhuizen of the Center for Human Reproduction in Leiden for collecting the fetal tissues after informed consented elective abortions and Prof Dr J. L. Bloem, Dr C. M. Orschell-Traycoff, Dr S. Scherjon, and Dr K. Zwinderman for critical review of the manuscript.
Supported in part by a grant from the J. A. Cohen Institute for Radiopathology and Radiation Protection.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
J. H. F. Falkenburg, Dept of Hematology, Leiden University Medical Center, Bldg 1, C2R, PO Box 9600, 2300 RC Leiden, The Netherlands; e-mail: falkenburg.hematology@lumc.nl.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal