Abstract
Imatinib mesylate, an Abl kinase inhibitor, produces sustained complete hematologic responses (CHRs) in chronic myelogenous leukemia (CML) patients, but the sequence and timing of morphologic and cytogenetic changes in CML patients during prolonged imatinib mesylate treatment has not been described. In this report, we document sequential hematologic and bone marrow findings in 19 interferon-refractory/interferon-intolerant chronic phase CML patients on imatinib mesylate for at least 14 months. Patients treated at an effective oral dose (300 to 600 mg per day) were followed with peripheral blood (PB) counts, marrow examination, and cytogenetic studies at 0, 2, 5, 8, 11, and 14 months. By 2 months, 17 of 19 patients achieved CHR; 1 reached CHR by 5 months, and 1 at 11 months. Five of 19 patients developed cytopenias requiring treatment interruption and/or dose reduction, but all were able to continue in CHR on study. In contrast to interferon-alfa treatment, imatinib mesylate–treated CML patients achieved not only CHR but complete morphologic marrow response. Normalization of marrow lagged behind PB response; however, by 8 months, all marrows showed normal or reduced cellularity without morphologic evidence of CML. Eighteen of 19 patients continued in CHR and morphologic marrow remission at 14 months; 1 patient relapsed with chronic phase CML. Although hematologic and marrow responses were uniform, cytogenetic responses were variable. Complete cytogenetic responses occurred in 6 patients, with 4 also in remission by fluorescent in situ hybridization and/or reverse-transcription–polymerase chain reaction. Six of 19 had partial and 7 of 19 no cytogenetic response. Several patients acquired additional clonal cytogenetic abnormalities during therapy, a finding with significant implications for prognosis and laboratory monitoring in imatinib mesylate–treated CML patients.
Introduction
The t(9;22) translocation in chronic myelogenous leukemia (CML) and in a subset of acute lymphoblastic leukemias results in production of a BCR-ABL fusion protein with Abl kinase activity. In experimental models, the expression of this Abl kinase activity causes expansion of pluripotent stem cells, preferentially favors myeloid lineage differentiation, and inhibits erythroid differentiation. Inhibition of BCR-ABL reverses these effects.1 2
The specific Abl kinase inhibitor imatinib mesylate, also called STI571 or Gleevec (Novartis, Hanover, NJ), which blocks the adenosine 5′-triphosphate–binding site of Abl kinase, has been shown to induce growth arrest or apoptosis in BCR-ABL–expressing hematopoietic cells.3-5 In phase 1 clinical trials, imatinib mesylate produced sustained complete hematologic responses (CHRs) in chronic-phase CML patients treated with effective daily doses of 300 mg or greater.6
The morphologic and cytogenetic findings in chronic phase CML patients treated with imatinib mesylate over a prolonged time period have not been previously described. In this report, we document the sequential hematologic, bone marrow morphologic, and cytogenetic findings in 19 chronic-phase CML patients treated with imatinib mesylate for at least 14 months. These patients are part of the initial cohort of interferon-refractory/interferon-intolerant chronic-phase CML patients treated with imatinib mesylate; they represent the longest experience with this novel therapy.
Patients and methods
Patients
Review of the Leukemia Program records at Oregon Health Sciences University (Portland) and the University of California at Los Angeles identified 19 chronic-phase CML patients who had been treated with imatinib mesylate for at least 14 months at the time of the review. The clinical responses of these 19 patients have been previously reported as part of the larger phase 1 clinical trial cohort.6 In all patients, the diagnosis of chronic-phase CML was confirmed prior to imatinib mesylate treatment by morphologic review of peripheral blood (PB) and bone marrow and by documentation of the presence of theBCR-ABL translocation by conventional metaphase cytogenetic analysis or molecular studies. The patients were treated with imatinib mesylate at oral doses of 300 to 600 mg per day and were followed with complete blood counts (CBCs) and bone marrow aspiration and biopsy at 0, 2, 5, 8, 11, and 14 months. Bone marrow specimens were subjected to morphologic evaluation, routine metaphase karyotyping, and fluorescent in situ hybridization (FISH) cytogenetic studies. Reverse-transcription–polymerase chain reaction (RT-PCR) analysis for the BCR-ABL translocation was not performed regularly as part of this study, but limited PCR results were available for some patients at later time points.
At the time of entry into the study, the patients had a mean age of 56.9 years (range, 19-70 years). There were 9 male patients and 10 female patients. The mean duration of disease was 3.5 years (range, 1.1 to 9.11 years). Thirteen patients (68%) were interferon refractory; 5 patients (26%) were interferon intolerant; and 1 patient was both. Of the 19 patients, 15 had only a classic t(9;22)(q34;q11) Philadelphia chromosome (Ph) translocation on routine karyotyping at the beginning of imatinib mesylate therapy, and 3 patients had complex translocations. One showed evidence of clonal evolution before imatinib mesylate therapy, with an additional t(7;8) translocation in 15 of 20 Ph+ cells. Pretreatment characteristics of specific patients, including age, sex, disease duration, and results of their karyotypic analysis (postinterferon/pre-imatinib mesylate therapy), are shown in Table1.
Pretreatment characteristics of chronic myelogenous leukemia patients
| Patient no. . | Age, y . | Sex . | Disease duration, y . | IFN status . | Karyotype [no. metaphases] . |
|---|---|---|---|---|---|
| 1 | 46 | F | 4.1 | Resistant | 46,XX, t(22;9)(9;1)(q11;q34-q32;q42) [18] |
| 2 | 19 | F | 3.7 | Resistant | 46,XX, t(9;22)(q34;q11)[17]/ 46,XX [9] |
| 3 | 54 | F | 1.6 | Intolerant | 46,XX, t(9;22)(q34;q11) [20] |
| 4 | 66 | M | 2.5 | Intolerant | 46,XY, t(9;22)(q34;q11) [20] |
| 5 | 60 | F | 4.2 | Resistant | 46,XX, t(9;22)(q34;q11) [19]/ 46,XX [1] |
| 6 | 51 | F | 1.8 | Intolerant | 46,XX, t(9;22)(q34;q11) [17]/ 46,XX [3] |
| 7 | 69 | M | 3.9 | Resistant | 46,XY, t(9;22)(q34;q11) [21] |
| 8 | 65 | M | 7.3 | Resistant | 46,XY, t(9;22)(q34;q11) [15] |
| 9 | 70 | M | 6.5 | Intolerant | 46,XY, t(9;22)(q34;q11)[23]/ 46XY [2] |
| 10 | 60 | F | 1.7 | Resistant | 46,XX, t(7;8)(q22;q13), t(9;22)(q34;q11) [15]/ 46,XX, t(9;22)(q34;q11) [5]/ 46,XX [1] |
| 11 | 52 | M | 1.1 | Resistant/Intolerant | 46,XY, t(9;22)(q34;q11) [21] |
| 12 | 64 | M | 3.9 | Resistant | 46,XY, t(22;9)(9;13;1)(q11;q34;q32;q21;q31) [19]/ 47,XY, t(22;9)(9;13;1)(q11;q34;q32;q21;q31),+ 8 [1] |
| 13 | 69 | F | 1.5 | Resistant | 46,XX, t(9;22)(q34;q11) [20] |
| 14 | 58 | F | 2.11 | Intolerant | 46,XX, t(5;9;22)(q35;q34;q11) [15]/ 46XX [5] |
| 15 | 65 | M | 1.2 | Resistant | 46,XY, t(9;22)(q34;q11) [22] |
| 16 | 54 | F | 9.11 | Resistant | 46,XX, t(9;22)(q34;q11) [17] |
| 17 | 53 | F | 2.8 | Resistant | 46,XX, t(9;22)(q34;q11) [20] |
| 18 | 38 | M | 6.2 | Resistant | 46,XY, t(9;22)(q34;q11) [20] |
| 19 | 69 | M | 1.3 | Resistant | 46,XY, t(9;22)(q34;q11) [20] |
| Patient no. . | Age, y . | Sex . | Disease duration, y . | IFN status . | Karyotype [no. metaphases] . |
|---|---|---|---|---|---|
| 1 | 46 | F | 4.1 | Resistant | 46,XX, t(22;9)(9;1)(q11;q34-q32;q42) [18] |
| 2 | 19 | F | 3.7 | Resistant | 46,XX, t(9;22)(q34;q11)[17]/ 46,XX [9] |
| 3 | 54 | F | 1.6 | Intolerant | 46,XX, t(9;22)(q34;q11) [20] |
| 4 | 66 | M | 2.5 | Intolerant | 46,XY, t(9;22)(q34;q11) [20] |
| 5 | 60 | F | 4.2 | Resistant | 46,XX, t(9;22)(q34;q11) [19]/ 46,XX [1] |
| 6 | 51 | F | 1.8 | Intolerant | 46,XX, t(9;22)(q34;q11) [17]/ 46,XX [3] |
| 7 | 69 | M | 3.9 | Resistant | 46,XY, t(9;22)(q34;q11) [21] |
| 8 | 65 | M | 7.3 | Resistant | 46,XY, t(9;22)(q34;q11) [15] |
| 9 | 70 | M | 6.5 | Intolerant | 46,XY, t(9;22)(q34;q11)[23]/ 46XY [2] |
| 10 | 60 | F | 1.7 | Resistant | 46,XX, t(7;8)(q22;q13), t(9;22)(q34;q11) [15]/ 46,XX, t(9;22)(q34;q11) [5]/ 46,XX [1] |
| 11 | 52 | M | 1.1 | Resistant/Intolerant | 46,XY, t(9;22)(q34;q11) [21] |
| 12 | 64 | M | 3.9 | Resistant | 46,XY, t(22;9)(9;13;1)(q11;q34;q32;q21;q31) [19]/ 47,XY, t(22;9)(9;13;1)(q11;q34;q32;q21;q31),+ 8 [1] |
| 13 | 69 | F | 1.5 | Resistant | 46,XX, t(9;22)(q34;q11) [20] |
| 14 | 58 | F | 2.11 | Intolerant | 46,XX, t(5;9;22)(q35;q34;q11) [15]/ 46XX [5] |
| 15 | 65 | M | 1.2 | Resistant | 46,XY, t(9;22)(q34;q11) [22] |
| 16 | 54 | F | 9.11 | Resistant | 46,XX, t(9;22)(q34;q11) [17] |
| 17 | 53 | F | 2.8 | Resistant | 46,XX, t(9;22)(q34;q11) [20] |
| 18 | 38 | M | 6.2 | Resistant | 46,XY, t(9;22)(q34;q11) [20] |
| 19 | 69 | M | 1.3 | Resistant | 46,XY, t(9;22)(q34;q11) [20] |
IFN indicates interferon alfa.
Morphologic review
PB smears from each time point and CBC reports from the participating institutions were reviewed for documentation of PB findings. PB smears were evaluated for basophilia, the presence of circulating myeloid precursors and blasts, and platelet abnormalities. Wright-stained bone marrow aspirate smears and hematoxylin and eosin–stained paraffin sections of bone marrow biopsy and aspirate clot specimens were examined for overall marrow cellularity, cell maturation and proportion of blasts, cellular atypia, basophilia, and the presence or absence of focal lesions. Bone marrow aspirate differential counts were used to derive a myeloid-to-erythroid cellular ratio (M/E ratio), which was correlated with overall cellularity to evaluate the total myeloid and erythroid cell compartments. Reticulin stains were performed on core biopsies of a subset of 11 patients to evaluate marrow reticulin fibrosis over the course of therapy.
Cytogenetic and molecular analysis
Complete karyotyping on metaphase spreads was performed in all patients at intervals of 0, 2, 5, 8, 11, and 14 months.BCR-ABL interphase FISH studies and qualitative RT-PCR analysis for BCR-ABL were performed in selected patients at the discretion of the investigator, but usually following a complete cytogenetic response. The results of bone marrow metaphase karyotyping, BCR-ABL FISH results, and, when available, BCR-ABL RT-PCR analyses from each institution were correlated with the hematologic and marrow findings. Procedural details of the specific analyses are given below.
Chromosome preparations.
Bone marrow aspirate was introduced into culture medium and 3 different culture methods were used: One culture was harvested at 24 hours, one synchronized with 10−7 M methotrexate and harvested at 24 hours, and one supplemented with giant cell tumor–conditioned medium and harvested at 48 hours. The harvest and slide preparations were done according to standard methods. Chromosomes were Giemsa-trypsin-Wright–banded. Cytogenetic responses, based on metaphase analysis of at least 20 cells, if possible, were defined as complete (no Ph+ cells), partial (1% to 65% Ph+ cells), or absent (more than 65% Ph+cells). In some patients, particularly at the beginning of therapy, it was not always possible to find 20 metaphases for analysis.
Fluorescent in situ hybridization.
Cells fixed in 3:1 methanol–to–acetic acid were dropped onto slides and treated to optimize spreading, in a manner similar to routine metaphase chromosome preparation. Slides were baked at 95°C for 5 to 6 minutes, incubated in 2 × SSC at 37°C for 30 minutes, dehydrated through an alcohol series (70%, 80%, and 95% for 2 minutes each), and dried. Direct labeled probes for the t(9;22) translocation breakpoints (Vysis single fusion BCR-ABL set, catalog no. 190022 [Downer Grove, IL] or Ventana Medical Systems [Tucson, AZ] [Oncor] double-fusion BCR-ABL D-FISH set, catalog no. P5161-DC) were co-denatured with the target DNA at 72°C for 2 minutes and allowed to renature overnight at 37°C. Slides were then rinsed in 0.5 × SSC at 72°C for 5 minutes and transferred to phosphate-nonadet buffer for 3 minutes. Preparations were counterstained with 4′6-diamidino-2-phenylindole-2HCl for visualization of red, green, and yellow probe signals and analyzed through a Zeiss Axiophot (Germany), and images captured by means of a CytoVision system (Applied Imaging, Santa Clara, CA).
At least 200 interphase cells were scored for signal patterns. FISH results for at least one metaphase cell were analyzed when possible, to aid in interpretation of complex signal patterns. The normal signal pattern for both probe sets is 2 red, 2 green. The common abnormal pattern for the single fusion system (S-FISH) is one red, one green, one yellow (representing the derivative chromosome 22). False-positive (background) for the one red, one green, one yellow pattern in S-FISH is 10% or lower. The test is considered positive for the Ph rearrangement if the number of fusion signals exceeds 10% and negative if the number of fusion signals is 10% or lower. The common abnormal pattern for the more sensitive double-fusion system (D-FISH) is 1 red, 1 green, and 2 yellow signals (representing both the derivative chromosome 9 and the derivative chromosome 22). Observation of a single interphase cell with 1 red, 1 green, and 2 yellow signals with the D-FISH kit is considered to be positive for the Ph rearrangement. Variations in the signal pattern may reflect underlying complex karyotypes.
RT-PCR analysis for BCR-ABL.
The reported RT-PCR analyses were performed on bone marrow aspirate specimens by means of a standard nested primer technique.7In brief, RNA was isolated by means of a commercially available RNA extraction kit (QIAgen RNeasy Blood Mini Kit [Valencia, CA]). Complementary DNA was generated by reverse transcription with random primers (Gibco BRL 48190-011, Carlsbad, CA), and nested PCR was performed with specific primers for ABL and the major breakpoint cluster region within BCR. Primer sequences for ABL were as follows: 5′-GTT ATC TCC ACT GGC CAC AA-3′ (outer) and 5′-AAC GAG CGG CTT CAC TCA GA-3′ (inner). Primer sequences used for the major BCR translocation were as follows: 5′-GCT GAC CAA CTC GTG TGT GA-3′ (outer) and 5′-CAG ACT GTC CAC AGC ATT CC-3′ (inner).7 The PCR products were visualized in ethidium bromide–stained gels after electrophoresis. A positive result for the major translocation is a band present at 103 or 178 base pairs. ABL was used as an internal RNA control. The assay used for these studies was capable of detecting 1 translocation-carrying cell in 105 total cells.
Results
PB findings
Typical PB findings in chronic-phase CML include mild anemia, leukocytosis with a characteristic left shift, basophilia, and frequent thrombocytosis with large and/or hypogranular platelets. Some or all of these findings were present in all of our patients prior to the initiation of therapy. At the time of their 2-month blood and bone marrow evaluation, 17 of 19 patients had a documented CHR, defined as a white blood cell (WBC) count that was below 10 × 109/L and a platelet count below 450 × 109/L. One patient, who was also on phenytoin and who had subtherapeutic levels of imatinib mesylate, reached CHR at 5 months after an increase in dose from 350 to 500 mg per day and discontinuance of phenytoin. The remaining patient (although transiently showing CHR at 4 weeks) had elevated WBC and/or platelet counts at the 2-, 5-, and 8-month evaluations, with documented CHR only at 11 and 14 months.
On imatinib mesylate, the mean WBC count decreased from 31 × 109/L pretreatment (range, 8.8-94.9 × 109/L) to a mean of 6.4 × 109/L at 2 months (range, 1.3-31.5 × 109/L). Circulating myeloid precursors disappeared in all patients within 2 to 5 months. Mean platelet counts decreased from 438 × 109/L pretreatment (range, 118-1090 × 109/L) to a mean of 285 × 109/L at 2 months (range, 48-1080 × 109/L). As the platelet counts decreased, the large, atypical platelets characteristic of CML also disappeared from the circulation. Absolute basophil counts decreased from a mean of 1.0 × 109/L (range, 0-2.8 × 109/L) pretreatment to a mean of 0.3 × 109/L (range, 0-4.4 × 109/L) at 2 months; basophil counts were typically maintained at below 0.2 × 109/L during the remainder of the study (range, 0-0.2 × 109/L). Mild anemia was an occasional finding in these CML patients' pretreatment, and most patients experienced a mild decrease in hemoglobin levels during treatment with imatinib mesylate. The mean pretreatment hemoglobin was 130 g/L (13 g/dL) (range, 97-166 g/L [9.7-16.6 g/dL]), which decreased to a mean of 123 g/L (12.3 g/dL) by 2 months (range, 89-156 g/L [8.9-15.6 g/dL]). By 14 months, 13 of 17 patients had mildly decreased hemoglobin compared with pretreatment values (mean decrease, 15 g/L [1.5 g/dL]), and 4 patients had slightly increased hemoglobin (mean increase, 5 g/L [0.5 g/dL]). The PB findings, including mean and ranges for hemoglobin, WBC count, basophils, and platelet counts at all time points are summarized in Table2.
Peripheral blood and bone marrow values at 0, 2, 5, 8, and 11 mo in 19 imatinib mesylate–treated chronic-phase chronic myelogenous leukemia patients
| . | 0 mo . | 2 mo . | 5 mo . | 8 mo . | 11 mo . | 14 mo . |
|---|---|---|---|---|---|---|
| WBC count × 109/L, mean (range) | 31 (8.8-94.9) | 6.4 (1.3-31.5) | 5.9 (2.2-15.3) | 5.7 (2.4-11.5) | 5.4 (2.4-10.5) | 5.3 (3.2-12.6) |
| Platelet count × 109/L, mean (range) | 438 (118-1090) | 285 (48-1080) | 217 (69-359) | 206 (66-494) | 196 (58-341) | 167 (37-329) |
| PB basophil count × 109/L, mean (range) | 1.008 (0-2.779) | .296 (0-4.410) | .032 (0-.237) | .015 (0-.084) | .024 (0-.105) | .018 (0-.064) |
| Hemoglobin, g/L [g/dL], mean (range) | 130 [13.0] (97-166) [9.7-16.6] | 123 [12.3] (89-156) [8.9-15.6] | 126 [12.6] (94-149) [9.4-14.9] | 125 [12.5] (87-158) [8.7-15.8] | 124 [12.4] (96-149) [9.6-14.9] | 121 [12.1] (90-151) [9.0-15.1] |
| Marrow cellularity, % (range) | 87 (50-100) | 52 (15-95) | 41 (5-95) | 36 (5-65) | 39 (10-60) | 40 (10-80) |
| Marrow myeloid-to-erythroid ratio (range) | 11.6 (3.3-48) | 2.5 (.8-10) | 2.7 (.4-8.7) | 1.8 (.8-3.5) | 2.2 (1.0-4.1) | 1.7 (.7-3.8) |
| Marrow blasts, % (range) | 3.5 (0-12) | 1.6 (0-5) | 1.6 (0-4) | 1.3 (0-5) | 0.6 (0-2) | 1.1 (0-3) |
| . | 0 mo . | 2 mo . | 5 mo . | 8 mo . | 11 mo . | 14 mo . |
|---|---|---|---|---|---|---|
| WBC count × 109/L, mean (range) | 31 (8.8-94.9) | 6.4 (1.3-31.5) | 5.9 (2.2-15.3) | 5.7 (2.4-11.5) | 5.4 (2.4-10.5) | 5.3 (3.2-12.6) |
| Platelet count × 109/L, mean (range) | 438 (118-1090) | 285 (48-1080) | 217 (69-359) | 206 (66-494) | 196 (58-341) | 167 (37-329) |
| PB basophil count × 109/L, mean (range) | 1.008 (0-2.779) | .296 (0-4.410) | .032 (0-.237) | .015 (0-.084) | .024 (0-.105) | .018 (0-.064) |
| Hemoglobin, g/L [g/dL], mean (range) | 130 [13.0] (97-166) [9.7-16.6] | 123 [12.3] (89-156) [8.9-15.6] | 126 [12.6] (94-149) [9.4-14.9] | 125 [12.5] (87-158) [8.7-15.8] | 124 [12.4] (96-149) [9.6-14.9] | 121 [12.1] (90-151) [9.0-15.1] |
| Marrow cellularity, % (range) | 87 (50-100) | 52 (15-95) | 41 (5-95) | 36 (5-65) | 39 (10-60) | 40 (10-80) |
| Marrow myeloid-to-erythroid ratio (range) | 11.6 (3.3-48) | 2.5 (.8-10) | 2.7 (.4-8.7) | 1.8 (.8-3.5) | 2.2 (1.0-4.1) | 1.7 (.7-3.8) |
| Marrow blasts, % (range) | 3.5 (0-12) | 1.6 (0-5) | 1.6 (0-4) | 1.3 (0-5) | 0.6 (0-2) | 1.1 (0-3) |
WBC indicates white blood cell; PB, peripheral blood.
Cytopenias are an important potential complication of imatinib mesylate treatment. During the trial, 5 of 19 patients developed grades 3-4 hematologic toxicity (4 with neutropenia, 1 with thrombocytopenia) that resolved with treatment interruption (2 patients) and/or dose reduction to a lower effective dose (3 patients). All 5 patients were able to resume or continue treatment and to maintain CHR.
Bone marrow findings
The bone marrow in chronic-phase CML typically shows marked hypercellularity with myeloid hyperplasia, frequent megakaryocytic hyperplasia with small hypolobated megakaryocytes, and basophilia. Eosinophilia is common, as are pseudo-Gaucher cells and sea-blue histiocytes. Marrow fibrosis and bone abnormalities are frequent, although usually mild in chronic-phase disease.8 The pretreatment bone marrow aspirates and biopsies of all patients in this study showed some or all of these characteristic morphologic findings of CML. The mean values and ranges for the various morphologic parameters measured in the marrow over the course of imatinib mesylate therapy, including marrow cellularity, M/E ratio, and marrow blast percentage, are also summarized in Table 2.
In individual patients, the normalization of the marrow findings tended to lag slightly behind the hematologic response. Of 17 patients showing CHR at 2 months, 12 had persistent morphologic marrow involvement by CML, characterized by myeloid and/or atypical megakaryocytic hyperplasia. However, by 8 to 11 months, the marrow typically showed no morphologic evidence of CML, with normal or mildly decreased cellularity and with resolution of both myeloid and atypical megakaryocytic hyperplasia.
These CHR and marrow responses were sustained in 18 of 19 (95%) patients for 14 months. A single patient, initially treated with 300 mg imatinib mesylate per day, showed a mildly increased WBC count and increased marrow cellularity with recurrent myeloid hyperplasia at 14 months, consistent with morphologic relapse of chronic-phase CML. This patient responded to an increased dose of imatinib mesylate of 600 mg per day and continues in CHR at 29 months after starting imatinib mesylate.
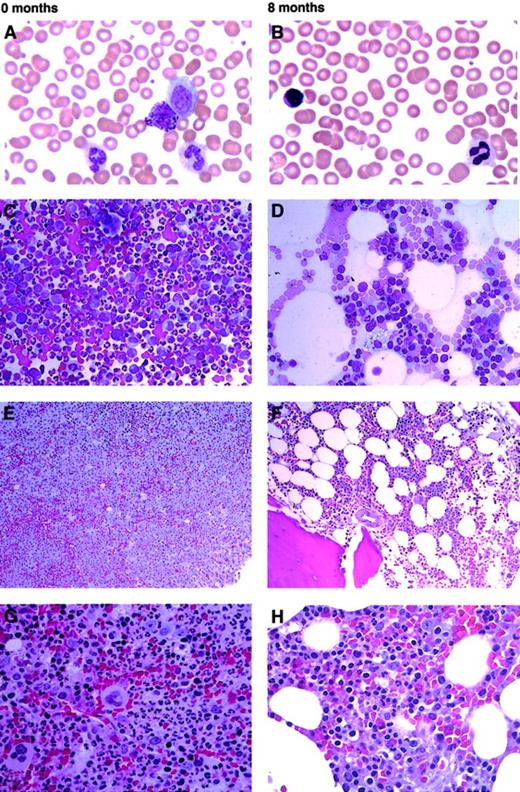
The marrows in imatinib mesylate–treated chronic phase CML patients showed a characteristic pattern of decreasing myeloid cellularity over time, manifested as decreases in overall marrow cellularity and M/E ratio, and resulting in at least a transient myeloid hypoplasia in 90% of patients. The decreased cellularity and proportion of myeloid precursors were most marked in the first 2 months of treatment. The marrow blast percentage also tended to decrease slightly over the same period. At 14 months, 9 patients (47%) had persistent myeloid hypoplasias, associated with normal or only mildly decreased WBC counts (range, 3.2-5.7 × 109/L). The characteristic PB and bone marrow morphologic findings before and following 8 months of imatinib mesylate treatment are illustrated in Figure1.
Typical morphologic findings in PB and marrow of a CML patient prior to treatment and after 8 months on imatinib mesylate.
(A) The pretreatment PB smear shows leukocytosis with circulating granulocytic precursors and basophilia, typical of chronic-phase CML (WBC count, 38.7 × 109/L; platelet count, 207 × 109/L). (B) After 8 months of imatinib mesylate treatment, the PB shows adequate counts and normal morphology (WBC count, 3.5 × 109/L; platelet count, 148 × 109/L). (C) The pretreatment bone marrow aspirate is hypercellular, with marked myeloid hyperplasia and M/E ratio exceeding 10:1. (D) The bone marrow aspirate at 8 months is mildly hypocellular with an M/E ratio of 1:1 owing to decreased myeloid precursors and a relative increase in erythroid precursors. Histiocytes are mildly increased. (E,G) Pretreatment bone marrow biopsy with hypercellularity (more than 95%) and marked myeloid and megakaryocytic hyperplasia, typical of chronic phase CML. (F,H) At 8 months, the biopsy shows borderline hypocellularity (30% to 40%) with mildly decreased myeloid and relatively increased erythroid precursors. Panels A, B show original magnification × 600; panels C, D, × 400; panels E, F, × 200; panels G, H, × 600. Panels A-D are Wright-Giemsa–stained; panels E-H are hematoxylin and eosin–stained.
Typical morphologic findings in PB and marrow of a CML patient prior to treatment and after 8 months on imatinib mesylate.
(A) The pretreatment PB smear shows leukocytosis with circulating granulocytic precursors and basophilia, typical of chronic-phase CML (WBC count, 38.7 × 109/L; platelet count, 207 × 109/L). (B) After 8 months of imatinib mesylate treatment, the PB shows adequate counts and normal morphology (WBC count, 3.5 × 109/L; platelet count, 148 × 109/L). (C) The pretreatment bone marrow aspirate is hypercellular, with marked myeloid hyperplasia and M/E ratio exceeding 10:1. (D) The bone marrow aspirate at 8 months is mildly hypocellular with an M/E ratio of 1:1 owing to decreased myeloid precursors and a relative increase in erythroid precursors. Histiocytes are mildly increased. (E,G) Pretreatment bone marrow biopsy with hypercellularity (more than 95%) and marked myeloid and megakaryocytic hyperplasia, typical of chronic phase CML. (F,H) At 8 months, the biopsy shows borderline hypocellularity (30% to 40%) with mildly decreased myeloid and relatively increased erythroid precursors. Panels A, B show original magnification × 600; panels C, D, × 400; panels E, F, × 200; panels G, H, × 600. Panels A-D are Wright-Giemsa–stained; panels E-H are hematoxylin and eosin–stained.
Megakaryocyte numbers also decreased with imatinib mesylate therapy; megakaryocyte clustering, and the number of atypical small hypolobated megakaryocytes decreased also. However, megakaryocytic hyperplasia was slower to resolve than myeloid hyperplasia, and in many marrows at the 2- and 5-month time points, the abnormalities of megakaryocytes were the only apparent residual manifestation of chronic-phase CML. Megakaryocytic hypoplasias also occurred during the course of imatinib mesylate treatment in 7 of 19 (37%) patients, 3 of whom also had persistent myeloid hypoplasia. Two of these patients were thrombocytopenic (platelet counts of 37 and 99 × 109/L). In contrast to the myeloid and megakaryocytic cell compartments, bone marrow erythroid cellularity typically increased after imatinib mesylate treatment. Relative or absolute erythroid hyperplasia occurred in most patients; the erythroid hyperplasia was usually transient and was seen most commonly at 2 months.
All of the patients had at least mild focal reticulin fibrosis before imatinib mesylate therapy. Eleven of 19 patients were studied over the course of imatinib mesylate therapy with sequential reticulin stains; in 6 of the 11 patients, the fibrosis decreased over time. Other incidental morphologic findings in marrows of imatinib mesylate–treated patients included occasional benign lymphoid aggregates in 42% of patients and the presence of sea-blue histiocytes on aspirates of 47% of patients. The sea-blue histiocytes persisted throughout the treatment, but the number of histiocytes observed tended to decrease. Occasional sea-blue histiocytes are commonly seen in the marrows of CML patients, but the finding is not specific to the disease and is not considered to be a manifestation of residual disease. Mild focal marrow necrosis with stromal injury was observed at 2 months in 4 patients, but was absent in subsequent marrows.
The sequential morphologic bone marrow evaluations in these imatinib mesylate–treated chronic-phase CML patients were complicated after the beginning of therapy by an increasing frequency of inadequate bone marrow aspirations. Good trephine biopsies were therefore critical in achieving adequate marrow evaluations in all patients over the course of the study.
Cytogenetic findings
Cytogenetic responses in this cohort of patients were extremely variable; the best cytogenetic responses at any time for each patient and the percentages of Ph+ metaphases in the marrow after 14 months of imatinib mesylate therapy are shown in Table3. Six patients (32%) had complete cytogenetic responses (no Ph+ metaphases) within 5 to 8 months of starting imatinib mesylate. Six additional patients (32%) had partial cytogenetic responses (1% to 65% Ph+metaphases), observed at 2 months (1 patient), 5 months (2 patients), 8 months (1 patient), and 11 months (2 patients). Of 6 patients with partial responses, 5 showed increasing numbers of Ph+ cells at 14 months. Despite hematologic and marrow morphologic remissions, 7 of the patients (36%) showed no cytogenetic response (more than 65% Ph+ metaphases at all time points).
Summary of hematologic, bone marrow morphologic, and cytogenetic responses to imatinib mesylate in 19 chronic-phase chronic myelogenous leukemia patients
| Patient no. . | Initial dose imatinib mesylate (mg/day) . | Time to CHR, mo . | Time to marrow morphologic remission, mo . | Hematologic status at 14 mo . | Best cytogenetic response . | Ph+ metaphases at best response, % (time observed, mo) . | Ph+ metaphases at 14 mo, % . |
|---|---|---|---|---|---|---|---|
| 1 | 400 | 2 | 5 | CHR | Complete | 0 (5) | 0 |
| 2 | 600 | 2 | 2 | CHR | Complete | 0 (5) | 0 |
| 3 | 600 | 2 | 2 | CHR | Complete | 0 (8) | 0 |
| 4 | 400 | 2 | 5 | CHR | Complete | 0 (8) | 0 |
| 5 | 600 | 2 | 2 | CHR | Complete | 0 (8) | 0 |
| 6 | 600 | 2 | 2 | CHR | Complete | 0 (5) | 0 |
| 7 | 300 | 2 | 8 | CHR | Absent | 100 | 100 |
| 8 | 400 | 2 | 5 | CHR | Absent | 67 | 100 |
| 9 | 350 | 2 | 5 | CHR | Absent | 100 | 100 |
| 10 | 500 | 2 | 5 | CHR | Absent | 95 | No growth |
| 11 | 500 | 2 | 8 | CHR | Absent | 93 | 93 |
| 12 | 500 | 2 | 5 | CHR | Absent | 90 | 90 |
| 13 | 400 | 11 | 5 | CHR | Absent | 100 | 100 |
| 14 | 300 | 2 | 8 | Relapse of CML | Partial | 20 (2) | 40 |
| 15 | 350 | 5 | 8 | CHR | Partial | 8 (11) | 30 |
| 16 | 300 | 2 | 5 | CHR | Partial | 5 (14) | 5 |
| 17 | 350 | 2 | 5 | CHR | Partial | 25 (8) | 35 |
| 18 | 400 | 2 | 5 | CHR | Partial | 5 (8) | 55 |
| 19 | 500 | 2 | 2 | CHR | Partial | 5 (11) | 15 |
| Patient no. . | Initial dose imatinib mesylate (mg/day) . | Time to CHR, mo . | Time to marrow morphologic remission, mo . | Hematologic status at 14 mo . | Best cytogenetic response . | Ph+ metaphases at best response, % (time observed, mo) . | Ph+ metaphases at 14 mo, % . |
|---|---|---|---|---|---|---|---|
| 1 | 400 | 2 | 5 | CHR | Complete | 0 (5) | 0 |
| 2 | 600 | 2 | 2 | CHR | Complete | 0 (5) | 0 |
| 3 | 600 | 2 | 2 | CHR | Complete | 0 (8) | 0 |
| 4 | 400 | 2 | 5 | CHR | Complete | 0 (8) | 0 |
| 5 | 600 | 2 | 2 | CHR | Complete | 0 (8) | 0 |
| 6 | 600 | 2 | 2 | CHR | Complete | 0 (5) | 0 |
| 7 | 300 | 2 | 8 | CHR | Absent | 100 | 100 |
| 8 | 400 | 2 | 5 | CHR | Absent | 67 | 100 |
| 9 | 350 | 2 | 5 | CHR | Absent | 100 | 100 |
| 10 | 500 | 2 | 5 | CHR | Absent | 95 | No growth |
| 11 | 500 | 2 | 8 | CHR | Absent | 93 | 93 |
| 12 | 500 | 2 | 5 | CHR | Absent | 90 | 90 |
| 13 | 400 | 11 | 5 | CHR | Absent | 100 | 100 |
| 14 | 300 | 2 | 8 | Relapse of CML | Partial | 20 (2) | 40 |
| 15 | 350 | 5 | 8 | CHR | Partial | 8 (11) | 30 |
| 16 | 300 | 2 | 5 | CHR | Partial | 5 (14) | 5 |
| 17 | 350 | 2 | 5 | CHR | Partial | 25 (8) | 35 |
| 18 | 400 | 2 | 5 | CHR | Partial | 5 (8) | 55 |
| 19 | 500 | 2 | 2 | CHR | Partial | 5 (11) | 15 |
CHR indicates complete hematologic response; Ph+, Philadelphia chromosome-positive metaphases.
Although our numbers are small and the findings are only preliminary, some differences were noted among patient groups showing different cytogenetic responses. Patients showing complete cytogenetic response to imatinib mesylate tended to be younger (mean, 49.3 years; range, 19-66 years) than patients showing absent cytogenetic response (mean, 64.1 years; range, 52-70 years). Patients with complete cytogenetic responses tended to have a shorter duration of disease before entry into the study (mean, 2.9 years; range, 1.6-4.2 years) than patients showing partial or absent response (mean, 3.7 years; range, 1.1-9.9 years). Patients with prior cytogenetic response to interferon alfa were more likely to show partial or complete response to imatinib mesylate; however, 4 patients with no prior cytogenetic response to interferon alfa achieved partial response (2 patients) or complete response (2 patients) on imatinib mesylate.
Differences in pretreatment laboratory values were also noted for the response groups. Patients showing complete cytogenetic responses tended to have lower WBC counts (mean, 19.2; range, 8.8-38.7 × 109/L) than patients with partial and absent responses (mean, 36.8 and 36.2 × 109/L, respectively; range, 12.9-94.9 × 109/L). Complete responders tended to have lower platelet counts (mean, 267 × 109/L; range, 177-528 × 109/L) than patients with partial and absent responses (mean, 454 and 573 × 109/L, respectively; range, 118-1090 × 109/L). All patients with at least a partial cytogenetic response had no more than 5% marrow blasts prior to starting therapy; the group of 7 patients without any cytogenetic response included 2 patients with slightly increased blasts (8% and 12%).
The presence or absence of a complex Ph karyotype at the beginning of imatinib mesylate therapy did not predict for later cytogenetic response; the 3 patients with complex Ph karyotypes were singly distributed among all 3 response groups. The only patient (no. 10) with clonal evolution identified at beginning of imatinib mesylate therapy was in the absent response group; interestingly, the subclone with the additional t(7;8) cytogenetic abnormality was apparently extremely susceptible to imatinib mesylate therapy and was not detected after treatment was started.
Patients in all 3 response groups typically reached CHR by 2 months. Patients in the complete cytogenetic response group showed resolution of morphologic marrow involvement by 2 months (4 patients) or 5 months (2 patients). Patients with partial and absent cytogenetic responses showed no morphologic evidence of marrow involvement by 2 months (1 patient), 5 months (8 patients), and 8 months (4 patients).
All patients who achieved a complete cytogenetic response at any time during the study were still cytogenetically negative at 14 months. In general, complete cytogenetic response correlated with negative FISH and RT-PCR analyses for BCR-ABL (Table4). Discrepant positive D-FISH and negative RT-PCR results were obtained in one patient with a complex Ph translocation. There are different possible explanations for this discrepancy. It is possible that the PCR primers used might not have detected a variant translocation, but it is more likely that the sample for PCR was compromised by a prolonged interval (more than 48 hours) between the time it was obtained and the time it was processed for RT-PCR. It is very important to establish effective mechanisms for rapid processing of blood and marrow specimens for RT-PCR analysis in CML patients being evaluated for minimal residual disease. There is not sufficient RT-PCR data from this particular cohort of patients for a valid comparison of D-FISH and RT-PCR results. However, a comparison in our laboratory of RT-PCR results with D-FISH assay results from other cohorts of imatinib mesylate–treated CML patients has shown a very good correlation overall, although it is clear that some patients who become D-FISH–negative retain low-level RT-PCR positivity for a variable additional time period (R.M.B., unpublished data, September 2001).
Summary of fluorescent in situ hybridization and reverse-transcription–polymerase chain reaction results in patients with complete cytogenetic responses
| Patient no. . | Best cytogenetic response (time achieved, mo) . | BCR-ABL FISH results (time tested, mo) . | Qualitative RT-PCR for BCR-ABL (time tested, mo) . |
|---|---|---|---|
| 1 | Complete (5) | Positive (5, 8, 11, 14) | Negative (8) |
| 2 | Complete (5) | Negative (5, 8, 11, 14) | Negative (5) |
| 3 | Complete (8) | Negative (8, 11, 14) | Negative (14) |
| 4 | Complete (8) | Negative (8) | N/A |
| 5 | Complete (8) | Negative (8) | N/A |
| 6 | Complete (5) | Negative (8) | N/A |
| Patient no. . | Best cytogenetic response (time achieved, mo) . | BCR-ABL FISH results (time tested, mo) . | Qualitative RT-PCR for BCR-ABL (time tested, mo) . |
|---|---|---|---|
| 1 | Complete (5) | Positive (5, 8, 11, 14) | Negative (8) |
| 2 | Complete (5) | Negative (5, 8, 11, 14) | Negative (5) |
| 3 | Complete (8) | Negative (8, 11, 14) | Negative (14) |
| 4 | Complete (8) | Negative (8) | N/A |
| 5 | Complete (8) | Negative (8) | N/A |
| 6 | Complete (5) | Negative (8) | N/A |
FISH indicates fluorescent in situ hybridization; RT-PCR, reverse-transcription– polymerase chain reaction; N/A, results not available for these patients.
Five patients with partial (4 patients) or absent (1 patient) cytogenetic response showed significant changes in karyotype while being treated with imatinib mesylate; the changes are detailed in Table5. Two patients showed evidence of clonal evolution in Ph+ cells while on therapy; one patient had acquisition of an isochromosome 17 and one developed a t(12;12) translocation. One patient developed trisomy 8 in a second clone, unrelated to the Ph+ clone. One additional patient, although not demonstrating evidence of a clonal trisomy 8 abnormality, has had single cells with +8 detected (with and without the Ph).
Changes in karyotype observed during course of treatment with imatinib mesylate
| Patient no. . | Pretreatment karyotype [no. positive metaphases] . | Karyotypic change during imatinib mesylate treatment (best cytogenetic response) . |
|---|---|---|
| 10 | 46,XX,t(7;8)(q22;q13), t(9;22)(q34;q11) [15]/ 46,XX,t(9;22)(q34;q11) [5]/ 46,XX [1] | Loss of 46,XX,t(7;8)(q22;q13), t(9;22) clone (0/20 cells by 8 mo) with persistent 46,XX, t(9;22)(q34;q11) clone (absent cytogenetic response) |
| 12 | 46,XY,t(22;9)(9;13;1)(q11;q34;q32;q21;q31)[19]/ | Loss of 47,XY,t(22;9)(9;13;1)+ 8 (1 cell only) with persistent 46,XY,t(22;9)(9;1;13) clone |
| 47,XY,t(22;9)(9;13;1)(q11;q34;q32;q21;q31),+ 8 [1] | Addition of 47,XY,+ 8 (1/20 cells at 14 mo) (absent cytogenetic response) | |
| 15 | 46,XY,t(9;22)(q34;q11) [22] | 46,XY, t(9;22)(q34;q11),− 9,+ l(17)(q11) (in 2/10 cells at 14 mo) (partial cytogenetic response) |
| 16 | 46,XX,t(9;22)(q34;q11) [17] | 47,XX,+ 8[5/8 cells at 11 mo;19/20 cells at 14 mo] |
| Persistent 46,XX, t(9;22)(q34;q11) 1/20 (partial cytogenetic response) | ||
| 17 | 46,XX,t(9;22)(q34;q11) [20] | 46,XX,t(9;22)(q34;q11),t(12;12)(q13;q24.3) (4/20 cells at 8 mo; 1/20 cells at 17 mo) (partial cytogenetic response) |
| Patient no. . | Pretreatment karyotype [no. positive metaphases] . | Karyotypic change during imatinib mesylate treatment (best cytogenetic response) . |
|---|---|---|
| 10 | 46,XX,t(7;8)(q22;q13), t(9;22)(q34;q11) [15]/ 46,XX,t(9;22)(q34;q11) [5]/ 46,XX [1] | Loss of 46,XX,t(7;8)(q22;q13), t(9;22) clone (0/20 cells by 8 mo) with persistent 46,XX, t(9;22)(q34;q11) clone (absent cytogenetic response) |
| 12 | 46,XY,t(22;9)(9;13;1)(q11;q34;q32;q21;q31)[19]/ | Loss of 47,XY,t(22;9)(9;13;1)+ 8 (1 cell only) with persistent 46,XY,t(22;9)(9;1;13) clone |
| 47,XY,t(22;9)(9;13;1)(q11;q34;q32;q21;q31),+ 8 [1] | Addition of 47,XY,+ 8 (1/20 cells at 14 mo) (absent cytogenetic response) | |
| 15 | 46,XY,t(9;22)(q34;q11) [22] | 46,XY, t(9;22)(q34;q11),− 9,+ l(17)(q11) (in 2/10 cells at 14 mo) (partial cytogenetic response) |
| 16 | 46,XX,t(9;22)(q34;q11) [17] | 47,XX,+ 8[5/8 cells at 11 mo;19/20 cells at 14 mo] |
| Persistent 46,XX, t(9;22)(q34;q11) 1/20 (partial cytogenetic response) | ||
| 17 | 46,XX,t(9;22)(q34;q11) [20] | 46,XX,t(9;22)(q34;q11),t(12;12)(q13;q24.3) (4/20 cells at 8 mo; 1/20 cells at 17 mo) (partial cytogenetic response) |
The patient (no. 14) who relapsed at 14 months while on 300 mg imatinib mesylate per day had a complex Ph translocation and was in the partial response group. On an increased dose of imatinib mesylate, she achieved a second CHR and bone marrow morphologic remission. She has shown no evidence of clonal evolution and continues in CHR with no morphologic evidence of marrow involvement and continued partial cytogenetic response after a total of 29 months on study.
Discussion
Imatinib mesylate, the first molecularly targeted therapy for CML, produces a rapid and dramatic resolution of the PB abnormalities associated with CML. The hematologic responses have been reported to occur typically within 2 weeks after initiation of imatinib mesylate treatment, with CHR by 4 weeks.6 However, details of the specific bone marrow morphologic and cytogenetic findings that occur in imatinib mesylate–treated patients over a prolonged treatment interval have not been previously described. In this study, we report the detailed sequence and timing of the PB and bone marrow changes that occur reproducibly in chronic phase CML patients treated with an effective dose of imatinib mesylate over a minimum period of 14 months. This cohort of patients is part of the first phase 1 trial of imatinib mesylate, and these patients have therefore been on imatinib mesylate longer than any other group of imatinib mesylate–treated patients.6
This study demonstrates the novel finding that, in contrast to other nontransplantation therapies for CML, the bone marrows of imatinib mesylate–treated CML patients show little or no morphologic evidence of CML beyond the first 5 months.9 10 Surprisingly, even in this cohort of interferon alfa–resistant or interferon alfa–intolerant CML patients, many of whom had also received treatment with hydroxyurea, imatinib mesylate produced rapid and complete bone marrow morphologic remission. The myeloid hyperplasia associated with CML typically resolves completely by 2 months, but atypical megakaryocytic hyperplasia persists for up to 5 months in some patients. These hematologic and marrow responses have been maintained in 18 of 19 (95%) of our patients for at least 14 months. This series of patients, to our knowledge, represents the longest follow-up interval of imatinib mesylate–treated patients.
The characteristic morphologic and cytogenetic findings in imatinib mesylate–treated chronic-phase CML patients are very different from those of CML patients receiving other therapies; this has important ramifications for hematologists, hematopathologists, and cytogeneticists following imatinib mesylate–treated patients. The normalization of the marrow seen in imatinib mesylate–treated chronic-phase CML patients is in marked contrast to CML patients treated with previous chemotherapeutic agents. Therapy of CML with hydroxyurea or interferon alfa typically results in improvement of peripheral counts, but bone marrow abnormalities usually persist.11,12 Between 40% and 80% of patients on hydroxyurea achieve CHR, but the bone marrows in these patients are persistently hypercellular, even in complete responders, and there are pronounced megaloblastic changes. On interferon alfa, 60% to 80% of chronic-phase CML patients achieve CHR, but marrows are still usually at least mildly hypercellular. In one study, a reversion to normal marrow histology was reported in only 14 of 51 (27%) of biopsies from patients treated with interferon alfa,9 clearly a much lower percentage of marrow remission than in our series of imatinib mesylate–treated patients. As the single instance of relapse in our series shows, relapse on imatinib mesylate is manifested by increasing WBC counts, increasing marrow cellularity, and recurrent myeloid and/or megakaryocytic hyperplasia.
Possible mechanisms of resistance to imatinib mesylate are largely unknown. Preliminary data in cell lines and in patients with advanced-stage CML have suggested that the resistance to imatinib mesylate may be mediated by BCR-ABL gene amplification or point mutations of the BCR-ABL kinase, with reactivation of kinase activity.11-14 The relapsed patient in our series, although having a complex Ph+ translocation, did not have additional cytogenetic abnormalities before or during therapy and has responded to an increased dose of imatinib mesylate.
It has been speculated that the acquisition of additional cytogenetics abnormalities may confer resistance to imatinib mesylate. However, in the single instance of pretreatment clonal evolution in our study, a Ph+ clone with an additional t(7;8) showed apparent increased susceptibility to imatinib mesylate, not resistance. The patient continues to have 95% Ph+ cells (absent cytogenetic response), but has no evidence of the t(7;8) clone, and continues with complete hematologic and marrow response.
It is important to note that, unlike chronic-phase CML patients in morphologic remission at 5 to 8 months after transplantation, most patients on imatinib mesylate will still have detectable disease not only by sensitive D-FISH and RT-PCR analyses, but also by routine metaphase cytogenetics at 5 to 8 months after beginning therapy. CHR and normalization of marrow findings do not predict cytogenetic response in imatinib mesylate–treated patients. In this study, 18 of 19 patients had CHR and bone marrow morphologic remissions, yet 6 of these patients (36%) remained 66% to 100% Ph+ at 14 months, and 7 additional patients (32%) still had 5% to 25% Ph+ cells at best response. These data suggest that despite persistence of the Ph+ clone, inhibition of the BCR-ABL tyrosine kinase activity of imatinib mesylate restores normal regulation of cell numbers to the myeloid compartment.
Complete cytogenetic responses did occur in one third of our patients by 5 to 8 months. This subset of patients has been stable, and they have not shown acquisition of other cytogenetic abnormalities to date. These preliminary observations suggest that such patients might be safely followed by CBC, RT-PCR, and FISH studies to monitor disease status.
Patients showing at best a partial cytogenetic response at 2, 5, 8, and 11 months tended to have an increasing number of Ph+metaphases at 14 months, suggesting that most will not reach a complete cytogenetic response. We also report the acquisition of additional clonal cytogenetic abnormalities in 3 patients while in complete morphologic remission on effective doses of imatinib mesylate. Thus, despite hematologic and marrow remissions, our series shows that patients with partial or absent cytogenetic responses are still at risk for clonal evolution, which occurred relatively late in imatinib mesylate therapy in our patients, at 8 to 14 months. The significance of the clonal evolution in this setting is uncertain, but it is certainly worrisome for disease progression or development of myelodysplasia. These observations suggest that optimal monitoring of patients with partial or absent cytogenetic response will require periodic metaphase cytogenetic analysis.
Interestingly, 2 of the patients presented in this series developed trisomy 8 independently of the Ph+ clone following treatment with imatinib mesylate. We have also seen this in 3 other patients in the phase 1 trial (M.E.O., unpublished data, April 2002). While the development of trisomy 8 is a common feature of clonal evolution with disease progression in CML, the finding of metaphases containing trisomy 8 as the sole abnormality, independent of the Ph+ clone, is distinctly unusual in these patients. Through its antileukemic effect, imatinib mesylate may be unmasking abnormal residual hematopoiesis in these patients. Because these patients all had late chronic-phase CML, and most had a history of extensive prior exposure to interferon alfa or other chemotherapy, it is tempting to speculate that prior therapy exerted an inhibitory effect on normal stem cells, providing the necessary selective pressure for outgrowth of an abnormal resistant Ph− clone.
The documentation of complete hematologic and marrow responses in all of these patients and major cytogenetic responses in 12 (63%) holds promise that molecularly targeted therapy with imatinib mesylate may actually alter the natural history of disease in chronic-phase CML. However, our experience is still limited and the observations are preliminary. Additional studies of this earliest cohort of patients treated with imatinib mesylate may continue to offer new insights into pathogenesis, management, and laboratory monitoring of CML.
The authors acknowledge the contributions of Dr P. Nagesh Rao (University of California at Los Angeles; BCR-ABL FISH results) to this work.
Supported by grants from the National Cancer Institute (B.J.D.), a Translational Research Award from the Leukemia and Lymphoma Society (B.J.D.), and a Clinical Scientist Award from the Burroughs Wellcome Fund (B.J.D.).
R.M.B. and T.M.L. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Rita M. Braziel, Dept of Pathology, L471, Oregon Health Sciences University, 3181 SW Sam Jackson Park Rd, Portland, OR 97201; e-mail: braziel@ohsu.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal