A recent article by Steffens et al1 assessed whether the IL-7 cytokine promotes productive HIV-1 infection in naive CD4+ T cells. This is an important biologic issue because, while most circulating peripheral T lymphocytes are in the G0 state, the productive infection of both naive and memory CD4+ T cells with HIV-1, at least outside the context of lymphoid tissues,2 requires a progression into the G1b stage of the cell cycle.3 It is assumed that, in vivo, HIV-1 infection occurs in CD4+ T cells that are cycling because they have recently been stimulated by their cognate antigen. One quandary that arises from this hypothesis is that those T cells stimulated through their T-cell receptor (TCR) attain characteristics of memory lymphocytes and lose their naive phenotype. The detection of HIV-1 in naive T cells in vivo, albeit at significantly lower levels than in the memory T-cell subset,4-7 raises questions regarding the mechanisms controlling HIV-1 infection in the former population. Thus it is possible that a cytokine such as IL-7, which does not significantly modify the naive phenotype of T cells,8-10 may be involved in this process.
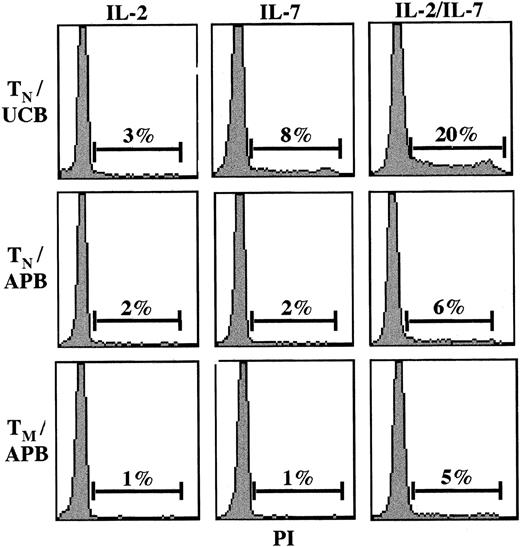
A study of HIV-1 infection in the context of IL-7 stimulation requires an understanding of the biologic effects of IL-7. But while Steffens et al reported that naive human CD4+ T cells proliferated in response to IL-7 in vitro, our 2 groups have independently found that naive CD4+ T cells do not proliferate under these conditions.11,12 The recent data of Geginat et al13 support these observations; they find that in vitro IL-7 synergizes with IL-15 in driving the proliferation of memory CD4+ T cells but has no effect on naive T cells.13 Indeed, we determined that only recent thymic emigrants, naive CD4+ T cells isolated from umbilical cord blood, proliferate significantly in response to IL-7 in vitro.11,12 Moreover, even under conditions where T lymphocytes are cultured in the presence of both IL-2 and IL-7 cytokines, only recent thymic emigrants, and not naive CD4+T cells, enter into the S or G2 phases of the cell cycle at significant levels (Figure 1). Finally, using a technique that permits simultaneous DNA and RNA quantitation, we previously demonstrated that, although 10% to 20% of IL-7–stimulated memory CD4+ T cells progress to the G1b phase of the cell cycle, only 3% to 5% of naive CD4+ T cells progress past G1a.12
The IL-2 and IL-7 cytokines preferentially induce cell-cycle entry in recent thymic emigrants.
Naive (CD45RA+; TN) and memory (CD45RO+; TM) CD4+-resting T lymphocytes were isolated from umbilical cord blood (UCB) and adult peripheral blood (APB) by negative selection. Cells were cultured in vitro in the presence of IL-2 (100 U/mL) and/or IL-7 (10 ng/mL), and cell-cycle entry was monitored at day 4 by assessing DNA content of propidium iodide–stained cells on a FACScan cytometer (Becton Dickinson, San Jose, CA). The percentage of cells in the S and G2/M phases of the cell cycle are indicated, and results are representative of data obtained in 1 of 3 representative experiments.
The IL-2 and IL-7 cytokines preferentially induce cell-cycle entry in recent thymic emigrants.
Naive (CD45RA+; TN) and memory (CD45RO+; TM) CD4+-resting T lymphocytes were isolated from umbilical cord blood (UCB) and adult peripheral blood (APB) by negative selection. Cells were cultured in vitro in the presence of IL-2 (100 U/mL) and/or IL-7 (10 ng/mL), and cell-cycle entry was monitored at day 4 by assessing DNA content of propidium iodide–stained cells on a FACScan cytometer (Becton Dickinson, San Jose, CA). The percentage of cells in the S and G2/M phases of the cell cycle are indicated, and results are representative of data obtained in 1 of 3 representative experiments.
It should nevertheless be noted that all studies assessing the effects of recombinant IL-7 on human T-cell subsets, including our own, may be biased because they have been performed ex vivo. In mice, homeostatic T-cell proliferation is readily detectable, but only under conditions of lymphopenia. In a lymphopenic environment, proliferation of naive T cells in mice is conditional on TCR/selfpeptide/major histocompatibility complex (MHC)–derived signals14-22 but is also strictly dependent on the presence of endogenous IL-7.23,24 Proliferation of T cells in nonlymphopenic mice can be induced by injection of high doses of recombinant IL-7, resulting in cell-cycle entry of a low percentage of CD4+ T cells (approximately 4%) and a higher percentage of CD8+ T cells (10%-11%).25 Thus, in interpreting the in vitro studies performed with human T cells, it is important to take into account the limitations resulting from the absence of in vivo factors that likely cooperate with IL-7 in driving cell-cycle progression in lymphoid organs.
One technical difference that may explain the apparent discrepancies between the respective in vitro human T-cell studies performed by our groups11,12 and that performed by Steffens et al concerns the manner in which CD4+ T cells were selected. In order to eliminate confounding effects due to in vivo activated T cells, we removed T lymphocytes expressing the HLA-DR activation marker with a monoclonal antibody. HLA-DR+ cells account for 2% to 10% of the T-cell population, depending on the donor. Under conditions where these cells are removed, purified CD4+ T cells do not proliferate in response to either IL-7, the phytohemagglutinin (PHA) lectin, or a mitogenic anti-CD3 mAb.3,11,12These data are pertinent to HIV-1 infection because Korin and Zack found that, under conditions where CD4+ T cells are stimulated with an α-CD3 mAb, the presence of HLA-DR+cells modulates HIV infection: productive HIV infection is observed in the presence of HLA-DR+ cells, whereas in their absence, infection is blocked.3 Thus the HIV-1 infection observed by Steffens et al in IL-7–treated naive CD4+ T cells may be specific to their experimental conditions. Furthermore, Steffens et al found that only naive T cells from 4 of 6 donors were responsive to IL-7–mediated HIV infection, and as such it is possible that this discrepancy results from differences in the percentages of activated T cells present in these individuals at a given time. This hypothesis is attractive since IL-7 is not likely to exert its effect at the level of enhanced viral entry; while IL-7 up-regulated expression of the HIV gp120 coreceptor CXCR4, this phenomenon was also observed in T cells from donors who were resistant to IL-7–mediated infection.1
In many of the experiments presented by Steffens et al, CD8+ T cells were maintained in the culture, and it is therefore possible that the capacity of naive CD4+ T cells to be infected by HIV-1 following IL-7 treatment is modulated by the presence of CD8+ T cells and/or activated T cells. Indeed, both in vitro and in vivo, CD8+ T cells appear to undergo significantly more division in response to IL-7.1,25Further studies will be necessary to distinguish the effects of IL-7 on naive CD4+ T cells in the context of distinct hematopoietic cell populations. Finally, it will be important to elucidate how the cellular environment in lymph nodes and the peripheral circulation differentially regulates the responsiveness of naive T cells to IL-7 and HIV-1 infection. Specifically, the in vivo data obtained in mice suggest that the effects of IL-7 may differ in conventional in vitro cultures and in the context of the 3-dimensional architecture and cellular environment present in lymphoid tissues.24 25 The data obtained from the ensemble of these experiments will allow us to determine whether, and under what conditions, recombinant IL-7 may be beneficial as an immune modulator in HIV-infected individuals.
IL-7 modulation of HIV-1 infection of naive T cells
In our published report, we provided data that IL-7 induces the productive infection of HIV-1 in the naive CD4+ T-cell compartment.1-1 Jaleco et al, based on a report by Korin and Zack,1-2 state that productive infection requires that the cell enter the G1b phase of the cell cycle. While we have not directly evaluated the stage of the cell cycle that these HIV+ naive T cells are in, others have recently reported that resting naive T cells, albeit in lymphoid tissue explant model, can still support HIV infection without progressing past the G1b phase of the cell cycle.1-3 This productive infection, however, was dependent on the environmental milieu of these lymphoid tissues.1-3 We believe that IL-7 is probably a key cytokine in promoting this productive infection. Kinter et al have also reported that the microenvironment of thymic ex vivo cultures mediates HIV infection of resting T cells.1-4
Another key point is that data from Jaleco and colleagues demonstrate that IL-7 does not induce the proliferation of naive T cells beyond G1a. This finding is not in conflict with our own published data. We have found that treatment of naive CD4+ T cells with IL-7 does lead to a small subpopulation of naive T cells, approximately 4%, that entered the cell cycle, as determined by intracellular Ki-67 staining. It is important to note that Ki-67 is a nuclear protein present in cells once they have passed G0and entered the cell cycle, but will not identify the exact stage of the cell cycle. Therefore, it is possible that this subpopulation may not have progressed past the G1a stage, as Jaleco et al report in their letter. We are in the process of determining whether this small population reflects the infected fraction of the naive CD4+ T cells. Nonetheless, this 4% cycling naive subpopulation in response to IL-7 has not acquired CD45RO expression (unpublished data). Preliminary phenotypic analysis from our laboratory of IL-7–treated naive T cells also indicates that they do not up-regulate CD69 or HLA-DR expression.
Jaleco et al suggest that the infected population may be that which initially expressed HLA-DR, which is an attractive hypothesis. But, though in the report published we have not depleted for HLA-DR expression, phenotypic analysis of the purified naive T-cell population reflected less than 2% of CD45RO-depleted cells to express HLA-DR. Others have depleted for HLA-DR expression, to isolate resting cells, and have been able to detect intracellular p24 expression within resting/quiescent naive T cells.1-3 1-4 Jaleco et al also suggested that maintaining CD8+ T cells in these experiments may impact the ability of IL-7 to modulate HIV infection of CD4+ naive T cells. But even in experiments where CD8+ T cells were depleted, we were still able to demonstrate IL-7 enhancement of HIV replication in the naive CD4+ T-cell compartment, as defined by CD4+CD45RA+CD45RO− expression (unpublished data). Additionally, we were able to also demonstrate that IL-7 can induce HIV replication in CD4+ memory (CD4+CD45RA−CD45RO+) and even total peripheral blood mononuclear cells (PBMCs).
IL-7 is an attractive immune-modulator. But prior to clinical studies utilizing this cytokine, a clear understanding of its role in modulating HIV replication in resting/naive T cells and even on other cellular fractions needs to be elucidated.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal