The biologic mechanisms involved in the pathogenesis of multiple myeloma (MM) bone disease are not completely understood. Recent evidence suggests that T cells may regulate bone resorption through the cross-talk between the critical osteoclastogenetic factor, receptor activator of nuclear factor-κB ligand (RANKL), and interferon γ (IFN-γ) that strongly suppresses osteoclastogenesis. Using a coculture transwell system we found that human myeloma cell lines (HMCLs) increased the expression and secretion of RANKL in activated T lymphocytes and similarly purified MM cells stimulated RANKL production in autologous T lymphocytes. In addition, either anti–interleukin 6 (anti–IL-6) or anti–IL-7 antibody inhibited HMCL-induced RANKL overexpression. Consistently, we demonstrated that HMCLs and fresh MM cells express IL-7 mRNA and secrete IL-7 in the presence of IL-6 and that bone marrow (BM) IL-7 levels were significantly higher in patients with MM. Moreover, we found that the release of IFN-γ by T lymphocytes was reduced in presence of both HMCLs and purified MM cells. Furthermore, in a stromal cell–free system, osteoclastogenesis was stimulated by conditioned medium of T cells cocultured with HMCLs and inhibited by recombinant human osteoprotegerin (OPG; 100 ng/mL to 1 μg/mL). Finally, RANKL mRNA was up-regulated in BM T lymphocytes of MM patients with severe osteolytic lesions, suggesting that T cells could be involved at least in part in MM-induced osteolysis through the RANKL overexpression.
Introduction
Multiple myeloma (MM) is a plasma cell malignancy localized in the bone marrow (BM) and characterized by the high capacity to induce bone destruction. Almost all patients with MM show early osteolytic lesions that result from an increased bone resorption due to the stimulation of osteoclast recruitment and activity in close contact with myeloma cells.1 However, the biologic mechanisms involved in the pathogenesis of MM-induced bone disease are still unclear.
In recent years, the osteoprotegerin ligand, namely, receptor activator of nuclear factor-κB ligand (RANKL) or tumor necrosis factor (TNF)–related activation-induced cytokine (TRANCE), has been identified as critical in the activation of osteoclastic cells.2 RANKL is expressed by stromal/osteoblastic cells and activated T lymphocytes and directly induces osteoclast differentiation and activation by binding to its receptor (RANK) on osteoclastic cells.2,3 The effects of RANKL are blocked by osteoprotegerin (OPG), a soluble decoy receptor produced by osteoblastic cells to preserve bone mass.3 Our recent data4 demonstrate that human myeloma cells do not directly express RANKL mRNA or produce RANKL protein but induce RANKL overexpression and inhibit OPG production in the BM environment, as reported by Pearse et al.5 In contrast, other authors have shown the presence of RANKL protein in BM myeloma cells and in myeloma cell lines.6 7 However, high RANKL production in the BM of patients with MM seems to be involved in the activation of osteoclastic cells.
Growing evidence suggests that T lymphocytes may regulate bone resorption and maintain bone homeostasis through the cross-talk between RANKL and interferon γ (IFN-γ), a cytokine that strongly suppresses osteoclastogenesis.8-10 In physiopathologic conditions, such as arthritis, activated T cells are capable of regulating bone loss through the expression of RANKL.11 In addition, recent data highlight the involvement of RANKL expressed by T lymphocytes in the mechanism of hypercalcemia in adult T-cell leukemia.12 These observations prompted us to investigate the potential effect of human myeloma cells on RANKL expression and IFN-γ secretion by T lymphocytes. We found that myeloma cells up-regulate RANKL production and decrease IFN-γ secretion in T lymphocytes and that BM CD3+ cells, obtained from MM patients with extensive skeletal destruction, express RANKL mRNA. These data highlight, for the first time, the possible contribution of T lymphocytes to the pathogenesis of MM-induced osteoclastic cells activation and confirm the critical role of RANKL in MM bone disease.
Patients, materials, and methods
Reagents
Recombinant human interleukin 6 (rhIL-6) was obtained from Endogen (Woburn, MA). Recombinant human OPG (rhOPG) was purchased from R & D Systems (Minneapolis, MN). Anti-CD3 and anti-CD28 monoclonal antibodies (mAbs) were obtained from Becton Dickinson Immunocytometry Systems (San Jose, CA).
Cells and culture conditions
Human cell lines.
Human myeloma cell line (HMCL) XG-6 was established in Dr Bataille's laboratory (INSERM U463, Nantes, France) and cultured as previously described.13 HMCL U266 and osteosarcoma cell line Saos-2 were obtained from the American Type Culture Collection (Rockville, MD); HMCL RPMI-8226, OPM-2, and human B-cell leukemia cell line REH were purchased from DSM (Brunswick, Germany). Epstein-Barr virus-positive (EBV+) cells, established from healthy subjects, were a kind gift from Dr P. Sansoni (University of Parma, Italy). For some experiments, human cell lines XG-6, RPMI-8226, U266, REH, and EBV+ cells (5 × 106 cells) were incubated in the presence or absence of IL-6 (20 ng/mL) in 5 mL RPMI 1640 medium supplemented with 2% heat-inactivated fetal bovine serum (FBS; Gibco Invitrogen, Milan, Italy), penicillin (50 U/mL), streptomycin (50 μg/mL), and glutamine (2 mM) for 24 hours.
Cell cultures.
CD3+ cells, T-lymphocyte subsets CD4+ and CD8+, and CD19+ B cells were purified using an immunomagnetic method (MACS Miltenyi, Bergisch Gladbach, Germany) from peripheral blood (PB) and BM mononuclear cells (MNCs) obtained from healthy subjects and patients with MM, acute B-cell leukemia, and B lymphoma with BM involvement. T-cell activation was performed incubating 2 × 106 MNCs or CD3+ cells in 6-well plates precoated with anti-CD3 (0.1 μg/well) plus CD28 (5 μg/well) mAb for 2 to 4 days. U266 or RPMI-8226 (5 × 106 cells) were cocultured with activated T cells (2 × 106) in a transwell system (0.45-μM pore size; Falcon, Becton Dickinson, Oxford, United Kingdom) for 24 to 48 hours in 5 mL complete medium in the presence or absence of anti–human IL-6 mAb (clone 6708.111; R & D Systems; 10 μg/mL) or anti–IL-7 polyclonal antibody (Peprotech, London, United Kingdom; 0.03 μg/mL) or anti-IgG. At the end of the coculture period mRNA and cellular proteins were extracted from T lymphocytes. Fresh MM cells were purified from patients at diagnosis or relapse using an anti-CD138 mAb as previously described.14 Purified MM cells (5 × 106) were cocultured with autologous purified CD3+ T lymphocytes (2 × 106) in the above-mentioned experimental conditions.
Osteoclastogenesis
Human osteoclastogenesis was assessed using both previously described methods.15,16 Briefly, PB human CD34+-enriched stem cells (5 × 105), obtained by a single leukapheresis of healthy donors treated with granulocyte colony-stimulating factor (G-CSF) for 3 to 5 days,15 or human PB MNCs at high density (1.5 × 106), were incubated in a stromal cell–free system with conditioned medium of activated T cells cocultured with HMCLs or purified MM cells in the presence or absence of rhOPG (100 ng/mL and 1 μg/mL) or anti–IL-7 polyclonal antibody (0.03 μg/mL) or anti-IgG for 10 days. At the end of the culture period osteoclasts (OCs) were identified as tartrate-resistant acid phosphatase (TRAP+) multinucleated cells (Sigma-Aldrich, Milan, Italy) according to the manufacturer's instructions. More than or equal to 3 nuclei TRAP+ cells were defined as mature OCs. Multinucleated TRAP+ cells also expressed calcitonin receptor and RANK.
Patients
We studied 8 women and 7 men (mean age, 62 years; range, 53-73 years) with newly diagnosed MM (stages I-III) and 20 sex- and age-matched controls. BM and PB samples were obtained after informed consent was given. Approval was obtained from the Institutional Review Board of the University of Parma for these studies. Informed consent was provided according to the Declaration of Helsinki. BM CD3+ T lymphocytes were purified from patients at diagnosis or relapse and from controls. Control lymphocytes were obtained from diagnostic BM samples for nonneoplastic diseases without any skeletal involvement. Only cell populations with a purity of more than 95% were tested.
RNA isolation and RT-PCR amplification
For reverse transcription–polymerase chain reaction (RT-PCR) analysis, total cellular RNA was extracted from cells using Trizol reagent (Gibco). Then, 1 μg RNA was reverse-transcribed with 400 U Moloney murine leukemia virus reverse transcriptase (Gibco) according to the manufacturer's protocol. cDNAs were amplified by PCR with the following specific primer pairs. RANKL: sense: 5′-AGCACATCAGAGCAGAGAAAGC-3′, antisense: 5′-CAGTAAGGAGGGGTTGGAGACC-3′; IL-7: sense: 5′-TTTTATTCCGTGCTGCTCGC-3′, antisense: 5′-GCCCTAATCCGTTTTGACCA-3′; and β2-microglobulin: sense: 5′-CTCGCGCTACTCTCTTCTCTTTCTGG-3′, antisense: 5′-GCTTACATGTCTCGATCCCACTTAA-3′. PCRs were performed in a thermal cycler (MiniCycler MyResearch, Watertown, MA) for 30 cycles (annealing temperature: 60°C, 66°C, 63°C for RANKL, IL-7, and β2-microglobulin, respectively). Then, 10 μL of each amplified reaction was electrophoresed through a 2% agarose gel, stained with ethidium bromide (1 μ/mL) in 1 times TBE buffer (0.1 M Tris [tris(hydroxymethyl)aminomethane], 90 mM boric acid, 1 mM EDTA [ethylenediaminetetraacetic acid], pH 8.4) and visualized under UV light. Product size (464 base pair (bp) for RANKL, 429 bp for IL-7, and 334 bp for β2-microglobulin) was established by comigration with a 100-bp ladder marker (Gibco). Pictures of the electrophoresed cDNAs were recorded with a digital DC 120 Kodak camera and quantified by ID Image Analysis Software (Kodak Digital Science-Eastman Kodak, Rochester, NY). To analyze the effects of the experimental conditions independently of variability due to the amount of cDNA amplified, the signal of specific cDNAs was normalized to the respective signal of β2-microglobulin which expression was found to be comparable under all the conditions used. To confirm the proper identity, PCR products of the appropriate size were directly sequenced.
Western blot analysis
Western blot analysis for RANKL expression was performed as previously described.4 T lymphocytes alone or in transwell cocultures with HMCLs or fresh MM cells were resuspended in 100 μL lysis buffer (10 mM Tris-HCL, pH 7.6, 150 mM NaCl, 5 mM EDTA, 2 mM phenylmethylsulfonyl fluoride [PMSF], 2 mg/mL aprotinin [Sigma, St Louis, MO], and 1% Triton X-100). COS-7 cells lipofected with plasmid expression vector pCEP4, pCEP4-murine RANKL (Amgen, Thousand Oaks, CA), were used as positive controls. Protein levels were determined using a standard procedure (Uptima, Interchim, France). After 40 minutes on ice, lysates were cleared by centrifugation at 12 000g for 30 minutes at 4°C. Proteins (70 μg) were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) using 10% polyacrylamide gels and transferred onto polyvinylidene difluoride membrane. After blocking, membranes were incubated overnight with a monoclonal anti-RANKL antibody (1.5 μg/mL) obtained from R & D Systems. After washing, membranes were incubated with a horseradish peroxidase (HRP)–conjugated goat antimouse antibody (1:10 000; Becton Dickinson) at room temperature for 30 minutes. Blots were then developed using the Supersignal West Dura Extended Duration Substrate detection system (Pierce, Rockford, IL).
Immunoreactive bands were visualized by a 5-minute exposure (Kodak X-OMAT). The intensity of each band was quantified by ID Image Analysis Software. To detect soluble RANKL (sRANKL), immunoprecipitation of conditioned media was performed using RANK-Fc (R & D Systems). Briefly, 1 mL supernatant was incubated with 0.5 μg RANK-Fc for 1 hour at 4°C; then, 20 μL protein G PLUS–agarose (Santa Cruz Biotechnology, Santa Cruz, CA) was added and incubated with mixing for 2 hours at 4°C. Pellets were collected by centrifugation and washed 4 times with RIPA buffer (Tris-HCL 100 mM, NaCl 150 mM, EDTA 1 mM, sodium deoxycholate 1%, SDS 0.1%, Triton X-100 1%). After the final wash, the immunoprecipitated material was recovered by boiling in sample loading buffer and separated by electrophoresis. Gel was stained with a silver stain plus kit (Bio-Rad Laboratories, Milan, Italy) to detect the presence of sRANKL. In addition, an immunoblot analysis was performed using the above-mentioned procedure.
ELISA
The amount of sRANKL, IFN-γ, IL-6, IL-1, TNF-α, IL-7, and IL-11 in conditioned media, BM plasma, and PB serum was determined by commercially available enzyme-linked immunosorbent assay (ELISA) kits. The assay kit for RANKL was from Biomedica (Vienna, Austria). IFN-γ, IL-6, IL-1, and TNFα assay kits were obtained from Endogen. IL-7 and IL-11 kits were purchased from R & D Systems. The sensitivity range of ELISA tests was 0.4 to 50 pmol/L (8-1000 pg/mL) for RANKL, 25.6 to 1000 pg/mL for IFN-γ, 10.24 to 400 pg/mL for IL-6, 10.2 to 400 pg/mL for IL-1, 15.6 to 1000 pg/mL for TNF-α, 0.156 to 20 pg/mL for IL-7, and 15.6 to 1000 pg/mL for IL-11. The intra-assay and interassay coefficients of variation (CV) were of 5% and 7% for RANKL; 2% and 6% for IFN-γ; less than 10% for IL-6, IL-1, and TNFα; 3.3% and 7.8% for IL-7; and 2.4% and 6.6% for IL-11. ELISA for sRANKL is an enzyme immunoassay designed to determine soluble uncomplexed human RANKL. sRANKL binds to the precoated recombinant OPG and forms a sandwich with the detection antibody. The detection antibody is a rabbit antihuman soluble RANKL that is specific for RANKL with a negligible cross-reactivity (< 1%) to human TNF-related apoptosis-inducing ligand (TRAIL), as certified by the producer.
Statistical analysis was performed using analysis of variance (ANOVA) for repeated measurements, followed by a Tukey-Kramer test.P < .05 was considered significant. Results are expressed as the mean ± SE.
Fluorescence-activated cell sorting analysis
Fluorescein isothiocyanate (FITC)–conjugated and phycoerythrin (PE)–conjugated mAbs or CyChrome-conjugated mAbs recognizing CD138, CD126, CD3, CD4, CD8, CD25, or HLA-DR (human leukocyte antigen-DR) and the negative controls of IgG1 or IgG2a isotype of irrelevant specificity were purchased from Becton Dickinson. Purified cells were resuspended in phosphate-buffered saline (PBS) containing 1% fetal calf serum (FCS), 1% human serum, 10% mouse serum, and 0.01% sodium azide, then stained for 20 minutes at 4°C with combinations of saturating amounts of fluorochrome-conjugated mAbs. After staining, cells were washed extensively and analyzed. Flow cytometry analysis was performed using a fluorescence-activated flow cytometer (FACScan, Becton Dickinson) as previously reported.17
Results
Effect of HMCLs and fresh MM cells on RANKL production
We have previously demonstrated that HMCLs and fresh MM cells purified from several patients with MM do not express RANKL mRNA and protein.4
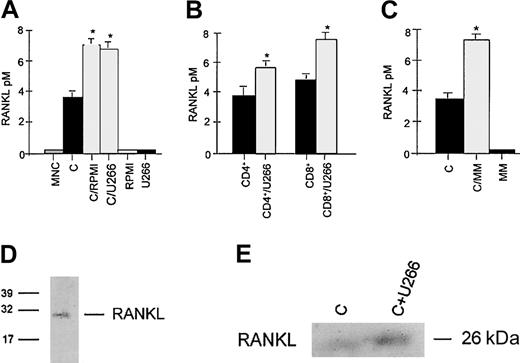
As expected, T-cell activation with anti-CD3 plus anti-CD28 mAbs induces RANKL mRNA in both CD4+ and CD8+lymphocytes as shown by RT-PCR analysis (Figure1A); consistently, RANKL protein was induced in CD3+-activated T lymphocytes, with a maximal effect observed after 4 days of activation (Figure 1B).
Effect of myeloma cells on RANKL expression in activated T lymphocytes in cocultures.
Purified CD4+, CD8+, and CD3+ T lymphocytes were activated with coated CD3 plus CD28 mAb. After 4 days mRNA and proteins were tested for RANKL expression by RT-PCR (A) and Western blot analysis (B), respectively. The 24-hour activated CD3+ T lymphocytes were cocultured in a transwell system with HMCLs and RANKL mRNA expression was evaluated in T cells 24 hours later (panel C). Instead RANKL protein was assessed after a further 48 hours of cocultures in activated T cells (D) or activated CD4+/CD8+ subsets (E) and in the presence or absence of anti–IL-6 mAb (F). Similarly, RANKL protein was evaluated in autologous T lymphocytes cocultured with fresh myeloma cells from MM patients (G). The figures are representative of 3 independent experiments; C indicates control (activated CD3+lymphocytes). Graphs represent the mean optical density (OD) of RANKL normalized to OD of β2-microglobulin (panel C) or actin (D-G; named “OD ratio”) of representative experiments.
Effect of myeloma cells on RANKL expression in activated T lymphocytes in cocultures.
Purified CD4+, CD8+, and CD3+ T lymphocytes were activated with coated CD3 plus CD28 mAb. After 4 days mRNA and proteins were tested for RANKL expression by RT-PCR (A) and Western blot analysis (B), respectively. The 24-hour activated CD3+ T lymphocytes were cocultured in a transwell system with HMCLs and RANKL mRNA expression was evaluated in T cells 24 hours later (panel C). Instead RANKL protein was assessed after a further 48 hours of cocultures in activated T cells (D) or activated CD4+/CD8+ subsets (E) and in the presence or absence of anti–IL-6 mAb (F). Similarly, RANKL protein was evaluated in autologous T lymphocytes cocultured with fresh myeloma cells from MM patients (G). The figures are representative of 3 independent experiments; C indicates control (activated CD3+lymphocytes). Graphs represent the mean optical density (OD) of RANKL normalized to OD of β2-microglobulin (panel C) or actin (D-G; named “OD ratio”) of representative experiments.
To investigate the potential effect of HMCLs on RANKL expression in activated T lymphocytes, a series of cocultures was performed in a transwell system. We found that HMCL U266 further increased RANKL mRNA expression in PB-activated T lymphocytes (Figure 1C) after 24 hours of coculture. Consistently, RANKL protein expression was stimulated by U266 and RPMI-8266 after 48 hours of coculture (Figure 1D). The stimulatory effect was more pronounced in CD8+ cells rather than CD4+ T lymphocytes as shown in Figure 1E, where a typical experiment with U266 is presented.
The addition of anti–IL-6 mAb was able to reduce RANKL induction by HMCL RPMI-8226 (Figure 1F) as well as by U266 cells (data not shown). Consistently, IL-6 concentration was significantly higher in the supernatant of cocultures with RPMI-8226 or U266 (1.80 ± 0.03 ng/mL and 1.20 ± 0.02 ng/mL, respectively, versus 0.63 ± 0.01 ng/mL activated T lymphocytes; P < .001). Fresh purified MM, similarly to HMCLs, stimulated RANKL expression by autologous T lymphocytes obtained from MM patients in a transwell system. Densitometric analysis showed a 3.8-fold increase of RANKL in T lymphocytes cocultured with myeloma cells (Figure 1G).
The concentrations of sRANKL were significantly increased in supernatants of activated T lymphocytes cocultured with RPMI-8226 or U266 myeloma cells in comparison with control (Figure2A). This effect was observed in both CD8+ and CD4+ T cells (Figure 2B). Similarly, fresh purified MM cells stimulated RANKL secretion (Figure 2C) by autologous T lymphocytes; sRANKL levels were undetectable in supernatants of MM cell cultures.
Effect of myeloma cells on RANKL secretion by activated T lymphocytes.
Aliquots of conditioned media were assessed for sRANKL by ELISA. Activated CD3+ T lymphocytes (A) or CD4+/CD8+ subsets (B) were cocultured with HMCL RPMI-8266 or U266 placed in a transwell insert. Autologous CD3+ T cells were cocultured with fresh MM cells (panel C). (C indicates activated CD3+ T cells.) Graphs represent the mean levels ± SE of 3 repeated experiments. *P < .05. Secretion of RANKL into conditioned medium was checked by immunoprecipitation with RANK-Fc followed by gel electrophoresis. RANKL protein was detected either by gel staining with silver stain plus in conditioned medium of activated T cells cocultured with HMCL (D) or by immunoblot analysis using anti-RANKL mAb (E). Data are representative of 3 independent experiments. C indicates activated CD3+ lymphocytes).
Effect of myeloma cells on RANKL secretion by activated T lymphocytes.
Aliquots of conditioned media were assessed for sRANKL by ELISA. Activated CD3+ T lymphocytes (A) or CD4+/CD8+ subsets (B) were cocultured with HMCL RPMI-8266 or U266 placed in a transwell insert. Autologous CD3+ T cells were cocultured with fresh MM cells (panel C). (C indicates activated CD3+ T cells.) Graphs represent the mean levels ± SE of 3 repeated experiments. *P < .05. Secretion of RANKL into conditioned medium was checked by immunoprecipitation with RANK-Fc followed by gel electrophoresis. RANKL protein was detected either by gel staining with silver stain plus in conditioned medium of activated T cells cocultured with HMCL (D) or by immunoblot analysis using anti-RANKL mAb (E). Data are representative of 3 independent experiments. C indicates activated CD3+ lymphocytes).
To confirm the presence of sRANKL in conditioned medium of CD3+ cells and in the cocultures with HMCL, an immunoprecipitation with RANK-Fc was performed. sRANKL was identified as a band with a molecular weight of about 26 kDa (Figure 2D). Western blot analysis confirmed that HMCL U266 stimulates sRANKL production by activated T lymphocytes (Figure 2E).
In contrast to the stimulatory effect on sRANKL production, a slight inhibitory effect was observed on TNF-α secretion by activated T lymphocytes cocultured with HMCL (mean level ± SE: 4.7 ± 0.32 versus 6.2 ± 0.44 ng/mL; P < .05). No effect was found on IL-1 secretion (180 ± 33 versus 151 ± 26 pg/mL;P = NS), whereas IL-11 levels were undetectable in our coculture system.
Effect of HMCLs and fresh MM cells on IFN-γ secretion by T lymphocytes
HMCLs (RPMI-8226 and U266) significantly reduced IFN-γ secretion by activated CD3+ T cells (mean percent of inhibition ± SD versus control: 69% ± 3%; P < .001; Figure3A). Similarly fresh MM cells inhibited IFN-γ secretion by both activated and nonactivated autologous T lymphocytes in a transwell coculture system (mean percent of inhibition ± SD: −43% ± 7%; P < .001 and −28 ± 6%; P < .01, respectively; Figure3B,C).
Effect of HMCLs and fresh purified MM cells on IFN-γ secretion by T lymphocytes.
Soluble IFN-γ levels were detected by ELISA in aliquots of conditioned medium of activated CD3+ T lymphocytes cocultured with HMCL RPMI-8266 or U266 in a transwell system for 48 hours (A) and in aliquots of conditioned medium of activated (B) and nonactivated (panel C) autologous T lymphocytes cocultured with fresh MM cells; C indicates nonactivated CD3+ cells. Graphs represent the mean levels ± SE of 6 repeated experiments. *P < .05; **P < .01.
Effect of HMCLs and fresh purified MM cells on IFN-γ secretion by T lymphocytes.
Soluble IFN-γ levels were detected by ELISA in aliquots of conditioned medium of activated CD3+ T lymphocytes cocultured with HMCL RPMI-8266 or U266 in a transwell system for 48 hours (A) and in aliquots of conditioned medium of activated (B) and nonactivated (panel C) autologous T lymphocytes cocultured with fresh MM cells; C indicates nonactivated CD3+ cells. Graphs represent the mean levels ± SE of 6 repeated experiments. *P < .05; **P < .01.
T-cell–mediated osteoclastogenesis induced by HMCL: effect of OPG
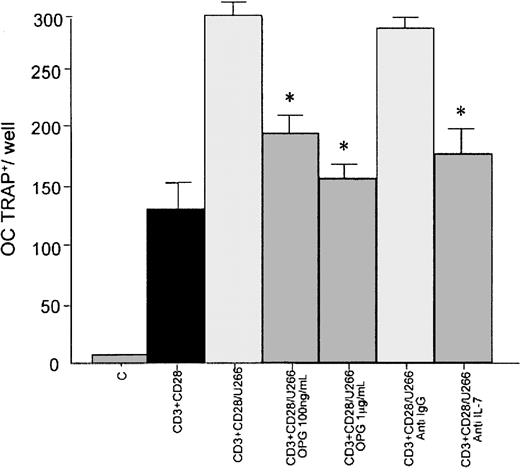
To demonstrate that RANKL overexpression in T lymphocytes is able to induce osteoclast formation, we assessed human CD34+-derived osteoclastogenesis in a stromal cell–free condition. In this system we observed that conditioned medium of CD3- plus CD28-activated T lymphocytes cocultured with U266 significantly increased the formation of multinucleated TRAP+ cells after 10 days of culture (56% ± 6%; P < .01) in comparison with conditioned medium of activated T lymphocytes cultured alone. A significant inhibition on osteoclast formation was observed in the presence of rhOPG at 100 ng/mL (188 ± 11 versus 300 ± 8;P < .05 ) and 1 μg/mL (162 ± 10 versus 300 ± 8;P < .05). The presence of a neutralizing antibody anti–IL-7 inhibited the osteoclastogenesis induced by conditioned medium of cocultures (176 ± 16 versus 300 ± 8;P < .05; Figure 4).
CD34+-derived osteoclastogenesis by T-cell–conditioned medium cocultured with a HMCL: effect of OPG.
PB human CD34+ (5 × 105) cells were incubated with the conditioned medium of activated CD3+cells cocultured for 10 days with U266 in presence or absence of rhOPG (100 ng/mL and 1 μg/mL) or neutralizing polyclonal anti–IL-7 antibody or irrelevant anti-IgG antibody. Multinucleated (nuclei > 3) TRAP+ cells were identified as osteoclastic cells; C indicates nonactivated T lymphocytes. Graphs represent the mean levels ± SE of 3 repeated experiments. *P < .05.
CD34+-derived osteoclastogenesis by T-cell–conditioned medium cocultured with a HMCL: effect of OPG.
PB human CD34+ (5 × 105) cells were incubated with the conditioned medium of activated CD3+cells cocultured for 10 days with U266 in presence or absence of rhOPG (100 ng/mL and 1 μg/mL) or neutralizing polyclonal anti–IL-7 antibody or irrelevant anti-IgG antibody. Multinucleated (nuclei > 3) TRAP+ cells were identified as osteoclastic cells; C indicates nonactivated T lymphocytes. Graphs represent the mean levels ± SE of 3 repeated experiments. *P < .05.
Similar results were obtained using human PB MNCs at high density to generate osteoclasts (data not shown).
Role of IL-7 in RANKL overexpression by T cells in cocultures and IL-7 expression and production by HMCLs and MM patients
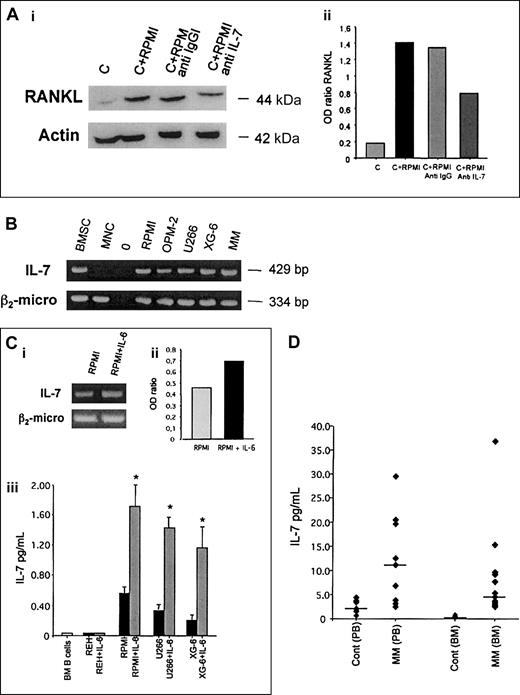
It has been recently demonstrated that IL-7 stimulates RANKL expression in T lymphocytes18; therefore, we hypothesized an involvement of this cytokine in the induction of RANKL in T cells by HMCLs. The addition of a neutralizing anti–IL-7 polyclonal antibody reduced RANKL overexpression induced by an HMCL (RPMI-8226) in activated T lymphocytes, whereas an irrelevant anti-IgG polyclonal antibody did not produce any effect (Figure5A).
Effect of anti–IL-7 mAb in cocultures and IL-7 expression and production by HMCLs and MM patients.
(A) Activated CD3+ cells were cocultured with HMCL RPMI-8226 in the presence or absence of anti–IL-7 polyclonal antibody (0.03 μg/mL) for 24 hours (i); a polyclonal anti-IgG was used as control. RANKL expression was analyzed by Western blot analysis. The figure is representative of 3 independent experiments; C indicates control (activated CD3+lymphocytes). Band intensity was quantified by densitometry and graphically presented (ii) as OD ratio of a representative experiment (optical density of RANKL normalized to the OD of actin). (B) Analysis of IL-7 mRNA expression in HMCLs (RPMI-8226, OPM-2, U266, XG-6) and fresh purified MM cells by RT-PCR. Bone marrow stromal cells (BMSCs) were used as positive control and PB MNCs from healthy subjects as negative control. (C) RT-PCR analysis of IL-7 expression in HMCL RPMI-8266 stimulated with IL-6 (20 ng/mL) for 24 hours. The intensity of the bands was quantified by densitometry; graph (ii) represents the mean OD of IL-7 normalized to the OD of β2-microglobulin (OD ratio) of a representative experiment. Aliquots of conditioned media of normal bone marrow B cells or B leukemia cell line REH and HMCLs (RPMI-8226, U266, XG-6) incubated in presence or absence of IL-6 (20 ng/mL) were assessed for IL-7 levels by ELISA (iii). Graph at bottom represents the mean levels ± SE of 6 repeated experiments. *P < .001. (D) PB serum and BM plasma were obtained from MM patients and healthy subjects. IL-7 levels were detected by ELISA (plots represent individual value and bars the median levels).
Effect of anti–IL-7 mAb in cocultures and IL-7 expression and production by HMCLs and MM patients.
(A) Activated CD3+ cells were cocultured with HMCL RPMI-8226 in the presence or absence of anti–IL-7 polyclonal antibody (0.03 μg/mL) for 24 hours (i); a polyclonal anti-IgG was used as control. RANKL expression was analyzed by Western blot analysis. The figure is representative of 3 independent experiments; C indicates control (activated CD3+lymphocytes). Band intensity was quantified by densitometry and graphically presented (ii) as OD ratio of a representative experiment (optical density of RANKL normalized to the OD of actin). (B) Analysis of IL-7 mRNA expression in HMCLs (RPMI-8226, OPM-2, U266, XG-6) and fresh purified MM cells by RT-PCR. Bone marrow stromal cells (BMSCs) were used as positive control and PB MNCs from healthy subjects as negative control. (C) RT-PCR analysis of IL-7 expression in HMCL RPMI-8266 stimulated with IL-6 (20 ng/mL) for 24 hours. The intensity of the bands was quantified by densitometry; graph (ii) represents the mean OD of IL-7 normalized to the OD of β2-microglobulin (OD ratio) of a representative experiment. Aliquots of conditioned media of normal bone marrow B cells or B leukemia cell line REH and HMCLs (RPMI-8226, U266, XG-6) incubated in presence or absence of IL-6 (20 ng/mL) were assessed for IL-7 levels by ELISA (iii). Graph at bottom represents the mean levels ± SE of 6 repeated experiments. *P < .001. (D) PB serum and BM plasma were obtained from MM patients and healthy subjects. IL-7 levels were detected by ELISA (plots represent individual value and bars the median levels).
Moreover, to support our hypothesis we investigated whether myeloma cells were able to express or produce IL-7. We found that several HMCLs (RPMI-8226, OPM-2, XG-6, U266) and fresh purified CD138+ MM cells expressed IL-7 mRNA, whereas B-cell leukemia REH as well as PB MNCs and purified BM CD19+ B lymphocytes obtained from healthy donors and from patients with B-cell lymphoblastic leukemia did not (Figure 5B). However, IL-7 mRNA was detected in a portion of healthy donors as well as in some patients with infectious disease (data not shown). IL-7 was undetectable in conditioned medium of MNCs or normal B cells or the B-cell leukemic cell line REH. A slight stimulatory effect on IL-7 mRNA expression in HMCLs stimulated by IL-6 was found (Figure 5C, top). In addition IL-7 was significantly up-regulated when HMCLs were cultured in the presence of IL-6 (Figure 5C). In contrast, IL-6 did not induce IL-7 production in normal B cells and REH (Figure 5C) as well as in EBV+cells and in B cells obtained from patients with acute lymphoblastic leukemia or B lymphoma with BM involvement (data not shown). All cells were previously evaluated for CD126 expression by flow cytometry.
When we tested IL-7 levels in vivo we found that IL-7 serum levels were significantly higher in MM patients in comparison to healthy subjects (median, 12.15 pg/mL; range, 2.41-29.5 pg/mL versus 1.91 pg/mL; range, 0-3.43 pg/mL; P < .05). Similarly, IL-7 levels in BM plasma were significantly increased in MM patients in comparison with healthy subjects (median, 8.67 pg/mL; range, 2.68-36.8 pg/mL versus 0.40 pg/mL; range, 0-0.46 pg/mL; P < .05; Figure 5D).
RANKL expression by T lymphocytes from MM patients
Flow cytometry analysis demonstrated a significantly increased number of activated T lymphocytes bearing activation markers indicated by the presence of CD25, HLA-DR, and CD38 (data not shown). To address our in vitro results toward a clinical perspective we evaluated RANKL expression in CD3+ cells purified from 11 BM samples from 15 MM patients previously studied for skeletal involvement. Using RT-PCR we found that CD3+ cells obtained from BM of MM patients with extensive osteolysis and pathologic fractures (5 patients) expressed RANKL mRNA, whereas MM patients without osteolysis (6 patients) or control subjects did not (Figure6B).
RANKL mRNA expression by BM T lymphocytes from MM patients.
Analysis of IL-7 and β2-microglobulin mRNA by RT-PCR in purified bone marrow CD3+ T cells obtained from MM patients either with extensive osteolysis or without bone lesions. The osteosarcoma cell line Saos-2 was used as positive control.
RANKL mRNA expression by BM T lymphocytes from MM patients.
Analysis of IL-7 and β2-microglobulin mRNA by RT-PCR in purified bone marrow CD3+ T cells obtained from MM patients either with extensive osteolysis or without bone lesions. The osteosarcoma cell line Saos-2 was used as positive control.
Discussion
The present study shows that myeloma cells are able to induce an up-regulation of RANKL and a down-regulation of IFN-γ secretion by T lymphocytes at least in part through the mediation of IL-7. Our results confirm the critical role of the RANKL molecule in osteoclast activation and the potential involvement of T lymphocytes in MM-induced osteoclast activation.
The biologic mechanisms involved in the pathogenesis of MM bone disease are still not completely understood.1 Recently, in agreement with other authors,5 we have demonstrated that myeloma cells produce an imbalance in the OPG/RANKL expression in the BM environment.4 We show that myeloma cells are also able to affect RANKL expression in T lymphocytes. Using a coculture system, we demonstrate that HMCLs stimulate RANKL production and secretion by activated CD3+ cells.
In addition, we show that RANKL overexpression is mediated by the release of a soluble factor because the stimulatory effect was observed in a transwell system. Among the molecules that could be responsible for the stimulation of RANKL, we focused our attention on IL-7. The role of IL-7 in the regulation of T homeostasis and activation is well established19; more recently, it has been postulated that IL-7 might be involved in osteoclast activation because IL-7 stimulates RANKL production by T cells.18 We found that HMCLs and fresh MM cells express IL-7 mRNA in contrast to normal or leukemic B cells and that IL-7 secretion was up-regulated in the presence of IL-6. This stimulatory effect of IL-6 on IL-7 expression and secretion seems to be specific for myeloma cells because we did not find any stimulatory effect of IL-6 on BM normal B cells as well as in EBV+ cells or in B leukemia cells. The role of IL-7 on RANKL stimulation in T lymphocytes by myeloma cells was confirmed by the inhibitory effect exerted by an antibody anti–IL-7 in the cocultures; furthermore, we found that IL-7 neutralization inhibited MM-induced osteoclastogenesis.
An inhibitory effect on RANKL in the cocultures was also observed in the presence of anti–IL-6 mAb. It is likely that IL-6 is indirectly involved in the mechanism underlying RANKL stimulation by HMCLs because no evidence indicates that IL-6 stimulates RANKL in T lymphocytes or in other cell systems.4 On the other hand, activated T cells secrete IL-6 and we found that IL-6 levels were significantly higher in the supernatant of the cocultures. An increase of IL-6 production by T lymphocytes was also demonstrated in MM patients.20 In addition, our data indicate that IL-6 stimulates HMCLs to produce IL-7, which in turn stimulates RANKL in T lymphocytes.18 The potential involvement of IL-7 in the pathophysiology of MM is supported by the in vivo finding of higher IL-7 levels in PB serum and BM plasma of MM patients than in healthy subjects.
Our in vitro model of osteoclastogenesis widely used by others8,15,18 supports the possibility that the effect of myeloma cells on RANKL production and secretion by activated T cells is involved in MM-induced osteoclast formation. In fact, we observed that supernatants of CD3+ cells cocultured with HMCLs increased osteoclast formation inhibited by rhOPG. However, OPG did not completely blunt the stimulatory effect on RANKL production by conditioned medium of cocultures. The OPG inability to completely block the osteoclastogenetic activity of T cells, also reported by others,8 suggests that RANKL-independent mechanisms are also involved in this effect. Besides RANKL, other cytokines are secreted by activated T cells,21-23 either osteoclastogenetic such as TNF-α, IL-1, IL-6, IL-11, and macrophage inflammatory protein 1α (MIP-1α) or antiosteoclastogenetic such as IL-18 and IFN-γ.24,25 In our coculture system we found higher levels of IL-6 compared to control. In contrast, HMCLs had no effect on IL-1 and IL-11 secretion by T lymphocytes and induced a slight inhibitory effect on TNF production. Because recent data indicate a cross-talk between RANKL and IFN-γ in T lymphocytes,10,26 the potential effect of HMCLs on IFN-γ secretion was also examined. It is likely that the inhibitory effect shown by HMCLs on IFN-γ secretion may contribute to explaining the increased osteoclastogenetic activity of T cells cocultured with HMCLs and the loss of control of myeloma cell proliferation because an inhibitory effect of IFN-γ on IL-6–dependent myeloma cell growth was previously reported.27 In agreement with all these observations a defective IFN-γ secretion by T cells was observed in MM patients.28 The decrease in IFN-γ levels observed in our cocultures could be due to the production of immunosuppressive factors by myeloma cells such as transforming growth factor β (TGF-β). It has been demonstrated that TGF-β from MM cells inhibits proliferation and IL-2 responsiveness in T lymphocytes,29and it is known that TGF-β suppresses IFN-γ production in human T lymphocytes.30-32
On the other hand, it is unlikely that IL-7 produced by HMCLs could affect IFN-γ production by T cells in our coculture. Even if it has been reported that IL-7 up-regulates IFN-γ in T cells,33this effect has been observed only with a short IL-7 exposure (< 12 hours) and at higher concentrations than those detected in the conditioned medium of HMCLs and in MM patients.
Our in vitro model was supported by the in vivo finding of an increased number of activated T lymphocytes in the BM of MM patients. This observation confirms previous studies showing the presence of an activated T-cell subpopulation34 with a CD8 clonal expansion35-37 in peripheral blood of MM patients. In addition, the stimulatory effect on RANKL production by T cells was confirmed by the finding of RANKL mRNA expression in BM CD3+ T cells purified from MM patients with massive osteolysis (> 3 osteolytic lesions) in comparison with patients without osteolysis. Moreover, this observation should be confirmed in a larger cohort of patients.
In line with our observation, activated CD3+ cells were found by others to be positive for RANKL expression in bone biopsies of MM patients.5
Our results suggest that in MM the RANKL overexpression by T lymphocytes is involved in osteoclast activation, as has been observed in other pathophysiologic conditions, such as rheumatoid arthritis,11,38 periodontal infection,39osteoporosis,40 and hypercalcemia in adult T-cell leukemia.12
It is likely that myeloma cells induce osteoclastic cell activation through the induction of OPG/RANKL imbalance in BM stromal cells as has been recently demonstrated.4 5 The present study indicates that myeloma cells up-regulate RANKL and down-regulate IFN-γ secretion in T cells and that this mechanism too may contribute to MM-induced osteoclastogenesis.
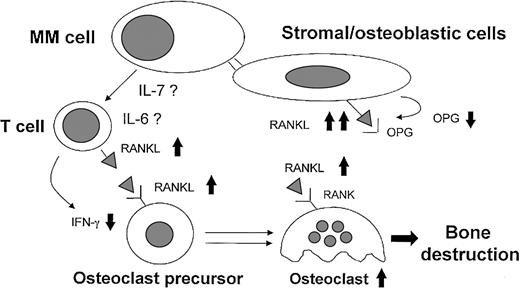
In conclusion, on the basis of these data and previous reports we can now propose a new physiopathologic mechanism (Figure7) underlying MM bone destruction that highlights the critical role of the RANKL system.
Model for MM-induced osteoclastogenesis through RANKL induction.
Myeloma cells induce an imbalance in the OPG/RANKL ratio in stromal/osteoblastic cells through the cell-to-cell contact. In addition, myeloma cells stimulate RANKL and down-regulate IFN-γ secretion by T cells at least in part through the direct release of IL-7 or indirect involvement of the high IL-6 levels induced by myeloma cells in the bone environment. The high BM expression and level of the critical osteoclastogenetic factor RANKL associated with lower levels of osteoclastogenetic inhibitors, such as OPG and IFN-γ, may be involved in MM-induced activation of osteoclasts.
Model for MM-induced osteoclastogenesis through RANKL induction.
Myeloma cells induce an imbalance in the OPG/RANKL ratio in stromal/osteoblastic cells through the cell-to-cell contact. In addition, myeloma cells stimulate RANKL and down-regulate IFN-γ secretion by T cells at least in part through the direct release of IL-7 or indirect involvement of the high IL-6 levels induced by myeloma cells in the bone environment. The high BM expression and level of the critical osteoclastogenetic factor RANKL associated with lower levels of osteoclastogenetic inhibitors, such as OPG and IFN-γ, may be involved in MM-induced activation of osteoclasts.
Prepublished online as Blood First Edition Paper, August 8, 2002; DOI 10.1182/blood-2002-04-1121.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Nicola Giuliani, Hematology Department of Internal Medicine and Biomedical Science, University of Parma, via Gramsci 14, 43100 Parma, Italy; e-mail:nicola.giuliani@unipr.it.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal