We examined isolated leukemia B cells of patients with chronic lymphocytic leukemia (CLL) for expression of zeta-associated protein 70 (ZAP-70). CLL B cells that have nonmutated immunoglobulin variable region genes (V genes) expressed levels of ZAP-70 protein that were comparable to those expressed by normal blood T cells. In contrast, CLL B cells that had mutated immunoglobulin variable V genes, or that had low-level expression of CD38, generally did not express detectable amounts of ZAP-70 protein. Leukemia cells from identical twins with CLL were found discordant for expression of ZAP-70, suggesting that B-cell expression of ZAP-70 is not genetically predetermined. Ligation of the B-cell receptor (BCR) complex on CLL cells that expressed ZAP-70 induced significantly greater tyrosine phosphorylation of cytosolic proteins, including p72Syk, than did similar stimulation of CLL cells that did not express ZAP-70. Also, exceptional cases of CLL cells that expressed mutated immunoglobulin V genes and ZAP-70 also experienced higher levels tyrosine phosphorylation of such cytosolic proteins following BCR ligation. Following BCR ligation, ZAP-70 underwent tyrosine phosphorylation and became associated with surface immunoglobulin and CD79b, arguing for the involvement of ZAP-70 in BCR signaling. These data indicate that expression of ZAP-70 is associated with enhanced signal transduction via the BCR complex, which may contribute to the more aggressive clinical course associated with CLL cells that express nonmutated immunoglobulin receptors.
Introduction
The leukemia B cells of patients with chronic lymphocytic leukemia (CLL) can be classified into 1 of at least 2 major subgroups on the basis of the mutational status of their expressed immunoglobulin variable (V) genes.1,2 Patients with CLL cells that express nonmutated genes tend to have a relatively aggressive clinical course when compared with patients who have CLL cells that express immunoglobulin V genes with somatic mutations.2-4 Moreover, patients with leukemia cells that express nonmutated immunoglobulin V genes are generally more likely to have CLL cells with atypical morphology, advanced clinical stage, and progressive disease.3 However, the mechanism accounting for the differences in clinical behavior of these 2 types of CLL is unknown.
Recent studies on the gene expression profiles of isolated CLL B cells have revealed a set of genes that is differentially expressed in these 2 subgroups.5,6 CLL cells from different patients share common expression levels of many genes and have a gene expression profile distinct from that of other B-cell malignancies or normal B cells. However, CLL cells that express nonmutated immunoglobulin V genes can be distinguished from leukemia cells that have mutated immunoglobulin V genes through their differential expression of a small subset of genes. One of these genes encodes zeta-associated protein 70 (ZAP-70), a 70-kDa ζ-chain CD3-receptor–associated protein tyrosine kinase (PTK) of T lymphocytes. In contrast to CLL cells that have mutated immunoglobulin receptors, CLL cells that use nonmutated immunoglobulin V genes express ZAP-70 RNA.5
The functional significance of ZAP-70 gene expression in this subgroup of CLL B cells is unknown. ZAP-70 is a PTK that is characterized by 2 tandem src homology 2 (SH2) domains and a C-terminal catalytic domain.7,8 Following ligation of the T cell receptor (TCR), there is activation of src family PTK that in turn phosphorylates tyrosine-containing immunoreceptor tyrosine-based activation motifs (ITAMs) within the cytoplasmic tails of the accessory molecules of the TCR.9 ZAP-70 is recruited to the phosphorylated ITAMs and subsequently becomes activated, in turn causing activation of Tec family PTKs and downstream signaling pathways, such as the phospholipase Cγ/Ca++ signaling pathway and the Ras/mitogen activated protein kinase (MAPK) pathway.10 B cells generally lack ZAP-70, but instead use another related PTK, Syk, for signal transduction via the B-cell receptor (BCR) complex.11 Similar to ZAP-70, Syk is recruited to the phosphorylated ITAMs of the activated BCR complex where it subsequently becomes activated.12 Thus, ZAP-70 and Syk play similar roles in membrane antigen-receptor signaling pathways.
Prior studies indicated that ZAP-70 could reconstitute BCR signaling in Syk-deficient B cells.13 In addition, Syk apparently could effect TCR signaling in patients deficient in ZAP-70.14However, it is not known whether ZAP-70 protein is expressed in CLL, let alone capable of undergoing phosphorylation or association with the CLL BCR complex upon surface immunoglobulin ligation.
In the present study, we examined purified CLL B cells for expression of ZAP-70 protein and examined whether ZAP-70 could potentially play a role in BCR signal transduction.
Materials and methods
Cell isolation
Blood samples were collected from patients who satisfied diagnostic and immunophenotypic criteria for common B-cell CLL15 after signing an informed consent form approved by the institutional review boards of the University of California, San Diego or Long Island Jewish Hospital. The patients were not previously treated and had not received recombinant growth factors or exogenous cytokines. Blood mononuclear cells were isolated by density-gradient centrifugation over Ficoll-Hypaque (Pharmacia, Uppsala, Sweden). Cells were suspended in fetal bovine serum (FBS) containing 5% dimethyl sulfoxide for storage in liquid nitrogen. The viability of the CLL cells was at least 85% at the initiation of culture, as determined by flow cytometric analyses of cells stained with 5 μg/mL propidium iodide (PI) (Molecular Probes, Eugene, OR) for 15 minutes at 37°C. All samples contained more than 90% CLL B cells as assessed by flow cytometric analyses.
Flow cytometry
The cells were stained with monoclonal antibodies (mAbs) conjugated with either fluorescein, phycoerythrin, allophycocyanin, or Cy-Chrome (BD PharMingen, La Jolla, CA) for direct, 4-color, multiparameter flow cytometry by means of a FACS-Calibur (Becton Dickinson, San Jose, CA), as described.16 The fluorescence intensity of each stained cell population was compared with that of the same population treated with an isotype control, fluorochrome-conjugated mAb of irrelevant specificity. To measure for expression of CD38, we calculated the median fluorescence intensity ratio (MFIR). This is the ratio of the median fluorescence intensity of gated CD19+CD5+ cells stained with CD38 versus that of CD19+CD5+ cells stained with the isotype control mAb. In addition, we set a threshold fluorescence intensity for considering cells positive for CD38, such that approximately 0.2% of the CD19+CD5+ cells treated with the isotype control mAb had fluorescence greater than this threshold. CLL populations were considered positive for CD38 when more than 20% of the CD38-stained CD19+CD5+ cells had a fluorescence intensity greater than this threshold.
CLL B cells were isolated to greater than 99% purity by flow cytometry by means of a FACS-Starplus (Becton Dickinson) (data not shown). For this, the isolated blood mononuclear cells were stained with fluorochrome-conjugated mAbs for CD19. CD19+cells were isolated and then examined for purity by staining a test aliquot of cells with fluorochrome-conjugated mAbs specific for CD3, CD5, and CD56.
Cell treatment and sample preparation
Frozen CLL cells were grown overnight in complete RPMI-1640 medium supplemented with 10% fetal bovine serum followed by 2 to 3 hours of serum starvation before BCR stimulation. The viability of the cells for each preparation always exceeded 90%, as assessed by trypan-blue exclusion. The cells were adjusted to 1 × 107 cells per milliliter in isotonic 0.1 M phosphate-buffered saline (pH, 7.2). BCR ligation was performed with the use of biotinylated goat antihuman immunoglobulin M (IgM) F(ab′)2 (Southern Biotechnology Associates, Birmingham, AL) at 10 μg/mL. We incubated this reagent with the leukemia cells for 10 minutes on ice and then added Avidin (Sigma, St Louis, MO) to a final concentration of 10 μg/mL. The cell mixture was incubated at 37°C for a defined number of minutes to effect BCR signaling. Preliminary time course studies that examined for increases in tyrosine phosphorylation after 1, 5, 10, 15, and 30 minutes at 37°C revealed optimal stimulation at 10 minutes (data not shown). Therefore, in all subsequent experiments, the anti-IgM/avidin-treated leukemia cells were incubated for 10 minutes at 37°C, placed on ice, and then immediately pelleted by centrifugation at 4°C. Cell pellets were lysed in 200 μL ice-cold 1% Nonidet P-40 (NP-40) lysis buffer (10 mM Tris-HCl [tris(hydroxymethyl)aminomethane]–Hcl], pH 7.4; 5 mM EDTA [ethylenediaminetetraacetic acid]; 150 mM NaCl; 0.1% sodium dodecyl sulfate [SDS]; 0.1% sodium deoxycholate; 10 mM NaF; 1 mM 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride [AEBSF]; 0.8 μM aprotinin; 20 μM leupeptin; 36 μM bestatin; 15 μM pepstatin; 14 μM leucylamido-(4-guanidino)butane [E-64]) for 20 minutes on ice. For coimmunoprecipitation experiments, 1% NP-40 lysis buffer (10 mM Tris-HCl, pH 7.4; 5 mM EDTA; 50 mM NaCl; 0.1% SDS; and 0.1% sodium deoxycholate) was used. Cell lysates were clarified by centrifugation at 20 000g for 15 minutes.
VH gene analyses
Total cellular RNA was isolated from 5 × 106 CLL B cells by means of RNeasy (Qiagen, Valencia, CA), per the manufacturer's instructions. First-strand cDNA was synthesized from one third of the total purified RNA by means of an oligodeoxythymidine (oligo-dT) primer and Superscript II RT (Life Technologies, Grand Island, NY). The remaining RNA was removed with RNase H, and the cDNA purified by means of QIAquik purification columns (Qiagen). The purified cDNA was poly-deoxyguanosine (poly-dG)–tailed by means of dG triphosphate (dGTP) and terminal deoxytransferase (Roche, Indianapolis, IN). The VH gene expressed by the CLL B cells was determined by reverse transcriptase–polymerase chain reaction (RT-PCR) enzyme-linked immunosorbent assay (ELISA) technique.17 The cDNA from each sample was amplified with the use of VH family–specific primers for the sense strand of the gene of interest and an antisense Cμ consensus oligonucleotide primer. The PCR products were size selected by electrophoresis in 2% agarose containing 0.5 μg/mL ethidium bromide (Life Technologies). The expected products were excised and purified by means of Geneclean III (BIO 101, Carlsbad, CA), per the manufacturer's instructions, and cloned into pGEM-T (Promega, Madison, WI). Following transformation of XL-1 Blue competent cells (Stratagene, La Jolla, CA), plasmid DNA was isolated from overnight cultures of randomly selected bacterial colonies by means of QIAprep purification columns (Qiagen). Sequencing was conducted by means of a fluorescence-dideoxy-chain-termination method and an Applied Biosystems 377 automated nucleic acid sequence analyzer (ABI, Foster City, CA). Nucleotide sequences were analyzed by means of DNASTAR (Madison, WI) and by comparison with sequences deposited in the V BASE and GenBank sequence databases. At least 3 independent clones were analyzed for each sample. Somatic mutations were identified by comparison with the most homologous germ line VH gene. The method of Corbett and colleagues18 was used to assign diversity (D) genes of the longer gene families (D2 and D3), and 7 consecutive nucleotides were used for the shorter D gene families.
Immune precipitation and immunoblotting
The protein concentration of each cell lysate was determined by means of the Bio-Rad Protein Assay (Bio-Rad Laboratories, Hercules, CA). First, 10 μg total cell lysate was loaded onto 10% SDS polyacrylamide gels for electrophoresis. The size-separated proteins were transferred to Immobilon-P (Millipore, Bedford, MA) transfer membranes. Membranes were blotted with primary antibody at 4°C overnight or at room temperature for 2 hours. For analysis of phosphotyrosine proteins, we used the phosphotyrosine-specific mAb 4G10 (Upstate Biotechnology, Lake Placid, NY). We incubated the blots with secondary antibodies that were conjugated with horseradish peroxidase for 1 hour at room temperature. Blots then were washed and prepared for enhanced chemiluminescence and subsequent autoradiography with Super RX film (Fuji, Tokyo, Japan). Defined sections of the film were scanned for density measurements by means of a Scanmaker IISP (Microtek, Carson, CA) and were analyzed with the use of National Institutes of Health (NIH; Bethesda, MD) Image software. To report the change in signal intensity following stimulation, we subtracted 1 from the ratio of signal intensity of the defined section for the stimulated sample over the signal intensity of the same sample before stimulation and multiplied this number by 100 to derive the percentage change in signal intensity.
For immune precipitation studies, we incubated the lysates of 0.5 to 1 × 107 cells with saturating amounts of mouse mAbs specific for ZAP-70 (clone 29; Transduction Laboratories, Lexington, KY), CD79b (C-19; Santa Cruz Biotechnology, Santa Cruz, CA), or human IgM (Southern Biotechnology Associates), or affinity-purified rabbit antibodies to Syk (C-20; Santa Cruz Biotechnology). The lysates and antibodies were allowed to incubate either overnight at 4°C or for 2 hours at room temperature. Immune complexes were precipitated with 20 μL protein-A agarose beads (Pierce, Rockford, IL) for an additional 2-hour incubation at 4°C. The beads were washed twice with phosphate-buffered saline (PBS) and once with lysis buffer. Bound proteins were eluted by boiling the samples in SDS sample buffer and were then resolved by 10% SDS polyacrylamide gel electrophoresis.
Results
ZAP-70 expression in CLL B cells
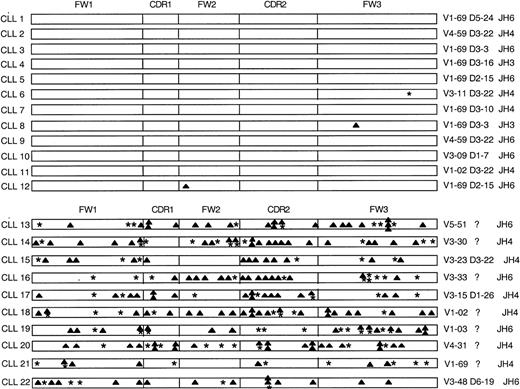
We examined the leukemia B cells from 22 patients with CLL for expression of ZAP-70 protein. Twelve of the patients had CLL cells that expressed nonmutated immunoglobulin receptors, whereas the remainder had leukemia cells expressing immunoglobulin that had undergone somatic mutation (Figure 1).
Analysis of the somatic mutations in the immunoglobulin VHgenes of 22 CLL patients.
The distribution of replacement (▴) and silent (*) mutations in the complementarity determining regions (CDRs) and framework regions (FWs) of each heavy chain are indicated. Listed on the left margin are the designations for the heavy chain of each CLL sample. Indicated on the right margin are the VH genes, known D segments, and JH genes that have the highest homology with each heavy-chain cDNA sequence.
Analysis of the somatic mutations in the immunoglobulin VHgenes of 22 CLL patients.
The distribution of replacement (▴) and silent (*) mutations in the complementarity determining regions (CDRs) and framework regions (FWs) of each heavy chain are indicated. Listed on the left margin are the designations for the heavy chain of each CLL sample. Indicated on the right margin are the VH genes, known D segments, and JH genes that have the highest homology with each heavy-chain cDNA sequence.
A high proportion of cases expressed the VH1-69 gene that prior studies had identified as being expressed at high frequency in B-cell CLL.1 19 Two of the patients who had leukemia cells with mutated immunoglobulin V genes (CLL16 and CLL17) were monozygous twins who were concordant for CLL. Both twins were diagnosed at the age of 57 years after they incidentally were found to have a leukocytosis of approximately 12 000/mm3 on a routine clinical exam. However, one twin (CLL16) subsequently experienced progressive disease, a lymphocyte-doubling time of 2 years, and hyperlymphocytosis (greater than 285 000/mm3), whereas the other (CLL17) has had nonprogressive disease and maintains a stable total white blood cell count of no more than 17 500/mm3. Cell lysates were prepared from CLL cells isolated to greater than 99% purity by means of the fluorescence-activated cell sorter.
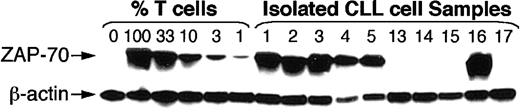
ZAP-70 protein was detected by immunoblotting with anti–ZAP-70 mAb in lysates of all 12 CLL cell samples that expressed nonmutated immunoglobulin V genes (Figure 2 and data not shown). Moreover, the level of ZAP-70 protein detected in each of the ZAP-70+ leukemia B-cell samples was comparable to that found in isolated blood T cells from healthy adults (Figure2). In contrast, CLL samples that expressed mutated immunoglobulin receptors generally did not have ZAP-70 protein at levels exceeding those found in control samples with 1% T cells (Figure 2). However, all samples contained equivalent levels of β-actin, indicating that all lanes had similar levels of protein loaded onto the gel (Figure 2).
ZAP-70 protein expression in CLL cell samples that expressed nonmutated (samples 1 through 5) or mutated (samples 13 through 17) immunoglobulin genes.
For immunoblot analyses, 10 μg protein lysate from each sample was loaded onto separate lanes of a polyacrylamide gel. Cell lysates were prepared from highly purified leukemia B cells (greater than 99% purity), Stable transfected Burkitt lymphoma B-cell line (BJAB), or mixtures of BJAB and isolated normal blood T cells containing 100%, 33%, 10%, 3%, or 1% T cells, as indicated at the top of the sample lanes. The immunoblots were probed with anti–ZAP-70 antibody to detect ZAP-70 protein, as indicated by the labeled arrow on the left-hand side of the Figure. The blots were stripped and reprobed with antibodies specific for β-actin, as indicated by the labeled arrow of the bottom row.
ZAP-70 protein expression in CLL cell samples that expressed nonmutated (samples 1 through 5) or mutated (samples 13 through 17) immunoglobulin genes.
For immunoblot analyses, 10 μg protein lysate from each sample was loaded onto separate lanes of a polyacrylamide gel. Cell lysates were prepared from highly purified leukemia B cells (greater than 99% purity), Stable transfected Burkitt lymphoma B-cell line (BJAB), or mixtures of BJAB and isolated normal blood T cells containing 100%, 33%, 10%, 3%, or 1% T cells, as indicated at the top of the sample lanes. The immunoblots were probed with anti–ZAP-70 antibody to detect ZAP-70 protein, as indicated by the labeled arrow on the left-hand side of the Figure. The blots were stripped and reprobed with antibodies specific for β-actin, as indicated by the labeled arrow of the bottom row.
Of the 10 CLL samples that expressed mutated immunoglobulin receptors, we found that only 1 (CLL16) expressed high levels of ZAP-70 protein. Through primary sequence analyses, we deduced that the immunoglobulin heavy chain variable region of CLL16 had overall sequence homology of only 91.2% with the germ line sequences of the nearest fit, namely, immunoglobulin VH3-33, D6-6, and JH6 (Figure 1). Samples CLL16 and CLL17 were obtained from monozygotic twins who were concordant for CLL. Like CLL16, CLL17 also expressed mutated immunoglobulin genes with only 92% overall sequence homology with the germ line sequences of the nearest fit, namely, immunoglobulin VH3-15, D1-26, and JH4 (Figure1). However, unlike CLL16, CLL17 did not have detectable levels of ZAP-70 protein (Figure 2).
We examined each leukemia cell sample for expression of CD38. Five of the 12 cases that used nonmutated immunoglobulin genes (CLL3, CLL5, CLL8, CLL9, and CLL11) had high-level expression of CD38 (average MFIR, 15 ± 8.6, with 76% ± 23% of the leukemia cells on average found to be CD38+), whereas only 1 of the 10 cases with mutated immunoglobulin genes (CLL16) expressed this surface molecule (MFIR, 2.3, with 37% of leukemia cells found to be CD38+). Therefore, a significantly higher proportion of the samples that used nonmutated immunoglobulin genes expressed CD38 (41%) than did samples with mutated immunoglobulin receptors (10%) (P < .05, Student t test). Furthermore, all samples found to express CD38 also had ZAP-70 protein, including CLL16. However, 7 of the 12 cases (59%) that used nonmutated immunoglobulin genes and that also expressed ZAP-70 protein had insignificant expression of CD38 (average MFIR, 1.2 ± 0.4, with an average of 6.5% ± 6.5% of the leukemia cells found to be CD38+). All 9 cases other than CLL16 that expressed mutated immunoglobulin genes also had negligible expression of CD38 (average MFIR, 1.0 ± 0.2, with an average 0.9% ± 0.7% of the leukemia cells found to be CD38+), including CLL17 obtained from the twin sibling of CLL16.
Functional analyses of BCR signaling
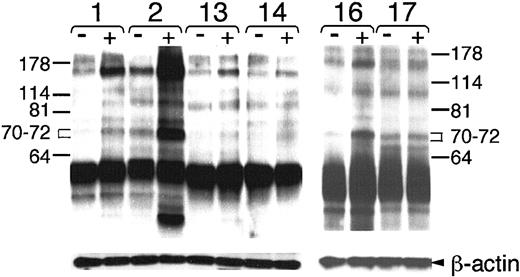
We examined whether ligation of the BCR could induce tyrosine phosphorylation of cytosolic proteins in the CLL cases examined for expression of ZAP-70. Each of the freshly thawed leukemia cell samples had comparable expression of surface IgM, allowing us to use anti-μ F(ab′)2 (anti-μ) as an agonist to effect BCR signaling (data not shown). We found that ligation of the BCR on CLL cell samples that expressed ZAP-70 (n = 13) induced tyrosine phosphorylation of many cytosolic proteins (Figure 3 and data not shown). This was observed even for CLL16, which expressed mutated immunoglobulin genes. Moreover, the increase in detected tyrosine phosphorylation following BCR ligation appeared greater in CLL cells that expressed ZAP-70 than in CLL cells that did not express ZAP-70 (Figure 3, and data not shown). In fact, ligation of the BCR on 8 of the CLL cell samples that did not express ZAP-70, including CLL17, did not induce any apparent changes in tyrosine phosphorylation, as detected by the antiphosphotyrosine antibody 4G10 (Figures 3 and4, and data not shown).
Immunoblot analysis of tyrosine-phosphorylated proteins in the lysates of CLL cells.
Cell lysates from 5 × 106 cells were loaded onto separate lanes of the polyacrylamide gel for immunoblot analysis with the anti–P-Tyr antibody 4G10. Each cell sample is indicated by a number above the pair of lanes for lysates made from cells before (−) or after (+) BCR stimulation with anti-μ F(ab′)2. Lysates from ZAP-70+ cases CLL1 (1) and CLL2 (2) are compared with those of CLL13 (13) and CLL14 (14) in the left panel. Lysates from samples obtained from monozygous twins, CLL16 (16) and CLL17 (17), but that were discordant for expression of ZAP-70, are provided in the right panel. Molecular weight markers are indicated on the left and right margins. The brackets define the areas between 70 and 72 kDa in size that were scanned for density with NIH image software. The blots were stripped and then probed with anti–β-actin to monitor for uniformity of protein loading (bottom row labeled “β-actin” on the right-hand margin).
Immunoblot analysis of tyrosine-phosphorylated proteins in the lysates of CLL cells.
Cell lysates from 5 × 106 cells were loaded onto separate lanes of the polyacrylamide gel for immunoblot analysis with the anti–P-Tyr antibody 4G10. Each cell sample is indicated by a number above the pair of lanes for lysates made from cells before (−) or after (+) BCR stimulation with anti-μ F(ab′)2. Lysates from ZAP-70+ cases CLL1 (1) and CLL2 (2) are compared with those of CLL13 (13) and CLL14 (14) in the left panel. Lysates from samples obtained from monozygous twins, CLL16 (16) and CLL17 (17), but that were discordant for expression of ZAP-70, are provided in the right panel. Molecular weight markers are indicated on the left and right margins. The brackets define the areas between 70 and 72 kDa in size that were scanned for density with NIH image software. The blots were stripped and then probed with anti–β-actin to monitor for uniformity of protein loading (bottom row labeled “β-actin” on the right-hand margin).
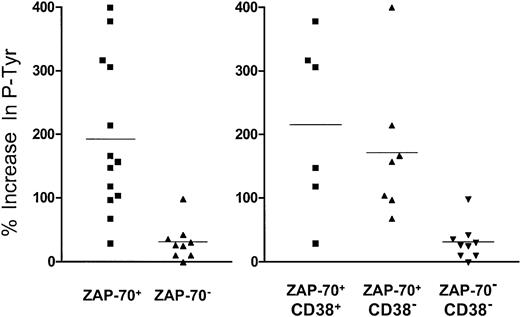
Increase in signal intensity of tyrosine phosphoproteins of 70 to 72 kDa following BCR ligation.
Each symbol represents one CLL sample. The height of each symbol corresponds to the percentage increase in phosphoprotein signal intensity caused by BCR ligation, as indicated on the left-hand axis. The graph on the left segregates the symbols of leukemia cell samples that did (ZAP-70+) or did not (ZAP-70−) express ZAP-70, whereas the graph on the right segregates samples on the basis of whether they expressed ZAP-70 and CD38 (ZAP-70+/CD38+, left group); ZAP-70 without CD38 (ZAP-70+/CD38−, middle group); or neither ZAP-70 nor CD38 (ZAP-70−/CD38−, right group). The horizontal line drawn among the symbols of each group represents the average fold-increase in signal intensity for that group.
Increase in signal intensity of tyrosine phosphoproteins of 70 to 72 kDa following BCR ligation.
Each symbol represents one CLL sample. The height of each symbol corresponds to the percentage increase in phosphoprotein signal intensity caused by BCR ligation, as indicated on the left-hand axis. The graph on the left segregates the symbols of leukemia cell samples that did (ZAP-70+) or did not (ZAP-70−) express ZAP-70, whereas the graph on the right segregates samples on the basis of whether they expressed ZAP-70 and CD38 (ZAP-70+/CD38+, left group); ZAP-70 without CD38 (ZAP-70+/CD38−, middle group); or neither ZAP-70 nor CD38 (ZAP-70−/CD38−, right group). The horizontal line drawn among the symbols of each group represents the average fold-increase in signal intensity for that group.
To evaluate the relative increase in tyrosine phosphorylation, we measured the changes in density of bands identified on the immunoblot after BCR ligation. For example, signal intensity measurements of a defined area that included proteins of 70 to 72 kDa on the immunoblot allowed us to compare the relative increase in the density of the bands following BCR ligation (Figure 4). Following BCR ligation, the 13 cases that expressed ZAP-70 had an average 190% increase (± 120%) in band intensity. This was significantly greater than the 30% increase (± 30%) in band intensity observed for the 9 cases that did not express ZAP-70 protein (P < .01, Student ttest; Figure 4, left panel).
Expression of ZAP-70 also appeared more relevant than expression of CD38 in defining the cases that responded to BCR ligation. The CLL cell samples that expressed CD38 (n = 6) had on average a 220% increase (± 140%) in the intensity of bands in this area following BCR ligation. Linear regression analysis of the increase in band intensities relative to the CD38 MFIR for each of these 6 CD38+ cases did not reveal a significant relationship, providing a squared Pearson product-moment correlation coefficient(R2) of only 0.4661 (data not shown). Although the average increase in band intensity for these 6 CD38+cases was significantly greater than the 30% average increase (± 30%) noted for the 9 CLL cell samples that expressed neither CD38 nor ZAP-70 (P < .05, Bonferroni ttest), it was not significantly greater than the 170% average increase (± 110%) noted for the 7 CD38− CLL cell samples that expressed ZAP-70 (Figure 4, right panel). Moreover, following BCR ligation, the CLL cases that expressed ZAP-70 but not CD38 (n = 7) had a significantly greater increase in signal intensity (m = 170% ± 110%) than did the 9 CD38− samples that did not express ZAP-70 (m = 30% ± 30%) (P < .01, Student t test).
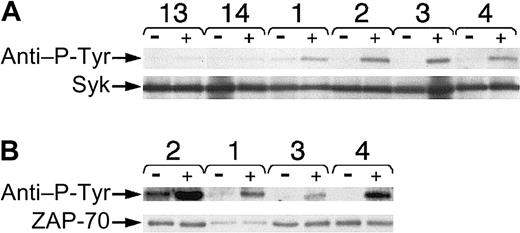
We examined samples for BCR-induced increases in tyrosine phosphorylation of proteins of this size, namely p72Syk. For this, we performed Syk immune precipitation of leukemia cell lysates with anti-Syk antibodies before and after BCR ligation and then probed the immunoblot with the antiphosphotyrosine antibody 4G10 (Figure 5A). Following BCR ligation, we noted an average 180% increase (± 40%) in the band intensity of P-Tyr Syk in the 5 examined cases that expressed ZAP-70. This fold-ncrease was significantly greater than that induced in the 5 examined leukemia cells that did not express ZAP-70 (m = 40% ± 20%) (P < .05, Student ttest). Stripping the blots and then reprobing them for Syk revealed that all of the 10 studied cases had similar amounts of Syk protein (Figure 5A, bottom panel).
Phosphorylation of Syk or ZAP-70 in CLL B cells after BCR ligation.
Lysates were prepared before and 10 minutes after BCR ligation with anti-μ F(ab′)2 and then used for immune precipitation with antibodies specific for Syk (A) or ZAP-70 (B). Each pair of lanes represents the lysates prepared from cells before (−) or after (+) BCR ligation, as indicated at the top of each sample lane. The numbers of the CLL samples are provided above each pair of lanes. CLL13 (13) and CLL14 (14) did not express ZAP-70, whereas CLL cases 1 through 4 were ZAP-70+. The top row in panels A and B provides the proteins of approximately 70 to 72 kDa that were detected by antiphosphotyrosine antibody 4G10, as marked by the left-hand arrow labeled “anti–P-Tyr.” The bottom rows in panels A and B provide the same bands identified on the stripped blots that reacted with anti-Syk (Syk) or anti–ZAP-70 (ZAP-70) antibodies, respectively, as indicated by the arrows on the left-hand side of the Figure.
Phosphorylation of Syk or ZAP-70 in CLL B cells after BCR ligation.
Lysates were prepared before and 10 minutes after BCR ligation with anti-μ F(ab′)2 and then used for immune precipitation with antibodies specific for Syk (A) or ZAP-70 (B). Each pair of lanes represents the lysates prepared from cells before (−) or after (+) BCR ligation, as indicated at the top of each sample lane. The numbers of the CLL samples are provided above each pair of lanes. CLL13 (13) and CLL14 (14) did not express ZAP-70, whereas CLL cases 1 through 4 were ZAP-70+. The top row in panels A and B provides the proteins of approximately 70 to 72 kDa that were detected by antiphosphotyrosine antibody 4G10, as marked by the left-hand arrow labeled “anti–P-Tyr.” The bottom rows in panels A and B provide the same bands identified on the stripped blots that reacted with anti-Syk (Syk) or anti–ZAP-70 (ZAP-70) antibodies, respectively, as indicated by the arrows on the left-hand side of the Figure.
Similarly, we prepared immune precipitates from lysates of ZAP-70+ leukemia cases before and after BCR ligation using antibodies to ZAP-70. Immunoblot analyses of these samples with antiphosphotyrosine antibodies revealed that the signal intensity of ZAP-70 increased following anti-μ stimulation (Figure 5B). ZAP-70 protein was found in each lane when the blots were stripped and then reprobed with anti–ZAP-70 antibody (Figure 5B, bottom panel).
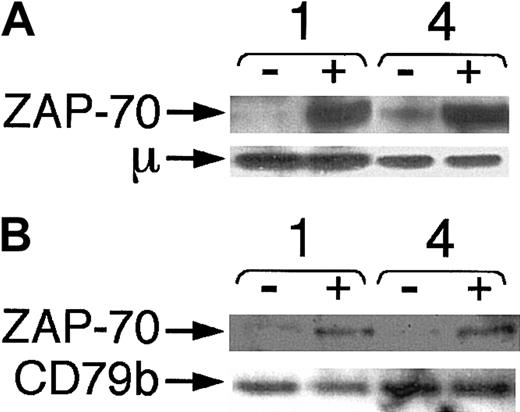
That ZAP-70 undergoes tyrosine phosphorylation within minutes of BCR ligation suggests that ZAP-70 may associate with the surface BCR complex. To examine for this, we prepared immune precipitates with antihuman IgM (Figure 6A) or anti-CD79b (Figure 6B) from ZAP-70+ cases before and after anti-μ stimulation. Immunoblot analyses with ZAP-70 antibodies revealed ZAP-70 in lanes with lysates prepared from anti-μ–stimulated cases. There was little or no detectable ZAP-70 signal in the samples prior to BCR ligation. In contrast, both prestimulated and poststimulated samples contained similar levels of μ (Figure 6A) or CD79b protein (Figure 6B), indicating that all immune precipitates had captured similar levels of target protein.
Association of ZAP-70 with IgM and CD79b in CLL B cells after BCR ligation.
Lysates were made before and 10 minutes after BCR ligation with anti-μ F(ab′)2 and prepared for immune precipitation with antibodies specific for human IgM (A) or CD79b (B). Samples were loaded onto separate lanes of a polyacrylamide gel for immunoblot analysis with anti–ZAP-70, as indicated by the labeled arrow marked “ZAP-70.” Stripped blots were reprobed for anti-μ (A) or anti-CD79b (B), as indicated by the arrows marked “μ” or “CD79b,” respectively. Each pair of lanes represents the lysates prepared from cells before (−) or after (+) BCR ligation, as indicated at the top of each sample lane. The numbers of the CLL samples are provided above each pair of lanes.
Association of ZAP-70 with IgM and CD79b in CLL B cells after BCR ligation.
Lysates were made before and 10 minutes after BCR ligation with anti-μ F(ab′)2 and prepared for immune precipitation with antibodies specific for human IgM (A) or CD79b (B). Samples were loaded onto separate lanes of a polyacrylamide gel for immunoblot analysis with anti–ZAP-70, as indicated by the labeled arrow marked “ZAP-70.” Stripped blots were reprobed for anti-μ (A) or anti-CD79b (B), as indicated by the arrows marked “μ” or “CD79b,” respectively. Each pair of lanes represents the lysates prepared from cells before (−) or after (+) BCR ligation, as indicated at the top of each sample lane. The numbers of the CLL samples are provided above each pair of lanes.
Discussion
In this study, CLL B cells that expressed nonmutated immunoglobulin V genes were found to express levels of ZAP-70 protein that were comparable to those detected in blood T cells of healthy adults. In contrast, leukemia cells that expressed mutated immunoglobulin receptors generally did not express detectable levels of ZAP-70 protein. These results agree with those obtained with the use of microarray to assess the gene-expression profiles of CLL B cells.5 However, in this study, we found that the association between the use of nonmutated immunoglobulin genes and the expression of ZAP-70 was not absolute, in that one of the CLL samples that expressed mutated immunoglobulin receptors also expressed high levels of the T-cell receptor PTK. Nevertheless, we note that a significantly higher proportion of the 12 cases with nonmutated immunoglobulin genes expressed ZAP-70 (100%) than did the 10 cases with mutated immunoglobulin receptors (10%) (P < .01).
High-level expression of CD38 correlated with expression of ZAP-70. CD38 is a 45-kDa type II transmembrane glycoprotein with ectoenzymatic activity,20-22 which has been argued to play a complex role in B-cell activation and proliferation.23 CLL-cell expression of CD38 has been associated with the use of nonmutated immunoglobulin V genes and more aggressive clinical disease.2,24 However, similar to other studies,25 we found that the expression of CD38 was not invariably associated with immunoglobulin mutational status. Indeed, one of the samples that expressed mutated immunoglobulin V genes (CLL16) also expressed CD38. Because this case also was ZAP-70+, it is conceivable that CD38 may play a role in expression of ZAP-70. However, because 7 of the 13 CLL cases that were ZAP-70+ had only negligible levels of CD38, expression of ZAP-70 appears neither dependent on CD38 nor sufficient to induce its expression in CLL.
The use of samples obtained from monozygotic twins concordant for CLL allowed us to examine for differences in leukemia cell populations that are likely due to somatic events. The CLL cells from both twins expressed mutated immunoglobulin VH genes of the VH3 subgroup, but were discordant for expression of ZAP-70 and CD38. Because these twins were monozygous, this analysis suggests that the differences between patients in leukemia-cell expression of ZAP-70 and/or CD38 in CLL cannot be explained by genetic polymorphism.
We found that leukemia cell samples that expressed ZAP-70 had a greater increase in tyrosine phosphorylation following BCR ligation than did leukemia cell samples lacking expression of ZAP-70. Prior studies demonstrated that CLL cases were heterogeneous in their ability to respond to BCR cross-linking.26,27 Furthermore, CLL cells that expressed CD38 appeared more responsive to BCR ligation.28 This association between CD38 and signaling intensity was also observed in the leukemia cell samples examined in this study. However, this may just be a reflection of the noted association between CD38 and expression of ZAP-70. All cases that expressed ZAP-70 were noted to have an increase in tyrosine phosphorylation following BCR ligation. Moreover, the extent of the average BCR-induced increase in signal intensity for ZAP-70 cases, even for ZAP-70+ cases that had negligible expression of CD38 (Figure 4), was significantly higher than that noted for samples that did not express ZAP-70.
One of the proteins that is phosphorylated after BCR ligation is Syk. Syk is an important PTK in the BCR-signaling pathway.12,29,30 Prior studies noted that the CLL cells of different patients varied in the extent to which Syk was phosphorylated following BCR ligation.31,32 Moreover, the extent to which Syk underwent tyrosine phosphorylation appeared to be associated with the ability of the leukemia cells to respond to BCR ligation.32 However, as noted in this and a prior study,32 the differences between CLL cases in the levels of Syk phosphorylation following BCR ligation were not apparently related to differences in the levels of Syk protein found in each of the CLL cell populations. Instead, we found that the leukemia cells that were better able to respond to BCR ligation expressed ZAP-70 in addition to Syk. Moreover, the average level of BCR-induced Syk phosphorylation in such cases was significantly greater than that observed in cases that did not express ZAP-70.
It is not clear whether expression of ZAP-70 contributes to the enhanced BCR-signaling capacity noted for CLL samples that express this protein. Prior studies indicated that Syk has an approximately 100-fold greater intrinsic PTK activity than does ZAP-70.33 Also, in contrast to ZAP-70, Syk can undergo stimulation in the absence of src-related kinases, initiate immunoreceptor signaling, and promote tyrosine phosphorylation of ITAMs in the BCR complex.34 In addition, normal B cells do not express ZAP-70 and yet respond to BCR ligation as well as do the CLL cases that have the highest response to stimulation via the BCR complex.32 Therefore, ZAP-70 is not necessary for effective BCR signaling, at least by normal B cells. Nevertheless, the association between expression of ZAP-70 and magnitude of tyrosine phosphorylation induced by surface immunoglobulin cross-linking in CLL suggests that this PTK may enhance the signaling capacity of the BCR complex in B-cell CLL.
Consistent with this notion, we found evidence that ZAP-70 may function in BCR signaling. Following ligation of the BCR, we found that ZAP-70 undergoes tyrosine phosphorylation and complexes with the proteins of the BCR complex. Conceivably, expression of ZAP-70 may lower the threshold for BCR signaling. ZAP-70 has been reported to promote tyrosine phosphorylation of the T-cell–receptor signaling motifs in immature CD4+CD8+ thymocytes in response to TCR cross-linking.35 Therefore, ZAP-70 may function to enhance phosphorylation of such motifs in CLL B cells and thereby lower the threshold for Syk phosphorylation following BCR ligation. Alternatively, expression of ZAP-70 in CLL cells may enhance the stability of phosphorylated Syk, allowing accumulation of the functional form of Syk in CLL following BCR ligation. Transfection of leukemia cells that lack ZAP-70 may be required to test these hypotheses. Nevertheless, expression of ZAP-70 appears most closely associated with the relative capacity of the BCR complex to effect signaling in the CLL B cell. Such differences may contribute to the noted clinical heterogeneity among patients with this disease.
The authors appreciate the skilled advice and assistance of Ms Esther Avery.
Prepublished online as Blood First Edition Paper, August 8, 2002; DOI 10.1182/blood-2002-06-1683.
Supported in part by National Institutes of Health grants R37 CA49870 (T.J.K.) and PO1 CA81534 (CLL Research Consortium) and P01 AI45865 (A.W.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Thomas J. Kipps, 9500 Gilman Dr, UCSD School of Medicine, La Jolla, CA 92093; e-mail:tkipps@ucsd.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal