CD5 is a negative regulator of B-cell receptor (BCR) signaling that is up-regulated after BCR stimulation and likely contributes to B-cell tolerance in vivo. However, CD5 is constitutively expressed on the B-1 subset of B cells. Contrary to CD5− B-2 B cells, B-1 B cells are long-lived because of autocrine interleukin-10 (IL-10) production through unknown mechanisms. We demonstrate herein a direct relationship between CD5 expression and IL-10 production. Human peripheral blood CD5+ B cells produce more IL-10 than CD5− B cells after BCR activation. Introducing CD5 into CD5− B cells induces the production of IL-10 by activating its promoter and the synthesis of its mRNA. The cytoplasmic domain of CD5 is sufficient for this process. CD5 also protects normal human B cells from apoptosis after BCR stimulation while reducing the BCR-induced Ca2+ response. We conclude that CD5 supports the survival of B cells by stimulating IL-10 production and by concurrently exerting negative feedback on BCR-induced signaling events that can promote cell death.
Introduction
CD5 is a lymphoid marker—one of the earliest acquired during T-cell ontogeny. Its expression increases coordinately with that of cell surface CD3.1 Although expressed on lymphoid-committed progenitors, its expression is lost following natural killer (NK) cell differentiation.2 In contrast to its being a pan–T-cell marker, CD5 is only expressed on some B cells. Based on its coexpression with CD11b, immunogloublin M (IgM+) B cells in mouse and human are classically separated into different subsets, termed B-1a and B-1b, each of which expresses CD11b and B-2.3 The B-1a subset expresses CD5, and at a functional level these CD5+ B cells frequently produce polyreactive antibodies, mainly IgM, that recognize a variety of self-antigens and foreign antigens.4 The reason for the expression of CD5 on a particular B-cell subset—something Wortis and Berland5call “one of many intriguing and seemingly idiosyncratic features of B-1a cells”—has remained obscure. Two hypotheses are proposed to explain the origin of B-1 cells. The lineage hypothesis holds that only certain fetal progenitors are destined to become B-1 cells.6,7 In contrast, the differentiation hypothesis holds that every B cell is able to acquire B-1 cell characteristics. In brief, the notion of B-cell lineage based on differential CD5 expression is controversial because of the lack of clear identification of a fetal progenitor destined to become a B-1 B cell and the demonstration that CD5− cells can acquire CD5 expression in vivo8 or in vitro9 after B-cell receptor (BCR) stimulation This up-regulation supports the initial view that CD5 is an activation marker10; however, a definitive function for this antigen remains to be established.
It has been shown that CD5 up-regulation in B cells plays a role in tolerance to autoantigens. By setting the threshold level for activation signals, CD5 prevents B cells from activation-induced cell death and maintains tolerance in anergic B cells in vivo.11 The reason for keeping potentially autoreactive cells alive is that these cells are also necessary for an effective immune response to some pathogens.12 This supported the role of CD5 as a negative regulator of BCR signaling, which was later demonstrated by the generation of CD5-null mice.13 In these animals, peritoneal B cells, which are poorly responsive to BCR stimulation, restored their capacity to fully proliferate to anti-IgM.13
The molecular basis for BCR inhibition by CD5 has been extensively investigated. The physical association of CD5 to the BCR was demonstrated,14 and our recent work further documented the structural basis of CD5 inhibition of BCR-mediated signals. Using a reconstitution approach, in a murine lymphoma B cell line we showed that Ca2+ response, extracellular signal-related kinase–2 (ERK-2) activation, and the production of interleukin-2 (IL-2) induced by BCR activation were antagonized by CD5.15 The role of the src autophosphorylationlike motif within the cytoplasmic domain was demonstrated.16 Of interest, this domain is highly conserved between mice and humans.3
We investigated the role of CD5 in human B cells. We surmised that either the constitutive or the BCR-induced expression of CD5 would play a role in B-cell survival, possibly through the induction of cytokines. IL-10 is the most relevant cytokine for B-cell survival in mice and humans. In mice, CD5+ B-1 B cells, unlike B-2 cells, are long-lived in vivo and in vitro.6,7 IL-10 is involved in the long-term maintenance of peritoneal B cells17 because it is produced mainly by B-1 cells, suggesting a positive autocrine regulatory loop.18 19 However, the precise mechanism of IL-10 production is unknown, and a possible direct relationship between CD5 expression and IL-10 secretion has never been addressed. When studying human peripheral B cells, we noticed that the CD5+subset constituting 10% of B cells produced more IL-10 and survived longer in vitro after activation than the CD5− subset. In addition, BCR activation elicited a lower Ca2+ response in CD5+ than in CD5− cells. This demonstrated that human B cells behave like murine B cells in this respect. We therefore reconstituted CD5− human B cells from the Epstein-Barr virus (EBV)–positive, Burkitt lymphoma-derived Daudi cell line with CD5. Our results demonstrate that CD5 helps B cells to survive after BCR engagement through 2 distinct mechanisms: (1) down-regulation of BCR-mediated early events, preventing B cells from overactivation and leading to BCR-mediated cell death, and (2) enhanced IL-10 production, providing a survival factor to B cells. The implications in CD5+ malignancies are discussed.
Materials and methods
Cells and cell cultures
The human B-lymphoma cell line Daudi was grown in RPMI 1640 medium (Seromed; Biochrom KG, Berlin, Germany) supplemented with 10% fetal calf serum (FCS), antibiotics (50 U/mL penicillin, 50 μg/mL streptomycin), 2 mM l-glutamine, and 1 mM sodium pyruvate (complete medium). Daudi-LZRS and Daudi-LZRS CD5 cells were grown in complete medium. Daudi-pNT.CD5 and Daudi-pNT.FcR-CD5 cells expressing a chimeric receptor made of the FcγRIIB1 extracellular and transmembrane domains, fused to the human CD5 cytoplasmic tail, were cultured in the same medium supplemented with 1 mg/mL G-418 (Geneticin; Invitrogen, Groningen, The Netherlands). This medium was also used for selection and culture of the pNT-neo vector stable transfectants (see “Constructs”).
Peripheral B lymphocytes were obtained from blood donors with their informed consent. Blood was centrifuged on lymphocyte separation medium, density 1077 (Eurobio, Les Ullis, France), and the resultant peripheral blood mononuclear cells (PBMCs) were submitted to B-cell–negative isolation kit (Dynal Biotech, Oslo, Norway). B cells comprised 8% to 15% of PBMCs and were up to 95% pure as assessed by CD19 staining. Cells were double stained with anti-CD5 and anti-CD3 to exclude residual T cells. CD5+ and CD5− B cells were then separated with a FACStar Plus cell sorter (Becton Dickinson, San Jose, CA).
Antibodies and reagents
For blockade of IL-10 in culture, we used rabbit polyclonal neutralizing antihuman IL-10 (SA Innotest, Besançon, France). Fluorescein isothiocyanate (FITC)– or phycoerythrin (PE)–labeled mouse antihuman CD5 (clone BL1A), CD19-B4 RD1, CD45RA-FITC, CD80-FITC, and CD86-PE were purchased from Beckman Coulter (Marseille, France). AffiniPure F(ab′)2 fragment rabbit IgG fraction to human IgM and Affinipure rabbit IgG fraction (whole molecule) to human IgM were obtained from Jackson ImmunoResearch Laboratories (West Grove, PA). CD27-PE was from PharMingen (BD Biosciences, France), and IgD-FITC was from DAKO (Copenhagen, Denmark).
Apoptosis was determined by staining cultured cells with Annexin V and propidium iodide (PI) using the FITC-labeled Annexin V kit (Beckman Coulter). CD40 ligand trimer (500 ng/μL) is a kind gift of Dr E. K. Thomas (Immunex, Seattle, WA).
Constructs
LZRS.GFP was constructed by inserting the internal ribosomal entry site–enhanced green fluorescence protein (IRES-EGFP) sequence from the pIRES2.EGFP plasmid (catalog no. 6029.1; Clontech) into the G Nolan LZRSpBMN-Z retroviral vector.20 The bicistronic retroviral vector LZRS.CD5.GFP was constructed by inserting the CD5 sequence at the XhoI site. Vectors encode EGFP downstream of the IRES sequence that reinitiates translation from the full-length premessenger RNA. Retroviral particles were produced by transient transfection of the helper-free retrovirus packaging cell line Phoenix by calcium phosphate coprecipitation. The defective retroviral supernatant was collected, passed through a 0.45-μm filter, and stored at −80°C until used to infect the Daudi cells. Infected Daudi cells were sorted based on their expression of EGFP.
Full-length cDNA of human CD5 from the pRC vector (kindly given by Dr Laurence Boumsell, INSERM U448, Créteil, France) was used for subcloning into the pNT-neo vector after NotI restriction enzyme digestion and ligation. This construct was linearized byScaI restriction enzyme digestion and was purified by SS-phenol extraction and ethanol precipitation. Daudi cells (5 × 106) were mixed with 20 μg digested plasmid DNA and electroporated at 260 V, 960 microfarads (μF) before selection with G-418 and sorting of CD5+ cells. An FcγRIIB-CD5cyt construct was generated as previously described,15 and a stable Daudi cell line expressing FcγRIIB-CD5cyt chimera (Daudi-pNT.FcR-CD5) was established as described above.
Ca2+ response analysis
Intracellular Ca2+ measurements on cell suspensions were performed with Fura-2/AM (Molecular Probes, Eugene, OR) as described previously.13 For Ca2+ response and CD5 expression correlation experiments, purified B cells were placed on gridded coverslips (CELLocate; Eppendorf, Hamburg, Germany), marking the localization of individual cells. Single-cell imaging was analyzed with a Diaphot 300 microscope (Nikon, Melville, NY) and an IMSTAR imaging system, as described previously.14 After a 20-minute Ca2+ measurement, cells were stained directly with PE-conjugated anti-CD5 and were fixed with 4% paraformaldehyde. Fluorescence analysis was made with Metavue software (Universal Imaging, Wester Chester, PA). Ca2+ responses and CD5 expression analysis were made independently, and results from the same cells used in the 2 experiments were compared.
Cytokine detection
IL-10 levels in supernatants of peripheral blood or Daudi B cells were measured by enzyme-linked immunosorbent assay (ELISA) using a detection kit (Diaclone, Besançon, France).
Analysis of IL-10 promoter activity using the luciferase assay
Then 107 Daudi-LZRS or Daudi-LZRS.CD5 cells were cotransfected by electroporation using 10 μg pGL2-Basic vector (without insert) or the pGL2-1308 vector previously described21 (containing the promoter/enhancer region of the human IL-10 gene inserted upstream of the luciferase gene), with 5 ng pRL-SV40 (Promega, Madison, WI) as an internal control plasmid. Daudi cells were electroporated at 280 V, 960 μF. If required, cells were stimulated after electroporation with 2 μg/mL F(ab′)2 anti-IgM for 48 hours. Cells were harvested 48 hours after electroporation and were lysed according to the manufacturer's instructions before promoter activities were analyzed using the Dual-Luciferase Reporter Assay System (Promega). These assays were repeated in quadruplicate to account for different transfection efficiencies.
Results
CD5+ peripheral B cells produce more IL-10 than CD5− B cells
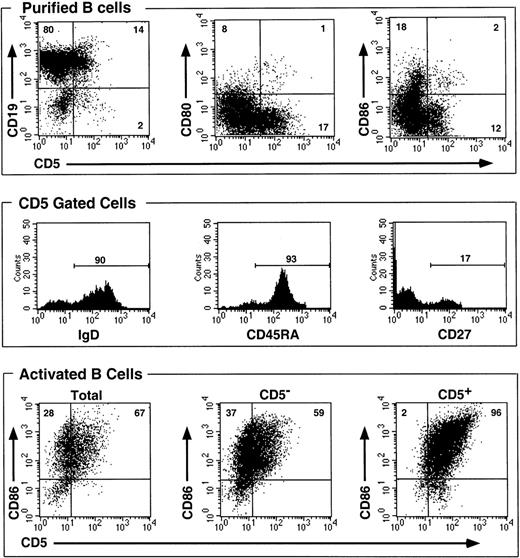
We purified B cells from normal peripheral blood by negative selection using magnetic beads. This method yielded on average a 94% pure CD19+ B-cell population. B cells were mostly CD5− (mean fluorescence intensity [MFI], 7.01) and were composed of 85% to 90% CD5− B cells (MFI, 3.89) and 10% to 15% CD5+ B cells (MFI, 30.62), based on a 13.0 MFI positivity threshold. The latter cells were mostly (more than 90%) IgM+ IgD+ CD45RA+ and CD80− and CD86−, whereas less than 20% were CD27+ (Figure 1, top and middle).
CD5+ peripheral blood B cells do not belong to an activated subset, and only a fraction of activated B cells express CD5 following activation.
B cells were purified by negative selection on beads from normal PBMCs leading to a greater than 90% pure population. Cells were double stained with mAbs, before (top and middle) or after activation with F(ab′)2 anti-IgM (10 μg/mL) and CD40 ligand trimer (2.5 μg/mL final) for 48 hours (bottom). Percentages of positive cells are shown on bars or inside quadrants.
CD5+ peripheral blood B cells do not belong to an activated subset, and only a fraction of activated B cells express CD5 following activation.
B cells were purified by negative selection on beads from normal PBMCs leading to a greater than 90% pure population. Cells were double stained with mAbs, before (top and middle) or after activation with F(ab′)2 anti-IgM (10 μg/mL) and CD40 ligand trimer (2.5 μg/mL final) for 48 hours (bottom). Percentages of positive cells are shown on bars or inside quadrants.
The lack of expression of CD80 and CD86 activation markers22,23 ruled out the possibility that CD5+ B cells represented a predominantly activated subpopulation. Next, total purified B cells in bulk were stimulated or not stimulated with anti-IgM + CD40 ligand trimer and were reanalyzed for the expression of CD5 and CD86. Supernatants were harvested at different periods of time, and IL-10 was measured using ELISA (Figure 2A). IL-10 production peaked at 36 hours and started to decline after 48 hours. Stimulated cells produced 10- to 20-fold more IL-10 than unstimulated cells. Note that in BCR-activated cells,22 23 CD86 was induced as expected in all 3 populations after 48 hours of stimulation (Figure 1, bottom). CD5 was also up-regulated on B cells, especially in the B-cell population that already expressed CD5 before stimulation (MFI, 93.29). As can be seen (Figure 1, bottom), however, CD5 was expressed on 67% of total B cells (MFI, 32.52) and on 59% of B cells from CD5− cells (MFI, 23.42) and on all B cells from CD5+ cells. Thus, not all B cells expressed CD5 following BCR activation because 37% remained CD5− (MFI, less than 13.0).
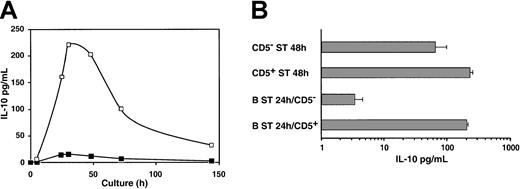
CD5+ B cells produce more IL-10 than CD5− B cells following BCR stimulation.
(A) Kinetic analysis of IL-10 production. Purified B cells were cultured in medium alone or with F(ab′)2 anti-IgM (10 μg/mL) and CD40 ligand trimer. Supernatants of 105 cells were harvested sequentially until day 7, and IL-10 was measured using ELISA. Shown are the means from 2 experiments. (B) Respective IL-10 production by CD5− and CD5+ activated blood B cells. CD5− ST and CD5+ ST 48 hours. Negatively selected B cells were stained with anti-CD3 and anti-CD5 mAbs and were sorted based on CD3−CD5− and CD3−CD5+ expression. Thereafter 105 cells were cultured in medium with F(ab′)2anti-IgM (10 μg/mL) and CD40 ligand trimer for 48 hours, and supernatants were harvested for IL-10 production assay.
CD5+ B cells produce more IL-10 than CD5− B cells following BCR stimulation.
(A) Kinetic analysis of IL-10 production. Purified B cells were cultured in medium alone or with F(ab′)2 anti-IgM (10 μg/mL) and CD40 ligand trimer. Supernatants of 105 cells were harvested sequentially until day 7, and IL-10 was measured using ELISA. Shown are the means from 2 experiments. (B) Respective IL-10 production by CD5− and CD5+ activated blood B cells. CD5− ST and CD5+ ST 48 hours. Negatively selected B cells were stained with anti-CD3 and anti-CD5 mAbs and were sorted based on CD3−CD5− and CD3−CD5+ expression. Thereafter 105 cells were cultured in medium with F(ab′)2anti-IgM (10 μg/mL) and CD40 ligand trimer for 48 hours, and supernatants were harvested for IL-10 production assay.
To analyze the respective contribution of the CD5+ and CD5− subsets in IL-10 production, 2 types of experiments were conducted. First, freshly isolated CD5+ and CD5− cells were sorted, and IL-10 production was compared after 48 hours of stimulation. We observed that CD5+ cells produced significantly more IL-10 than sorted CD5− cells (Figure 2B, top panels).
Second, unfractionated B cells were stimulated for 24 hours, a period sufficient for the acquisition of CD5 by more than 50% of B cells (not shown). Then CD5+ and CD5− B-cell fractions were sorted and restimulated for 48 hours before IL-10 measurements. We found a 70-fold higher production of IL-10 by CD5+ cells than by cells that remained CD5− (Figure 2B, bottom panels). Strikingly, although CD5− cells were activated (also evidenced by blastic shape and activation markers; not shown), they produced lower amounts of IL-10 than in the previous experiments in which they were sorted before any stimulation. Thus, only cells expressing CD5 before stimulation or that acquired CD5 de novo contributed to the production of IL-10. As demonstrated below, this does not reflect the inability of CD5− cells to respond to BCR stimulation.
CD5+ human peripheral B cells display a lower BCR-mediated Ca2+ response than CD5− B cells
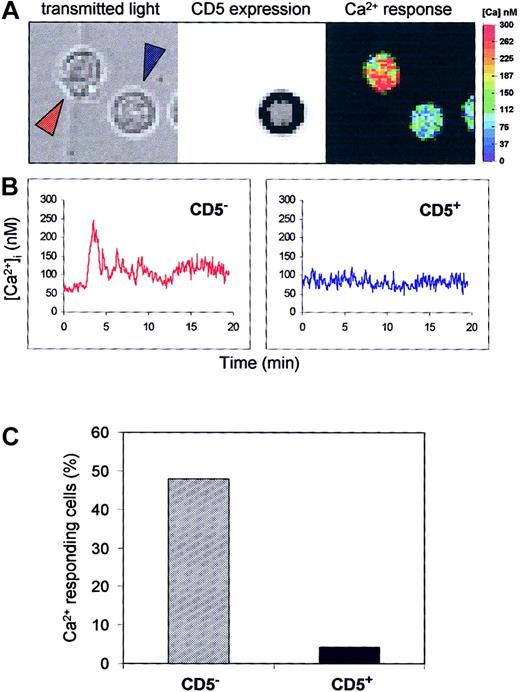
The demonstration that CD5 antagonizes early signaling events mediated by the BCR was made by other investigators in CD5-null mice13 and by us in murine cell lines.14,15We investigated whether this was true in human blood B cells. We based our experiments on negative purification of B cells instead of CD19-based sorting to avoid any signaling through the CD19 molecule.24 Unfractionated (more than 90% pure) B cells were allowed to settle on a gridded coverslip and were stimulated with anti-IgM, and the Ca2+ response was analyzed using an imaging system. Thereafter, cells were stained on coverslips with anti-CD5 monoclonal antibody (mAb) and fixed, and CD5 expression was revealed by microscopy. An example is illustrated in Figure3, showing a Ca2+ response and staining of CD5 in 2 individual cells. Only the cell that did not stain with anti-CD5 elicited a clear calcium response. The percentage of responding cells was then calculated. It was much lower in the CD5+ cells. The mean basal intracellular calcium concentration was calculated for each cell for 1 minute before the antibody was added, and the average level for the CD5− and CD5+ populations was analyzed. Data were expressed as mean ± SD, and the significant differences between the 2 series of results were assessed with the 2-tailed Student ttest.
Peripheral blood CD5+ B cells are less calcium responsive than CD5− B cells after BCR activation.
Purified blood B cells were loaded with Fura-2/AM, and the Ca2+ response of individual cells to anti-IgM (25 μg/mL) stimulation was performed before anti-CD5 staining. (A) Ca2+ levels were analyzed by single cell imaging (right), CD5 expression was revealed by immunofluorescence (middle), and the corresponding transmitted light image (left) of 2 B cells is shown. (B) Individual Ca2+ response of the 2 B cells indicated by blue and red arrows. The CD5− cell (red) shows an intracellular Ca2+ increase, but the CD5+ cell (blue) does not. (C) Occurrence of Ca2+ response in CD5−and CD5+ B cells. Data are from 254 CD− cells and 23 CD5+ cells from 3 independent experiments.
Peripheral blood CD5+ B cells are less calcium responsive than CD5− B cells after BCR activation.
Purified blood B cells were loaded with Fura-2/AM, and the Ca2+ response of individual cells to anti-IgM (25 μg/mL) stimulation was performed before anti-CD5 staining. (A) Ca2+ levels were analyzed by single cell imaging (right), CD5 expression was revealed by immunofluorescence (middle), and the corresponding transmitted light image (left) of 2 B cells is shown. (B) Individual Ca2+ response of the 2 B cells indicated by blue and red arrows. The CD5− cell (red) shows an intracellular Ca2+ increase, but the CD5+ cell (blue) does not. (C) Occurrence of Ca2+ response in CD5−and CD5+ B cells. Data are from 254 CD− cells and 23 CD5+ cells from 3 independent experiments.
These results show that the basal calcium level, albeit slightly higher in CD5+ cells (82.7 ± 37.8 vs 68.9 ± 36.9), was not significantly different between the 2 B-cell populations. Thus, as in B-1a murine B cells, CD5 is a negative regulator of BCR signaling in peripheral blood human B cells.
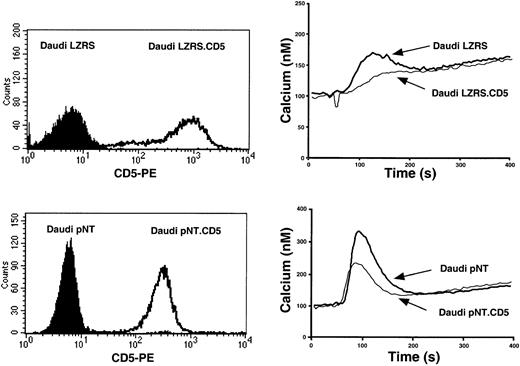
Introduction of CD5 into Daudi cells inhibits BCR signaling and triggers IL-10 production
To further prove the role of CD5 in IL-10 production, we used a CD5− human B-cell line, Daudi, unable to express surface CD5 even after BCR + CD40 ligand trimer stimulation (A.H.D., unpublished observations, April 2001). Daudi B cells were stably transfected with 2 different vectors: pNT-neo and retroviral LZRS encoding the full-length CD5 molecule. Stably transfected cells were selected for the high expression of surface CD5 (Figure 4, left). Cells were also selected for similar density of surface IgM (data not shown). Cells transfected with the vectors containing or not containing CD5 were stimulated with rabbit anti-IgM, and the Ca2+ response was analyzed. Although BCR triggering induced a sustained Ca2+ response in control cells transfected with vectors alone, the peak was dramatically reduced in CD5-transfected cells (Figure 4, right). The effect was clearly specific for CD5 and was not influenced by the vector. Note that Ca2+ responses are lower in GFP-containing LZRS-transfected cells. This may reflect the parallel overexpression of GFP in these cells with this bicistronic vector (see “Materials and methods”). These results are in keeping with those observed previously in a murine B-lymphoma line, IIA1.6.15 16 Thus, at least for the Ca2+response, CD5 exerted a comparable effect in a transformed human B-cell line and in normal B cells.
Introduction of CD5 inhibits BCR-induced calcium release in human Daudi B lymphocytes.
(Left) FACS analysis of CD5 expression. (Right) Ca2+response intensity following BCR stimulation. CD5− Daudi cells were transfected with pNT-neo or LZRS vectors containing or not containing full-length human CD5 cDNA. Transfected Daudi cells were stained with CD5 mAbs or loaded with the fluorescent Ca2+indicator Fura-2/AM, and the fluorescence of the suspension was monitored with a spectrometer in 1 mM Ca2+-containing medium at 37°C and was stimulated 50 seconds later with 2 μg/mL rabbit antihuman IgM.
Introduction of CD5 inhibits BCR-induced calcium release in human Daudi B lymphocytes.
(Left) FACS analysis of CD5 expression. (Right) Ca2+response intensity following BCR stimulation. CD5− Daudi cells were transfected with pNT-neo or LZRS vectors containing or not containing full-length human CD5 cDNA. Transfected Daudi cells were stained with CD5 mAbs or loaded with the fluorescent Ca2+indicator Fura-2/AM, and the fluorescence of the suspension was monitored with a spectrometer in 1 mM Ca2+-containing medium at 37°C and was stimulated 50 seconds later with 2 μg/mL rabbit antihuman IgM.
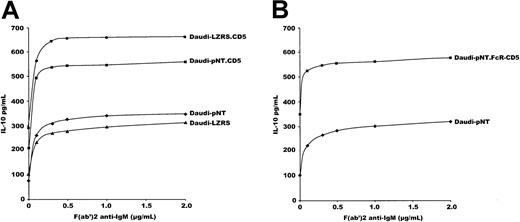
We next measured IL-10 production in vector-transduced and in CD5-transduced cells. Note that wild-type Daudi cells produce IL-10 in culture (unpublished data). As shown (Figure5A), stimulating the cells with increasing concentrations of F(ab′)2 anti-IgM augmented the production of IL-10 in CD5− (vector-transduced) and CD5-transfected Daudi cells. The latter produced 3 times more IL-10 than cells transfected with vectors alone. Results were similar to those produced with whole anti-IgM antibody (data not shown). Anti-IgM induced the production of IL-10 in a dose-response manner, reaching a plateau for 1 to 2 μg/mL F(ab′)2.
CD5 constitutively induces IL-10 production in Daudi cells.
(A) 5 × 104 Daudi cells transfected with CD5-containing vectors or with empty vectors were stimulated with increasing amounts of F(ab′)2 anti-IgM, and IL-10 was measured in the supernatants by ELISA after 48 hours of culture. (B) The same experiments with 5 × 104 Daudi cells transfected with pNT-containing an FcγRIIb-cytoplasmic CD5 chimera (Daudi-pNT.FcR-CD5). Data of IL-10 production are expressed in pg/mL. Results are representative of 6 experiments.
CD5 constitutively induces IL-10 production in Daudi cells.
(A) 5 × 104 Daudi cells transfected with CD5-containing vectors or with empty vectors were stimulated with increasing amounts of F(ab′)2 anti-IgM, and IL-10 was measured in the supernatants by ELISA after 48 hours of culture. (B) The same experiments with 5 × 104 Daudi cells transfected with pNT-containing an FcγRIIb-cytoplasmic CD5 chimera (Daudi-pNT.FcR-CD5). Data of IL-10 production are expressed in pg/mL. Results are representative of 6 experiments.
Interestingly, most of the increment in IL-10 production was observed with 0.5 μg/mL F(ab′)2; however, the slope was steeper in CD5-transfected than in vector-transfected cells. Thus, CD5-transfected cells reached a plateau for IL-10 production with less anti-IgM than vector-transfected cells. One interpretation is that CD5 has a synergistic effect on BCR-mediated IL-10 production. Importantly, and surprisingly, the increase in IL-10 production in CD5+Daudi cells compared with CD5− cells also occurred in the absence of any BCR stimulation, as shown from the baseline IL-10 production (Figure 5A). Thus, CD5 constitutively induces IL-10 production and augments BCR-mediated IL-10 production.
We next transfected Daudi cells with the FcγRIIB-CD5cyt chimera devoid of the external and transmembrane domains of CD5. Again, a 3-fold enhancement in IL-10 production was observed with cytoplasmic CD5 compared with pNT vector (Figure 5B).
CD5 directly enhances IL-10 promoter activity
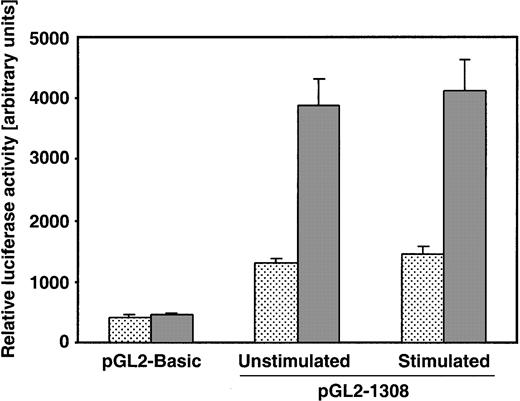
To gain more insight into the mechanism of IL-10 stimulation, we cotransfected Daudi-LZRS cells and Daudi-LZRS.CD5 cells with a pGL2 vector containing the promoter/enhancer region of the human IL-10 gene21 and with Renilla Luciferase control reporter vector. As shown (Figure 6), the basal activity of the IL-10 promoter was augmented up to 3 times more in CD5+ cells than in CD5− cells (1306.5 ± 80 vs 3879 ± 449 arbitrary units).
CD5 constitutively activates human IL-10 promoter.
Daudi-LZRS or Daudi-LZRS.CD5 cells stimulated or not stimulated with 2 μg/mL F(ab′)2 anti-IgM were cotransfected withRenilla Luciferase control reporter vector pRL-SV40 and each of the different pGL2 vectors, pGL2-Basic and pGL2-1308 (containing the human IL-10 promoter/enhancer region). To take into account different transfection efficiencies, firefly luciferase activity of the pGL2 vectors was normalized to Renilla luciferase activity within each experimental setting and displayed as arbitrary units. Four different electroporation assays for each condition were conducted. Shown are the control activity of pGL2 basic vector and the basal activity (unstimulated) and anti-IgM–induced activity of pGL2-1308 vector (stimulated) in CD5− (dotted) and CD5+cells (gray). Data shown are means ± SD of 4 experiments.
CD5 constitutively activates human IL-10 promoter.
Daudi-LZRS or Daudi-LZRS.CD5 cells stimulated or not stimulated with 2 μg/mL F(ab′)2 anti-IgM were cotransfected withRenilla Luciferase control reporter vector pRL-SV40 and each of the different pGL2 vectors, pGL2-Basic and pGL2-1308 (containing the human IL-10 promoter/enhancer region). To take into account different transfection efficiencies, firefly luciferase activity of the pGL2 vectors was normalized to Renilla luciferase activity within each experimental setting and displayed as arbitrary units. Four different electroporation assays for each condition were conducted. Shown are the control activity of pGL2 basic vector and the basal activity (unstimulated) and anti-IgM–induced activity of pGL2-1308 vector (stimulated) in CD5− (dotted) and CD5+cells (gray). Data shown are means ± SD of 4 experiments.
In agreement with the increase in IL-10 production, this effect was observed in unstimulated cells. Strikingly, BCR stimulation with anti-IgM had no significant additional effect on the promoter activity. The amount of IL-10–specific mRNA produced under the same conditions was also evaluated by reverse transcription–polymerase chain reaction (RT-PCR) and was higher after BCR activation and in CD5+ unstimulated B cells than their CD5−counterparts (data not shown). These data further strengthened the role of CD5 as an autonomous inducer of IL-10 mRNA and protein synthesis.
CD5 and peripheral B-cell survival
It has been shown that strong BCR-mediated activation ultimately leads to programmed cell death.25 Meanwhile, CD5, which is up-regulated after BCR engagement, exerts a negative feedback on the BCR, thus contributing to B-cell survival. On the other hand, IL-10 was shown to promote B-cell survival.17-19 Our results suggested that IL-10 may also contribute to the enhancement of B-cell survival mediated by CD5 up-regulation. We tried here to address the physiologic relevance of our observations by studying BCR-mediated cell death in peripheral blood B cells. We activated B cells and cultured them in medium alone or with neutralizing anti–IL-10 antibodies (Figure 7).
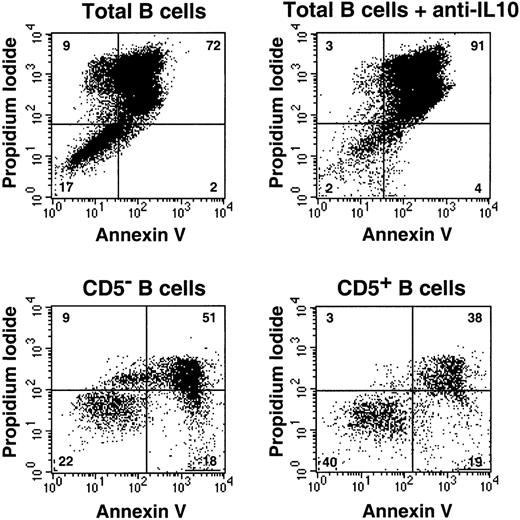
IL-10 protects B cells from cell death.
(Top) Bulk B cells were stimulated with F(ab′)2 anti-IgM (10 μg/mL) and CD40 ligand trimer with or without neutralizing anti–IL-10 antibodies. Cells were then stained at day 7 with FITC-labeled Annexin V and PI and were analyzed using fluorescence-activated cell sorting (FACS). (Bottom) Purified CD5− and CD5+ B cells were activated as above for 48 hours, and apoptosis was measured at day 4. Results are representative of 3 experiments.
IL-10 protects B cells from cell death.
(Top) Bulk B cells were stimulated with F(ab′)2 anti-IgM (10 μg/mL) and CD40 ligand trimer with or without neutralizing anti–IL-10 antibodies. Cells were then stained at day 7 with FITC-labeled Annexin V and PI and were analyzed using fluorescence-activated cell sorting (FACS). (Bottom) Purified CD5− and CD5+ B cells were activated as above for 48 hours, and apoptosis was measured at day 4. Results are representative of 3 experiments.
B-cell survival was investigated by double staining with PI and Annexin V. As shown, the survival rate at day 7 of culture was 17% with medium compared with 2% with anti–IL-10. Although we did not intend to study the mechanism of B-cell death, we could observe PI+ Annexin V+ necrotic cells in our cultures. Earlier analysis would possibly have shown predominantly PI− Annexin V+ apoptotic cells, as expected.25 Because CD5+ cells are the principal IL-10 producers, we would expect them to have a longer lifespan after stimulation. Indeed, when sorted and activated as above, twice as many CD5+ cells as CD5− cells were alive at day 4. Therefore, CD5+ peripheral blood B cells survive longer than CD5− cells after BCR stimulation.
Discussion
B cells up-regulate CD5 after interaction with an autoantigen26 or a T-independent antigen.9 In turn, because CD5 is physically associated with the BCR14and down-regulates BCR-induced early events,13,15 it prevents further B-cell activation. This would explain, for instance, why anergic B cells broke tolerance when transplanted into a CD5-null background.11 However, negative signaling does not answer why CD5+ B cells have a longer lifespan than CD5− cells. In this work, we have shown for the first time that the CD5 molecule induces and enhances IL-10 production in human B cells and, consequently, that its expression may be associated with increased B-cell survival. Because the function of CD5 is similar in human and murine B cells, our findings facilitate an understanding of why autoantibody-producing B-1a cells express CD5 and have a longer lifespan because of the autocrine production of IL-10 in mouse models. However, because CD5 up-regulation is also observed on B-2 cells after BCR activation, it may also be a general mechanism involved in the control of B-cell survival after antigen recognition. Strong evidence for the potentiation by CD5 of BCR-induced IL-10 production is the ability of human CD5+ peripheral B cells to produce more IL-10 than their CD5− counterparts. This neither reflects the activation state of cells before BCR stimulation, which is not different between the 2 subsets, nor indicates that CD5−cells are anergic. Indeed, CD5− cells strongly up-regulate cell-surface activation markers after stimulation, and they show, in contrast to CD5+ cells, a sustained calcium increase after BCR triggering. Note that, although low, the spontaneous IL-10 secretion by fresh CD5+ B cells was repeatedly found to be higher than that of their CD5− counterparts (data not shown). In conclusion, CD5 is not strictly an activation marker because it is present on a few blood B cells that do not coexpress CD80 or CD86. CD5 cannot be considered a lineage marker for B-1a cells because it is up-regulated on most B cells after BCR stimulation. It is possible that some B1-peritoneal B cells may be derived from activated B-2 cells.
Using a reconstitution approach, we showed that CD5 also exerted a positive effect on BCR-mediated IL-10 production in the human Daudi B-cell line. This can be inferred from CD5-transduced Daudi cells, which responded better to low anti-IgM stimulus and steeper dose-response assays than CD5− cells. Strikingly, an “autonomous” production of IL-10 was also induced by CD5 in the absence of any BCR stimulation. It could be argued that this production was mediated through signals delivered by a CD5 ligand present at the surfaces of Daudi cells. However, this possibility can be excluded by our data showing that the cytoplasmic domain is as effective as the full-length molecule to trigger IL-10 production. Moreover, culturing the cells with mAb against the CD5 molecule or against CD72—a putative CD5 ligand—has no influence on IL-10 production (A.H.D., unpublished data, November 2001).
Additional evidence suggesting that, in this cellular model, CD5 has a direct influence on IL-10 production independent of BCR stimulation came from studies on the IL-10 promoter. We found that anti-IgM stimulation failed to further increase the activity induced by CD5. Nevertheless, anti-IgM stimulation increased IL-10 mRNA (not shown) and protein production in CD5-transduced cells, suggesting that although CD5 directly enhances IL-10 transcription, BCR mediates its effect at a posttranscriptional level, possibly by stabilizing mRNA. How CD5 works to activate the IL-10 promoter is not yet understood, and it should be emphasized here that we have presented no evidence that CD5 can also directly influence the activity of this promoter in normal B cells. We could speculate that, because the promoter is already significantly activated in Daudi B cells, it would be highly sensitive to additive signals triggered by CD5. The nature of this signal is unknown. However, although CD5 is not significantly phosphorylated on tyrosine residues in unstimulated Daudi cells (not shown), likely excluding a role for src homology (SH2) domain-containing proteins in the observed phenomenon, the CD5 cytoplasmic domain can also recruit signaling molecules independently of BCR stimulation.27,28This situation may be achieved in activated normal B cells that have up-regulated the CD5 molecule or in CD5+ B-cell subsets that produce IL-10, such as the one in the peritoneum.17-19
An important issue, therefore, is to understand the molecular mechanisms governing CD5 expression. Indeed, other inducers than BCR, such as EBV, are able to modulate CD5 expression.10 It could be argued that the distinction between CD5+ and CD5− subsets is an artifact because all B cells seem to express little CD5 if analyzed by using an amplification technique.10 This, however, does not raise questions about our results because we showed that normal human peripheral B cells that up-regulate CD5 also up-regulate IL-10 in parallel, making our results applicable to all B cells. The respective roles of BCR and CD5 in IL-10 production could not be directly addressed in de novo CD5+peripheral B cells, mainly because we were unable to dissociate CD5 expression from BCR stimulation using several inhibitors of BCR-signaling pathways (H.G.-G., unpublished data, March 2001). Nevertheless, our transfected Daudi cell model demonstrates the direct role of CD5 in IL-10 production inasmuch as activated Daudi cells failed to express endogenous CD5.
We think, therefore, that by down-modulating BCR signaling and up-regulating IL-10 production in parallel, CD5 uses these metabolic processes coordinately to control the fate of B cells in general after antigen encounter and that its expression may also explain the prolonged survival of human B-cell subsets equivalent to B-1a cells in the mouse. Human B-1 B cells, albeit less well-characterized than murine ones, are found in cord blood29 and in adult blood30 and are enriched for cells that spontaneously produce polyreactive antibodies.4 The prototypic disease with proliferation of CD5+ cells is chronic lymphocytic leukemia. There is some evidence that malignant chronic lymphocytic leukemia (CLL) cells belong to B-1 cells31,32 and that IL-10 is an autocrine growth factor needed for the expansion of malignant B cells.32,33 This hypothesis is substantiated by the recent demonstration that de novo CD5+ diffuse large B-cell lymphomas are more aggressive than their CD5−counterparts.34 As a matter of fact, the role of IL-10 as an autocrine growth factor needed for the expansion of malignant B cells expressing the CD5 molecule may not be fortuitous.
We thank Hélène Fohrer for help in cell sorting.
Prepublished online as Blood First Edition Paper, August 8, 2002; DOI 10.1182/blood-2002-05-1525.
Supported by a grant from the Association de la Recherche contre le Cancer. J.H. is a recipient of a Fondation pour la Recherche Médicale fellowship.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Ali Dalloul, Laboratoire d'immunologie, Institut National de la Santé et de la Recherche Médicale Unité 543, 83 Bd de l'Hôpital, 75013, Paris, France; e-mail: dalloul@ccr.jussieu.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal