Regulatory T cells have been shown to control animal models of immune-mediated pathology by inhibitory cytokine production, but little is known about such cells in human disease. Here we characterize regulatory T-cell responses specific for a human red blood cell autoantigen in patients with warm-type autoimmune hemolytic anemia. Peripheral blood mononuclear cells from patients with autoimmune hemolytic anemia were found either to proliferate and produce interferon-γ or to secrete the regulatory cytokine interleukin 10 when stimulated in vitro with a major red blood cell autoantigen, the RhD protein. Flow cytometric analysis confirmed that the majority of the responding cells were of the CD4+phenotype. Serial results from individual patients demonstrated that this bias toward proliferative or interleukin-10 responses was unstable over time and could reverse in subsequent samples. Epitope mapping studies identified peptides from the sequence of the autoantigen that preferentially induced interleukin-10 production, rather than proliferation, and demonstrated that many contain naturally processed epitopes. Responses to such peptides suppressed T-cell proliferation against the RhD protein, an inhibition that was mediated largely by interleukin 10 and dependent on cytotonic T lymphocyte–associated antigen (CTLA-4) costimulation. Antigenic peptides with the ability to stimulate specific regulatory cells may represent a new class of therapeutic agents for immune-mediated disease.
Introduction
Current treatments for patients with autoimmune diseases rely on inducing generalized immunosuppression and are associated with serious side effects. The rational design of more specific, effective approaches will depend on a better understanding of the responses to the relevant autoantigens, which are poorly characterized in most human autoimmune diseases.
Autoantigen-specific CD4+ helper T (Th) cells are pivotal in the development of animal models of autoimmunity and therefore provide a target for novel forms of immunotherapy.1,2 Th cell clones can be classified into different functional types on the basis of the cytokines they secrete,3 and it has become clear that the pathogenicity of autoimmune responses can be determined by mutual antagonism between these helper subpopulations.1,2 Initially, attention focused on Th1 and Th2 cells, which produce interferon-γ (IFN-γ) and interleukin 4 (IL-4), respectively.3 Many models of autoimmune disease, including those that are antibody mediated, are driven by helper responses that are strongly dominated by the Th1 subset, and inducing a corresponding Th2 bias can prevent or ameliorate the pathology.1,2 However, in experimental animals, further, T-regulatory (Tr) cell subpopulations with important roles in immunoregulation and tolerance have now been defined.4-6In particular, production of the Tr1 cytokine IL-10 can protect rodents against a wide range of immune-mediated diseases, including autoimmune diabetes,7,8 experimental encephalomyelitis,9-11 and colitis,12 while Th3 cell secretion of transforming growth factor-β (TGF-β) prevents spontaneous autoimmunity13 and mediates some forms of oral tolerance.14 Regulatory subpopulations characterized by CD25 expression also have been isolated from rodents5,6and, more recently, from human peripheral blood, but in most reports the suppressive effects of these cells are nonspecific and not dependent on cytokine production.5,6 15-18
Although important to the design of future therapy,4,19the subsets of autoantigen-specific Th cells in most human autoimmune diseases have not been defined and, in particular, virtually nothing is known about secretion of the regulatory cytokines IL-10 and TGF-β. Organ-specific, antibody-mediated conditions, such as autoimmune hemolytic anemia (AIHA), in which the targets are well characterized, provide an opportunity to study Th cell responses specific for autoantigens of pathogenic relevance. In AIHA, autoantibodies bind to red blood cells (RBCs) and reduce their life span in vivo.20 The disease can be classified according to the temperature reactivity and class of the autoantibodies20: “warm” IgG antibodies are the most common, have optimal affinity at 37°C, and promote RBC destruction by splenic macrophage phagocytosis and/or complement-mediated lysis.20 In the majority of patients, warm antibodies are specific for the Rh protein complex,21,22 which also expresses important blood groups. Current therapy for warm-type AIHA is reliant on corticosteroids, often given over a long period of time, with transfusion to treat any life-threatening hemolytic crises, and other immunosuppressive drugs or splenectomy used in refractory or relapsing cases.20 Since these approaches typically control, rather than cure, the disease and can cause serious side effects, there is a pressing need to develop more effective, safer methods of specific immunotherapy.
Examples of murine AIHA are helper dependent,23-27 with the spontaneous form of the disease affecting NZB mice being mediated by a Th1-biased autoreactive response.2,28 In human AIHA, Th cells have been studied29 in disease caused by warm RBC antibodies. The Rh complex has been shown to carry autoreactive helper determinants, since Rh peptide–specific Th cells that have been activated in vivo can be detected in the peripheral blood of all patients with anti-Rh autoantibodies.29 These autoreactive T cells were identified by their ability to proliferate in vitro, but their patterns of cytokine production, and thus the contribution of different Th subpopulations to the responses, have not yet been reported. The first aim was therefore to establish whether Rh-specific Th cells secreting the respective Th1 and Th2 cytokines IFN-γ and IL-4, or the regulatory cytokines IL-10 and TGF-β1, are present in AIHA. We then mapped the fine specificity of the respective Th cells and characterized any regulatory cytokine responses. The study not only demonstrates human autoantigen–specific regulatory T cells that secrete IL-10, but also identifies the peptides that they recognize, and therefore represents an important step in understanding the pathogenesis of autoimmune diseases and in developing specific immunotherapy.
Patients, materials, and methods
Patients
AIHA was diagnosed in patients attending the Aberdeen Royal Infirmary on the basis of clinical and hematological investigation and a positive Coombs test. The protocol for investigation was approved by the Grampian Health Board and the University of Aberdeen Joint Ethical Committee, and all patients gave informed consent. Details of the cases are summarized in Table1. Patients were included in the series only if they were considered to have warm-type primary AIHA with no evidence of underlying disease. Most blood samples were obtained when patients were well controlled and receiving low-to-moderate doses of steroid treatment. Control blood samples were taken from 7 healthy human volunteers with no serological evidence of warm RBC autoantibodies or anti-D alloantibodies.
Details of AIHA patients
| Patient no. . | Sex . | Date of birth . | Blood group . | Coombs test at presentation . | Antibody specificity . | HLA-DR type . | ||
|---|---|---|---|---|---|---|---|---|
| IgG . | C3 . | Rh . | Panagglutinin . | |||||
| 1 | F | 15/1/29 | O+ | +++++ | − | + | − | DRB1*11; 15 |
| 2 | F | 26/01/38 | O+ | +++++ | +++++ | + | + | DRB1*04; 15 |
| 3 | F | 23/4/22 | O+ | +++++ | +++ | + | − | DRB1*01; 12 |
| 4 | F | 18/4/45 | A+ | + | ++ | + | − | NT |
| 5 | F | 16/1/46 | A+ | ++ | +++++ | + | − | DRB1*01; 03 |
| 6 | M | 27/6/30 | O+ | +++++ | +++++ | + | − | DRB1*03; 15 |
| 7 | F | 27/7/77 | A+ | +++++ | +++ | + | + | DRB1*11; 15 |
| 8 | M | 26/10/34 | A+ | +++++ | +++++ | + | − | DRB1*01; 12 |
| 9 | M | 16/6/27 | B+ | +++++ | − | + | + | DRB1*03; 15 |
| 10 | F | 24/1/31 | O+ | +++++ | + | NT | NT | DRB1*03; 13 |
| 11 | F | 2/12/27 | O+ | +++++ | +++++ | + | − | NT |
| Patient no. . | Sex . | Date of birth . | Blood group . | Coombs test at presentation . | Antibody specificity . | HLA-DR type . | ||
|---|---|---|---|---|---|---|---|---|
| IgG . | C3 . | Rh . | Panagglutinin . | |||||
| 1 | F | 15/1/29 | O+ | +++++ | − | + | − | DRB1*11; 15 |
| 2 | F | 26/01/38 | O+ | +++++ | +++++ | + | + | DRB1*04; 15 |
| 3 | F | 23/4/22 | O+ | +++++ | +++ | + | − | DRB1*01; 12 |
| 4 | F | 18/4/45 | A+ | + | ++ | + | − | NT |
| 5 | F | 16/1/46 | A+ | ++ | +++++ | + | − | DRB1*01; 03 |
| 6 | M | 27/6/30 | O+ | +++++ | +++++ | + | − | DRB1*03; 15 |
| 7 | F | 27/7/77 | A+ | +++++ | +++ | + | + | DRB1*11; 15 |
| 8 | M | 26/10/34 | A+ | +++++ | +++++ | + | − | DRB1*01; 12 |
| 9 | M | 16/6/27 | B+ | +++++ | − | + | + | DRB1*03; 15 |
| 10 | F | 24/1/31 | O+ | +++++ | + | NT | NT | DRB1*03; 13 |
| 11 | F | 2/12/27 | O+ | +++++ | +++++ | + | − | NT |
NT indicates not tested.
Determination of autoantibody specificity
Autoantibody eluted by ether treatment21from the RBCs of AIHA patients or serum antibody was screened in hemagglutination assays for the ability to bind a panel of RBCs including R
Antigens and mitogens
A complete panel of 42 15-mer peptides, with 5 amino acid overlaps, was synthesized29 (Department of Biochemistry, University of Bristol, United Kingdom), corresponding to the sequence of the 30-kDa Rh protein associated with expression of the D blood group antigen.30 To ensure purity, peptides were synthesized by fluorenylmethoxycarbonyl chemistry31 on resin using a base-labile linker, rather than by pin technology, and screened by high-performance liquid chromatography (HPLC) and amino acid analysis. As previously optimized,29 32 the peptides were used to stimulate cultures at 20 μg/mL.
RhD protein was purified from RBCs by immunoprecipitation using a monoclonal anti-D (T19, Scottish National Blood Transfusion Service), which is specific for an epitope on loop 6.33 The immunoprecipitation method was adapted from that previously published.21 Briefly, 0.4 mL packed D-positive RBCs were incubated with 0.5 mL 250 μg/mL T19 for 1 hour at 37°C, washed, lysed in hypotonic solution at 4°C, and solubilized in 2% Triton X100 (Sigma, Dorset, United Kingdom). After removing insoluble debris by centrifugation, immune complexes were immobilized by incubation with magnetic beads (Biomag, PerSeptive Biosystems, MA). RhD protein was added to cultures at an estimated concentration of 5 μg/mL, still complexed to the beads, since antigen-presenting cells (APCs) efficiently take up and process proteins bound to an insoluble matrix.27 Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) analysis and Western blotting of the preparation21 revealed a major band estimated at 30 kDa, corresponding to the migration of RhD protein, but no evidence of the Rh-associated glycoprotein. In all experiments, control cultures were stimulated with magnetic beads coated with T19 antibody alone.
The control antigen Mycobacterium tuberculosis purified protein derivative (PPD) (Statens Seruminstitut, Denmark) was added to cultures at 20 μg/mL. PPD readily provokes recall T-cell responses in vitro,34 since most United Kingdom citizens have been immunized with Bacillus Calmette-Guérin (BCG).
Proliferation assays
As previously described,29,32 peripheral blood mononuclear cells (PBMCs) were separated from fresh blood samples by density gradient centrifugation and cultured at a concentration of 1.25 × 106 cells/mL. Cell proliferation was estimated from the incorporation of 3H-thymidine in triplicate microtiter wells 5 days after stimulation with antigen. Results are presented either as mean cpm = +/− SD of the triplicate sample, or as a stimulation index (SI), expressing the ratio of mean CPM in stimulated versus unstimulated control cultures. An SI > 3 is interpreted as representing a significant positive response.35
Measurement of cytokine production
The production of IFN-γ, IL-4, IL-10, and TGF-β in cultures was measured by a highly sensitive cellular enzyme-linked immunosorbent assay (ELISA).35 Briefly, 5 days after stimulation with peptide, PBMC cultures were transferred into duplicate wells in microtiter plates (Nunc, Roskilde, Denmark) coated with monoclonal anti–cytokine capture antibody (Pharmingen, Oxon, United Kingdom). After incubation of PBMC for 24 hours at 37°C, the plates were developed with the appropriate biotinylated monoclonal detection antibody (Pharmingen), ExtraAvidin-alkaline phosphatase conjugate (Sigma), and p-nitrophenyl phosphate substrate (Sigma). The absorbance at 405 nm was measured using a multiscan plate reader (Labsystems, Basingstoke, United Kingdom). Cytokine secretion was calculated by interpolation from a standard curve generated by incubating duplicate wells with doubling dilutions of recombinant human, IFN-γ, IL-4, IL-10, and TGF-β (Pharmingen). Results are presented either as the mean cytokine concentration in duplicate wells, or as SI, expressing the ratio of mean concentration in stimulated versus unstimulated control cultures. An SI > 2.0 is interpreted as representing a significant positive response.35
Blocking antibodies
To inhibit the effects of IL-10, a neutralizing antibody specific for this cytokine (Pharmingen) was added to newly established cultures at 1 ng/mL. As reported by others,16 36costimulation via cytotonic T lymphocyte–associated antigen (CTLA-4) was prevented by titrating a blocking antibody F(ab′)2 fragment (Alexis Biochemicals, Nottingham, United Kingdom) into newly established cultures.
Characterization of responding cells
The phenotypes of cultured cells that secrete cytokine in response to antigen were determined by flow cytometry. PBMCs from responding cultures were stained with anti-CD3 phycoerythrin-Texas Red-X (Beckman Coulter, Bucks, United Kingdom) and anti-CD4 fluorescein isothiocyanate (Beckman Coulter). Cells synthesizing IL-10 were labeled by incubating with anti–IL-10 phycoerythrin (Pharmingen) after inhibition of protein secretion with Brefeldin A (Sigma) and permeabilization with Intraprep (Beckman Coulter). Stained cells were analyzed using an EPICS XL cytometer (Beckman Coulter) and Expo v2 analysis software (Applied Cytometry Systems, Yorks, United Kingdom).
Statistical analysis
A nonparametric test, Spearman rank correlation, was used with the level for significance taken asP < .05 (2-tailed).
Results
Cytokine responses to RhD protein by Th cells from AIHA patients
PBMCs from a panel of AIHA patients were tested for the ability to respond to purified RhD protein by proliferating or secreting the respective Th1 and Th2 cytokines IFN-γ and IL-4, or the regulatory Tr1 and Th3 cytokines IL-10 and TGF-β1 (Figure1). The principal cytokine produced in response to the autoantigen by each PBMC sample was either IFN-γ or IL-10, with no IL-4 or TGF-β1 detectable in any of the stimulated cultures despite the use of well-validated, highly sensitive assays35 capable of measuring these cytokines in allo-responses to blood group antigens.37 Whether IFN-γ or IL-10 production predominated was related to the magnitude of the proliferative response. Thus, high levels of IFN-γ were associated with strong proliferation (Rs = 0.63,P < .01), while IL-10 predominated when proliferation was weak (Rs = −0.58, P < .005). Serial results from individual patients demonstrated that this bias toward proliferative or IL-10 responses was unstable over time and could reverse in subsequent samples. Such deviations in response type were specific to the autoantigen, since the control recall antigen PPD elicited strong proliferation, with IFN-γ the predominant cytokine produced, from all PBMC samples tested (results not shown).
Responses by PBMCs from AIHA patients to RhD autoantigen.
Proliferation and IL-10 production were measured after PBMCs from AIHA patients 1 (○), 2 (●), 9 (▾), 10 (▿), and 11 (▪) were stimulated in vitro with purified RhD protein. Results from serial samples from each patient, taken one month to one year apart, are included. The proliferative and IL-10 responses are inversely correlated (Rs = −0.58, P < .005, n = 22).
Responses by PBMCs from AIHA patients to RhD autoantigen.
Proliferation and IL-10 production were measured after PBMCs from AIHA patients 1 (○), 2 (●), 9 (▾), 10 (▿), and 11 (▪) were stimulated in vitro with purified RhD protein. Results from serial samples from each patient, taken one month to one year apart, are included. The proliferative and IL-10 responses are inversely correlated (Rs = −0.58, P < .005, n = 22).
The regulatory effects of the IL-10 produced in response to the RhD protein were tested by adding a neutralizing antibody specific for this cytokine to cultures of AIHA patients' PBMCs stimulated with the autoantigen. In each experiment, the blocking antibody resulted in markedly higher proliferative responses (mean increase in SI = 203% SD = 116, n = 6). These results lead us to propose that the patients are capable of mounting IL-10–mediated regulatory responses to the autoantigen, but that these are inconsistent and unable permanently to control the pathogenic Th1 cells in vivo. A close correlation between the cytokine responses and the clinical course of AIHA would not be expected because multiple factors influence the severity of the disease,20 38 and the anemia in most patients was well controlled during this study. Even so, analysis of cytokine responses by 17 samples from 5 patients, together with the corresponding blood hemoglobin levels, reveals that higher levels of IL-10 elicited by the RhD protein are associated with less severe anemia (Rs = 0.57, P < .02).
Mapping epitopes on the RhD protein that induce cytokine responses
Having demonstrated that the RhD protein is capable of eliciting different types of response in human AIHA, we next mapped the fine specificity of the respective Th cells. To determine the breadth of the responses and to avoid selection pressures inherent in cloning, we took advantage of techniques successfully developed for studying polyclonal T cells.27-29,32,34,35 Proliferation and the secretion of IFN-γ, IL-4, IL-10, and TGF-β1 were measured when PBMCs from the AIHA patients were stimulated with an overlapping panel of 15-mer peptides spanning the RhD protein sequence. Representative results from 3 of the 7 patients tested illustrate that multiple peptides were stimulatory, with the production of IFN-γ or IL-10 being the predominant cytokine responses (Figure 2A-C). By contrast, TGF-β1 induced by peptides was unusual, and IL-4 very rare. In 4 of the 7 patients tested, proliferative responses against peptides were positively associated with secretion of the Th1 cytokine IFN-γ (P < .05, Spearman rank correlation). It should be noted that, despite the complexity of the response profiles, particular peptides could be identified in each patient that selectively and reproducibly elicited IL-10, but not proliferation. These IL-10–inducing peptides include 652-66, 16152-166, 24232-246, and 34332-346for patient 1 and peptides 11102-116 and 24232-246 for patient 2, which were chosen for later investigation. In healthy control donors (n = 7, representative results shown in Figure 2D), proliferative, but not IL-10, responses to the RhD peptide panel were uncommon. In previous studies, recall T-cell proliferation in response to Rh peptides was also found to be rare.29 34
Responses by PBMCs to a panel of RhD autoantigen peptides.
Representative experiments showing proliferation and production of the Th cytokines γ-IFN, IL-10, and TGF-β by PBMCs from AIHA patients 1 (A), 2 (B), and 3 (C) and a healthy control donor (D) after stimulation with a panel of overlapping 15-mer peptides spanning the sequence of the RhD protein. Peptides are numbered 1-42 from the N-terminus. IL-4 was not detected in any of the cultures. The dashed line indicates the level of response taken as representing a positive response. Error bars represent SD. In 2 of the 3 patients, proliferative and γ-IFN responses are positively correlated (Rs = 0.42, P < .005; Rs = 0.1, P = .6; and Rs = 0.76, P < .001, respectively).
Responses by PBMCs to a panel of RhD autoantigen peptides.
Representative experiments showing proliferation and production of the Th cytokines γ-IFN, IL-10, and TGF-β by PBMCs from AIHA patients 1 (A), 2 (B), and 3 (C) and a healthy control donor (D) after stimulation with a panel of overlapping 15-mer peptides spanning the sequence of the RhD protein. Peptides are numbered 1-42 from the N-terminus. IL-4 was not detected in any of the cultures. The dashed line indicates the level of response taken as representing a positive response. Error bars represent SD. In 2 of the 3 patients, proliferative and γ-IFN responses are positively correlated (Rs = 0.42, P < .005; Rs = 0.1, P = .6; and Rs = 0.76, P < .001, respectively).
Regulatory activity by the Tr1 cytokine IL-10 secreted in response to RhD peptides
Since IL-10–secreting Th cells play an important role controlling animal models of immune-mediated disease,7-12 the production of this cytokine in response to the RhD protein and peptides was considered in detail. The first step was to determine the phenotype of the IL-10–secreting cells. Flow cytometry confirmed that most of the cells producing IL-10 when PBMCs from AIHA patients (n = 4) were stimulated with RhD protein (mean = 92%, SD = 11) or RhD peptides (mean = 93%, SD = 3, n = 36) were of the CD4+ helper phenotype (Figure 3). We next examined the relationship between the IL-10 responses to the RhD protein and to the peptide panel. In 19 samples from 7 patients, a significant correlation (Rs = 0.46, P < .05) was found between the level of IL-10 production induced by the purified autoantigen and the number of peptides that elicited this cytokine.
Flow cytometric analysis of PBMCs producing IL-10 in response to RhD peptides.
PBMCs from AIHA patients 1 and 9 were either left unstimulated in culture or incubated with RhD peptides that selectively induce IL-10 responses, before being stained for CD4 expression and intracellular IL-10. Similar results were obtained in another 2 patients.
Flow cytometric analysis of PBMCs producing IL-10 in response to RhD peptides.
PBMCs from AIHA patients 1 and 9 were either left unstimulated in culture or incubated with RhD peptides that selectively induce IL-10 responses, before being stained for CD4 expression and intracellular IL-10. Similar results were obtained in another 2 patients.
The dependence of the IL-10 response to the RhD protein on the breadth of specificities of the IL-10–secreting Th cells that are recruited, together with the identification of peptides that selectively stimulated IL-10 production, suggested a means to manipulate the type of response to the autoantigen. We tested whether peptides that induce only IL-10 production were able to alter the balance of the response to the RhD protein in vitro at times when this was dominated by proliferation. Cultures of PBMC from AIHA patients were stimulated to proliferate with the purified RhD protein, and the effect of adding RhD peptides that elicit IL-10 responses was determined. The results of 2 representative experiments demonstrate that IL-10–inducing peptides strongly inhibit proliferative responses to the purified RhD autoantigen (Figure 4A-B). Similar results were obtained in a total of 8 experiments, using samples from 3 patients that were obtained when the RhD protein elicited proliferation, and testing at least 2 IL-10–inducing peptides on each occasion. The dominant inhibition appears to be specific to the RhD protein, since no such effect was observed on Th cells stimulated to proliferate with the control recall antigen PPD. An alternative explanation for this difference between the 2 antigens is that PPD-induced proliferation is more resistant to the suppressive effects of IL-10. However, proliferative responses elicited by either RhD or PPD alone were equally inhibited by addition of the cytokine to cultures (> 90% inhibition by > 100 pg/mL IL-10 in experiments using PBMCs from 2 patients).
To determine whether the suppression of proliferative responses to the RhD protein mediated by peptides is IL-10 dependent, a neutralizing antibody specific for this cytokine was added to cultures. In each experiment (a total of 4 experiments in 3 patients, testing up to 3 peptides on each occasion), the inhibitory effects were entirely or largely blocked by this treatment (Figure5A). Furthermore, RhD peptides that failed to elicit IL-10 did not inhibit proliferative responses to the RhD protein (results not shown).
Autoantigen-specific inhibition by RhD peptides that induce IL-10.
RhD peptides that elicit IL-10 responses by PBMCs from AIHA patients were added to respective PBMC cultures that had been stimulated to proliferate by purified RhD protein or by the unrelated recall antigen PPD. The percentage inhibition of proliferation is shown in 2 representative experiments using samples from AIHA patients 1 (A) and 2 (B). Similar results were obtained in a total of 8 experiments, using samples from 3 patients and testing at least 2 IL-10–inducing peptides on each occasion.
Autoantigen-specific inhibition by RhD peptides that induce IL-10.
RhD peptides that elicit IL-10 responses by PBMCs from AIHA patients were added to respective PBMC cultures that had been stimulated to proliferate by purified RhD protein or by the unrelated recall antigen PPD. The percentage inhibition of proliferation is shown in 2 representative experiments using samples from AIHA patients 1 (A) and 2 (B). Similar results were obtained in a total of 8 experiments, using samples from 3 patients and testing at least 2 IL-10–inducing peptides on each occasion.
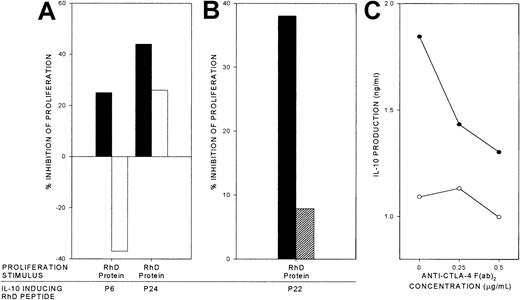
Dependency of specific inhibition by RhD peptides on IL-10 and CTLA-4.
RhD peptides that elicit IL-10 responses were used to inhibit proliferation by cultures of PBMCs from AIHA patient 1 that had been stimulated by purified RhD protein (▪). The effects on the inhibition of proliferation are shown when replicate cultures were treated with either (A) neutralizing anti–IL-10 antibody (■), or (B) blocking anti–CTLA-4 F(ab′)2 0.5 μg/mL (▨). The anti–CTLA-4 F(ab′)2 also inhibits IL-10 production by the PBMCs responding to the RhD peptides alone (●) compared with unstimulated cultures (○; C). In each panel, the mean of triplicate results from a representative experiment are illustrated (n = 4, from 2 patients).
Dependency of specific inhibition by RhD peptides on IL-10 and CTLA-4.
RhD peptides that elicit IL-10 responses were used to inhibit proliferation by cultures of PBMCs from AIHA patient 1 that had been stimulated by purified RhD protein (▪). The effects on the inhibition of proliferation are shown when replicate cultures were treated with either (A) neutralizing anti–IL-10 antibody (■), or (B) blocking anti–CTLA-4 F(ab′)2 0.5 μg/mL (▨). The anti–CTLA-4 F(ab′)2 also inhibits IL-10 production by the PBMCs responding to the RhD peptides alone (●) compared with unstimulated cultures (○; C). In each panel, the mean of triplicate results from a representative experiment are illustrated (n = 4, from 2 patients).
Evidence from animal models indicates that costimulation via ligation of CTLA-4 can be important in the induction of cytokine-mediated Tr responses.39 We therefore tested whether the regulatory responses elicited by the IL-10–inducing RhD peptides could be inhibited by a blocking antibody F(ab′)2 fragment specific for CTLA-4.16 36 Representative results (from a total of 4 experiments in 2 patients) demonstrate that anti–CTLA-4 antibody virtually abrogated the suppressive effect of these responses on proliferation stimulated by the purified RhD protein (Figure 5B), a reversal that was accompanied by a marked reduction in the level of IL-10 induced by the RhD peptides (Figure 5C).
Characterization of IL-10–inducing RhD peptides
The ability of particular RhD peptides to induce IL-10–mediated Tr responses in vitro raises the questions of whether it would be practical to exploit them therapeutically, and why these sequences have such a property. To examine these issues, we analyzed the results across the patient panel to determine whether the same peptides can elicit IL-10 responses in different individuals. Figure6 demonstrates that certain peptides preferentially induced IL-10 in many patients, despite variation in HLA type (Table 1). For example, peptides 322-36, 652-66, and 34332-346 each stimulated IL-10 responses in more than 50% of the patients and are therefore candidates to test for the ability to enhance autoantigen-specific regulation in vivo. Such peptides are predicted to bind to a number of different HLA-DR molecules (http://imtech.res.in/raghava/propred/index.html,http://www.csd.abdn.ac.uk/∼gjlk/MHC-Thread).
Summary of IL-10 responses by PBMCs from AIHA patients to RhD autoantigen peptides.
Shown here (▪) is the proportion of the AIHA patients tested (n = 11) with PBMCs that produced only IL-10 in response to each of the 42 peptides from the panel spanning the RhD protein. Also shown (■) are the results of parallel studies32 to map the dominant naturally processed epitopes from the RhD protein, which elicit proliferation by PBMCs from RhD-negative healthy donors who have been immunized with RhD-positive RBCs (n = 22). There is a significant correlation (Rs = 0.37,P < .02) between the abilities of each RhD peptide to elicit autoreactive IL-10 responses in the AIHA patients and alloreactive Th proliferation in the group of immunized healthy donors.
Summary of IL-10 responses by PBMCs from AIHA patients to RhD autoantigen peptides.
Shown here (▪) is the proportion of the AIHA patients tested (n = 11) with PBMCs that produced only IL-10 in response to each of the 42 peptides from the panel spanning the RhD protein. Also shown (■) are the results of parallel studies32 to map the dominant naturally processed epitopes from the RhD protein, which elicit proliferation by PBMCs from RhD-negative healthy donors who have been immunized with RhD-positive RBCs (n = 22). There is a significant correlation (Rs = 0.37,P < .02) between the abilities of each RhD peptide to elicit autoreactive IL-10 responses in the AIHA patients and alloreactive Th proliferation in the group of immunized healthy donors.
The criteria that determine the specificities of both pathogenic and regulatory autoreactive Th cells remain unresolved and, in particular, it is not clear whether they recognize epitopes that are normally naturally processed and presented. We therefore took advantage of a previous study32 of healthy RhD-negative blood donors who lack the RhD protein.40 These volunteers had been alloimmunized with RhD-positive RBCs, and we mapped the naturally processed RhD peptides that elicit proliferative responses by Th cells from their blood.32 Figure 6 demonstrates a positive correlation (Rs = 0.37, P < .02) between the abilities of each RhD peptide to elicit autoreactive IL-10 responses in the AIHA patients, and alloreactive Th proliferation in the group of immunized healthy donors. For example, peptide 652-66 is the most dominant sequence that stimulates autoimmune IL-10 production and also alloimmune proliferation. Our interpretation of these results is that naturally processed RhD peptides, some of which are presented promiscuously by a wide variety of HLA class II molecules, preferentially drive the selection of Tr cells capable of mounting IL-10 responses when the protein is expressed as an autoantigen.
Discussion
This report describes, for the first time, human autoantigen–specific regulatory cells. Although compelling evidence has accumulated that Tr cells secreting inhibitory cytokine are important in the control of pathogenic immune responses in animal models,7-14 the cells' existence and character in human autoimmune disease previously have been unclear. The work was facilitated in AIHA because, unlike many other human autoimmune conditions, the unequivocal identification of the major RBC autoantigens21,22 29 has enabled the relevant Th-cell responses to be studied in detail. The major findings are that IL-10–secreting Tr cells responsive to a major RBC autoantigen, the RhD protein, are detectable in the blood of patients with AIHA, and they specifically suppress proliferative Th1 responses elicited by the antigen in vitro, and they predominantly recognize peptides containing naturally processed epitopes. Such peptides therefore represent a new type of immunotherapeutic agent for AIHA and other autoimmune diseases.
PBMCs from AIHA patients would be predicted to secrete a wide variety of cytokines when stimulated with RBC antigens,41,42 and those measured here were selected to be characteristic of particular CD4+ Th subsets. The RhD protein was chosen as the autoantigen studied, since Th cells specific for epitopes from this protein are activated in AIHA patients with autoantibodies specific for the Rh complex,29 and it affords a unique comparison with alloimmune responses in RhD-negative healthy control donors who had been deliberately immunized with RhD-positive RBCs.32 The current results reveal complex patterns of proliferation and cytokine production by AIHA patients' CD4+ T cells when stimulated in vitro with the purified RhD protein or the panel of peptides spanning this RBC autoantigen. However, the predominant responses either were of the Th1 type, with proliferation accompanied by IFN-γ secretion, or marked by production of the regulatory cytokine IL-10. Most exogenously added, synthetic peptides are displayed to Th cells after uptake by APCs and binding to recycling HLA class II molecules, rather than direct exchange with sequences already presented on the surface.43 Any differences in profiles of the peptide responses between AIHA patients may be related to the variations in the HLA type of the individuals and reflect the ability of particular class II molecules to bind and present each peptide.43Conversely, the identification of peptides that elicited similar types of response in most patients may be due to promiscuity for binding different class II molecules and to processing mechanisms that can constrain the sequences available for presentation.43The relationships between the identity of sequences from the RhD protein that contain auto- and alloreactive T-cell epitopes, HLA restriction, and peptide affinity for class II molecules are clearly complex and are the subject of current investigation.
Although Th cells specific for at least some autoantigens are eliminated centrally in the thymus,11 it is nevertheless clear that peripheral mechanisms are critical in preventing autoaggressive responses, including those against RBCs.23,24,29,34,37,44-46 The current work supports the view2,46 that the balance between autoantigen-specific Th-cell subsets secreting different patterns of cytokines is an important mechanism for regulating autoimmune responses. In many examples of rodent autoimmunity, including AIHA, Th1 responses appear to be pathogenic, while Th2 responses can mediate protection from disease.1,2,28,46 Here, a Th1 bias is demonstrated in human AIHA patients whose Th cells commonly secreted IFN-γ but not IL-4 in response to the RhD protein or derived peptides. This contrasts with the results from parallel studies32 of healthy donors carrying the D-negative blood group who do not express the RhD protein.40 When such individuals are immunized with RhD-positive RBCs, the responses are characterized by a balanced Th0 profile, with both IFN-γ and IL-4 production in vitro.37
Antagonism between Th1 and Th2 responses may modulate the pathogenicity of autoantibody by providing help for different isotypes, rather than determine whether it is produced.2 However, autoantigen-specific regulatory Th cells secreting inhibitory cytokines such as IL-10 or TGF-β have the potential to restore peripheral self-tolerance.4,7-14 The presence of such cells in a human autoimmune disease was confirmed by the demonstration of Th cells from AIHA patients that were capable of producing IL-10 when stimulated with the RhD protein autoantigen. The finding that the RhD protein induced either IL-10 or proliferative responses in serial samples taken at different times leads us to propose that there is an unstable equilibrium between pathogenic Th1 and protective Tr cells in conditions such as AIHA, and that disease becomes chronic because of a failure to establish consistent, effective regulation. The existence of such a balance is supported by the finding that neutralization of IL-10 enhanced proliferation in response to the RhD protein by patients' T cells. A definitive relationship was not expected between changes in the type of response to RhD protein and the severity of AIHA, because multiple factors influence both hemolysis and erythropoiesis.20,38 Nevertheless, an analysis of all the samples obtained from the patient panel revealed that high IL-10 responses were associated with less severe anemia. Although IL-10 is a B-cell growth factor in addition to having regulatory functions and could, in principle, contribute to autoantibody production,2 the results are consistent with the view that production of IL-10 by RhD protein–specific T cells is protective in AIHA.
The Tr cells identified in this study have a number of characteristics that are relevant both to the understanding of the pathogenesis of autoimmune disease and to the design of future therapy. First, they include distinct sets of specificities from the Th1 cells that proliferate in response to the RhD protein, since particular peptides from the autoantigen sequence, for example, peptide 652-66, selectively stimulate Th IL-10 production in most of the AIHA patients. The notion that regulatory and pathogenic Th cells may recognize different epitopes is supported by recent analysis of T-cell responses to islet cell autoantigen in nonobese diabetic mice.47 A comparison with the results of a parallel study of healthy RhD-negative donors who have been immunized with the RhD protein as a foreign antigen32 revealed that the regulatory responses in the AIHA patients are focused on peptides that contain naturally processed epitopes, including those such as peptide 652-66 that are presented promiscuously by a variety of HLA class II molecules. This analysis confirms that at least some Th cells specific for naturally processed self-peptides attain a regulatory phenotype5 rather than being deleted in vivo. The data also support the belief that the pathogenic Th1 response in diseases such as AIHA2,28,46 targets epitopes that are normally cryptic or subdominant.48 49 Since the size of the IL-10 response to the RhD protein varies with the number of sequences that elicit IL-10, we now hypothesize that the progress of disease is determined by changes in antigen presentation that alter the relative abundance with which the different sets of epitopes are processed and presented.
The second characteristic of the Tr cells is that their ability to inhibit proliferative responses against the RhD protein is largely dependent on the secretion of IL-10. Such a population of autoreactive cells, here identified for the first time ex vivo, therefore resembles the murine and human Tr1 cells that can be driven to differentiate in vitro, which also mediate suppression via IL-10.12Furthermore, both the secretion of IL-10 in response to RhD peptides and the inhibition of proliferation were shown to be dependent on costimulation via CTLA-4, a requirement shared by other Tr cytokine responses.39 Despite the dependence on IL-10, the inhibitory effect appeared to be a form of linked suppression that was specific for proliferative responses induced by the RhD autoantigen, but not by a control recall antigen. Such specificity would be advantageous in the exploitation of this effect therapeutically and suggests that clustering of IL-10–secreting and –proliferating Th cells on the same APCs may be necessary for inhibition. Overall, the Tr responses characterized here differ from those recently shown to be mediated by CD4+CD25+ human peripheral blood cells, the effects of which are not antigen specific and neither IL-10 nor CTLA-4 dependent.15-18
This study opens the way for the evaluation of a novel form of treatment for AIHA and other autoimmune diseases. The identification of autoantigen-specific Tr cells secreting IL-10 raises the possibility that selective stimulation of this regulatory population could restore tolerance and lead to the resolution of disease. Since particular epitopes on the RhD protein preferentially induce such regulatory responses, the balance between Th subsets in autoimmune disease could be controlled therapeutically by administering autoantigenic peptides that induce only inhibitory cytokine production. The promiscuity of some such peptides for presentation by many different class II molecules raises the prospect that such treatments need not be individually matched to the HLA type of the patient.
The authors are grateful to Mr D. Wilson (Scottish National Blood Transfusion Service) for technical assistance with flow cytometry, to Mr John Duncan (Scottish National Blood Transfusion Service) for serological testing, and to Drs D. Culligan, J. Tighe, and H. Watson (Aberdeen Royal Infirmary) for help with patient recruitment.
Prepublished online as Blood First Edition Paper, August 1, 2002; DOI 10.1182/blood-2002-05-1383.
Supported by grants from the Wellcome Trust (United Kingdom), the Scottish National Blood Transfusion Service, and the Grampian University Hospitals Trust (United Kingdom).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
R. N. Barker, Department of Medicine and Therapeutics, Institute of Medical Sciences, University of Aberdeen, Foresterhill, Aberdeen AB25 2ZD, United Kingdom; e-mail:r.n.barker@abdn.ac.uk.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal