Treatment of chronic granulomatous disease (CGD) with myeloablative bone marrow transplantation is considered risky. This study investigated complications and survival according to different risk factors present at transplantation. The outcomes of 27 transplantations for CGD, from 1985 to 2000, reported to the European Bone Marrow Transplant Registry for primary immunodeficiencies were assessed. Most transplant recipients were children (n = 25), received a myeloablative busulphan-based regimen (n = 23), and had unmodified marrow allografts (n = 23) from human leukocyte antigen (HLA)–identical sibling donors (n = 25). After myeloablative conditioning, all patients fully engrafted with donor cells; after myelosuppressive regimens, 2 of 4 patients fully engrafted. Severe (grade 3 or 4) graft-versus-host disease (GVHD) disease developed in 4 patients: 3 of 9 with pre-existing overt infection, 1 of 2 with acute inflammatory disease. Exacerbation of infection during aplasia was observed in 3 patients; inflammatory flare at the infection site during neutrophil engraftment in 2: all 5 patients belonged to the subgroup of 9 with pre-existing infection. Overall survival was 23 of 27, with 22 of 23 cured of CGD (median follow-up, 2 years). Survival was especially good in patients without infection at the moment of transplantation (18 of 18). Pre-existing infections and inflammatory lesions have cleared in all survivors (except in one with autologous reconstitution). Myeloablative conditioning followed by transplantation of unmodified hemopoietic stem cells, if performed at the first signs of a severe course of the disease, is a valid therapeutic option for children with CGD having an HLA-identical donor.
Introduction
Chronic granulomatous disease (CGD) is an inherited disorder of phagocyte function, characterized by recurrent, often life-threatening bacterial and fungal infections and by granuloma formation in vital organs. Neutrophils, monocytes/macrophages, and eosinophils cannot generate microbicidal oxygen metabolites owing to a defect in 1 of the 4 subunits of the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase of phagocytes (gp91phox, p47phox, p67phox, and p22phox). Conventional treatment consists of lifelong anti-infectious prophylaxis with antibiotics such as cotrimoxazole,1 antimycotics such as itraconazole,2 and/or interferon gamma.3 Despite these measures, the annual mortality is still between 2% (autosomal recessive CGD) to 5% (X-linked CGD [X-CGD]).4 Therefore, there is a need for more effective therapies. The alternative to conventional treatment, hemopoietic stem cell transplantation (HSCT), considered to carry a high risk of complication and death, is usually postponed until the patient is chronically ill.
In this retrospective study, 27 patients (25 children, 2 adults) with CGD underwent transplantation with an unmodified hemopoietic allograft from a human leukocyte antigen (HLA)–identical donor. Transplant-associated complications and survival were analyzed according to the presence or absence of risk factors at transplantation: for example, therapy-refractory infection, acute inflammation, or sequelae due to chronic inflammation.
Patients, materials, and methods
Data collection
Between 1985 and 2000, 27 patients received an HSC transplant for CGD in 14 cooperating centers in Europe. Data of the allografts performed during this period were reported to the HSCT Registry for primary immunodeficiencies of the Working Party on Inborn Errors of the European Group for Blood and Marrow Transplantation (EBMT) and the European Society for Immunodeficiencies (ESID). A retrospective analysis was made of data reported up to March 2001, with a follow-up period of 4 months to 12 years (median, 2 years). Data of 7 patients were published previously.5-11
Patients
The characteristics of the 27 patients (23 male, 4 female; 25 children, 2 adults) are shown in Tables 1, 2, and 3.
HSCT for CGD with therapy-refractory infection
| Patient no. (reference) . | CGD type . | Age, y . | Sex . | Sex of donor . | Marrow MNCs infused per kg, × 108 . | Conditioning . | Risk factors (organs involved) . | Outcome (day of death) . | Myeloid engraftment (extent of hemopoiesis) . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | X-CGD | 11.5 | M | F | 1.7 | Bu, Cy | Disseminated aspergillosis (lung, heart, bone) | Multiorgan failure (died d +9) | (None) |
| 2 | X-CGD | 17 | M | F | 3.5 | Bu, Cy | Multifocal aspergillosis (lung, CNS) | Nodular pneumonia; skin GVHD IV (died d +90) | 2 mo (full) |
| 3 | X-CGD | 20 | M | F | 4.3 | Bu, Melphalan, alemtuzumab | Multifocal fungal infection* (lung, paraspinal) | Interstitial pneumonia Tracheostomy bleeding (died d +73) | 2 mo (full) |
| 4 | X-CGD carrier | 6.8 | F | M | 5† | Bu 8,‡ Flu 180, ATG | Aspergillosis (lung) | Nonengraftment; Aspergillus pneumonia; VOD (died d +46) | (None) |
| 5 | gp91phox | 5.9 | M | M | 81-153 | Cy 120,‡ Flu 125, ATG | Mycobacteriosis1-155 (lung, nodes) | Nonengraftment autologous reconstitution, alive | (None) |
| 6 (Ozsahin et al5) | gp91phox | 8 | M | F1-154 | 4 | Bu, Cy | Multifocal aspergillosis (lung, rib, psoas) | Alive and well | 5 y (full) |
| 7 | p22phox | 14.4 | F | M | 3.5 | Bu, Cy | Multifocal aspergillosis (lung, CNS) | Aspergilluspneumonia; skin GVHD 4 → chronic 4 y → resolved; alive and well | 4 y (full) |
| 8 (Bielorai et al6 | gp91phox | 3 | M | M | PBSCs | Bu, Cy, ATG | Aspergillosis (lung, rib) | Skin GVHD 3 → chronic → resolved, alive and well | 1.8 y (full) |
| 9 | X-CGD | 16.8 | M | F | 2 | Bu 16, Flu 200, ATG | Fungal gastritis# | Alive and well | 1 y (full) |
| Patient no. (reference) . | CGD type . | Age, y . | Sex . | Sex of donor . | Marrow MNCs infused per kg, × 108 . | Conditioning . | Risk factors (organs involved) . | Outcome (day of death) . | Myeloid engraftment (extent of hemopoiesis) . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | X-CGD | 11.5 | M | F | 1.7 | Bu, Cy | Disseminated aspergillosis (lung, heart, bone) | Multiorgan failure (died d +9) | (None) |
| 2 | X-CGD | 17 | M | F | 3.5 | Bu, Cy | Multifocal aspergillosis (lung, CNS) | Nodular pneumonia; skin GVHD IV (died d +90) | 2 mo (full) |
| 3 | X-CGD | 20 | M | F | 4.3 | Bu, Melphalan, alemtuzumab | Multifocal fungal infection* (lung, paraspinal) | Interstitial pneumonia Tracheostomy bleeding (died d +73) | 2 mo (full) |
| 4 | X-CGD carrier | 6.8 | F | M | 5† | Bu 8,‡ Flu 180, ATG | Aspergillosis (lung) | Nonengraftment; Aspergillus pneumonia; VOD (died d +46) | (None) |
| 5 | gp91phox | 5.9 | M | M | 81-153 | Cy 120,‡ Flu 125, ATG | Mycobacteriosis1-155 (lung, nodes) | Nonengraftment autologous reconstitution, alive | (None) |
| 6 (Ozsahin et al5) | gp91phox | 8 | M | F1-154 | 4 | Bu, Cy | Multifocal aspergillosis (lung, rib, psoas) | Alive and well | 5 y (full) |
| 7 | p22phox | 14.4 | F | M | 3.5 | Bu, Cy | Multifocal aspergillosis (lung, CNS) | Aspergilluspneumonia; skin GVHD 4 → chronic 4 y → resolved; alive and well | 4 y (full) |
| 8 (Bielorai et al6 | gp91phox | 3 | M | M | PBSCs | Bu, Cy, ATG | Aspergillosis (lung, rib) | Skin GVHD 3 → chronic → resolved, alive and well | 1.8 y (full) |
| 9 | X-CGD | 16.8 | M | F | 2 | Bu 16, Flu 200, ATG | Fungal gastritis# | Alive and well | 1 y (full) |
ATG indicates antithymocyte globulin; Bu, busulphan; CNS, central nervous system; Cy, cyclophosphamid; Flu, fludarabine; GVHD, graft-versus-host disease; MNCs, mononuclear cells; PBSCs, peripheral blood stem cells; VOD, veno-occlusive disease; →, evolves into.
Scedosporium apiospermum.
0.9 × 106 CD34 cells per kilogram.
Nonmyeloablative.
11 × 106 CD34 cells per kilogram.
Mycobacterium gordonae.
Heterozygous carrier of X-CGD.
#Ustilago species.
HSCT for CGD with active inflammation/inflammatory sequelae
| Patient no. (reference) . | CGD type . | Age, y . | Sex . | Sex of donor . | Marrow MNCs infused per kg, × 108 . | Conditioning . | Risk factors (lung function) . | Outcome (lung function) . | Myeloid engraftment (extent of hemopoiesis) . |
|---|---|---|---|---|---|---|---|---|---|
| 10 | gp91phox | 17 | M | F* | 3.2 | Bu, Cy | Colitis; pulmonary restriction (FVC 60%) | Colitis resolved; GVHD 4 → chronic → resolving | 1.3 y (full) |
| 11 | gp91phox | 4.3 | M | M | 1.9 | Bu, Cy | Colitis, steroid dependent; previous Aspergilluspneumonia | Colitis resolved; alive and well | 1.2 y (full) |
| 12 (Di Bartolomeo et al7) | gp91phox | 11 | M | F* | 4.7 | Bu 13, Cy | Pulmonary restriction (FVC 34%, O2dependent) | Restriction ↓ (FVC 72%, no O2); alive and well | 12 y (full) |
| 13 (Nagler et al8) | X-CGD | 6 | M | F | PBSCs | Bu 8,‡ Flu 240, ATG | Pulmonary restriction (FVC 28%, Sa O2 75%, clubbed) + lung cysts | Lung improved (Sa O2 87%); skin GVHD 2; alive and well | 4 y (full, after DLI) |
| 14 | p47phox | 5 | F | F | 1.1 | Bu, Cy | Pulmonary restriction (DLCO 40%, O2 dependent, clubbed) | Restriction ↓ (DLCO 76%, no O2, clubbing ↓); Catch-up-growth; alive and well | 3 y (full) |
| 15 | gp91phox | 4.4 | M | F | 5.9 | Bu, Cy | Pulmonary restriction (clubbed) + local bronchiectases | Restriction ↓ (clubbing ↓); catch-up-growth; skin GVHD 2; alive and well | 1.5 y (88%) |
| 16 | X-CGD | 38.7 | M | M | PBSCs | TBI 200,‡Flu 180 | Pulmonary restriction (FVC 38%, O2 dependent, clubbed, wheel chair) + bronchiectases | Lung improved (no O2, out of chair); alive and well | 1.6 y (full) |
| Patient no. (reference) . | CGD type . | Age, y . | Sex . | Sex of donor . | Marrow MNCs infused per kg, × 108 . | Conditioning . | Risk factors (lung function) . | Outcome (lung function) . | Myeloid engraftment (extent of hemopoiesis) . |
|---|---|---|---|---|---|---|---|---|---|
| 10 | gp91phox | 17 | M | F* | 3.2 | Bu, Cy | Colitis; pulmonary restriction (FVC 60%) | Colitis resolved; GVHD 4 → chronic → resolving | 1.3 y (full) |
| 11 | gp91phox | 4.3 | M | M | 1.9 | Bu, Cy | Colitis, steroid dependent; previous Aspergilluspneumonia | Colitis resolved; alive and well | 1.2 y (full) |
| 12 (Di Bartolomeo et al7) | gp91phox | 11 | M | F* | 4.7 | Bu 13, Cy | Pulmonary restriction (FVC 34%, O2dependent) | Restriction ↓ (FVC 72%, no O2); alive and well | 12 y (full) |
| 13 (Nagler et al8) | X-CGD | 6 | M | F | PBSCs | Bu 8,‡ Flu 240, ATG | Pulmonary restriction (FVC 28%, Sa O2 75%, clubbed) + lung cysts | Lung improved (Sa O2 87%); skin GVHD 2; alive and well | 4 y (full, after DLI) |
| 14 | p47phox | 5 | F | F | 1.1 | Bu, Cy | Pulmonary restriction (DLCO 40%, O2 dependent, clubbed) | Restriction ↓ (DLCO 76%, no O2, clubbing ↓); Catch-up-growth; alive and well | 3 y (full) |
| 15 | gp91phox | 4.4 | M | F | 5.9 | Bu, Cy | Pulmonary restriction (clubbed) + local bronchiectases | Restriction ↓ (clubbing ↓); catch-up-growth; skin GVHD 2; alive and well | 1.5 y (88%) |
| 16 | X-CGD | 38.7 | M | M | PBSCs | TBI 200,‡Flu 180 | Pulmonary restriction (FVC 38%, O2 dependent, clubbed, wheel chair) + bronchiectases | Lung improved (no O2, out of chair); alive and well | 1.6 y (full) |
DLCO indicates diffusing capacity for CO; DLI, donor lymphocyte infusion; FVC, forced vital capacity; TBI, total body irradiation. For other abbreviations see Table 1 footnote.
Heterozygous carrier of X-CGD.
Nonmyeloablative.
HSCT for CGD with no overt infection/inflammation
| Characteristic . | Data . |
|---|---|
| Patient no.3-150 | 17-27 |
| CGD type | 5 X-CGD; 4 gp91phox; 2 unknown |
| Age, y (median) | 0.8-14 (7) |
| Sex | 10 M; 1 F |
| Sex of donor | 8 F3-151; 3 M3-152 |
| Marrow MNCs infused per kg, × 108 (median) | 2.1-9.5 (4.7) |
| Conditioning | 9 Bu, Cy; 1 Bu, Cy, TNI; 1 Bu, Cy, TT, ATG |
| Risk factors | 7 none; 4 Aspergillus pneumonia (history) |
| Outcome | 1 GVHD II; all alive and well |
| Myeloid engraftment, extent of hemopoiesis (median) | 0.3-4 y, all full (2 y) |
| Characteristic . | Data . |
|---|---|
| Patient no.3-150 | 17-27 |
| CGD type | 5 X-CGD; 4 gp91phox; 2 unknown |
| Age, y (median) | 0.8-14 (7) |
| Sex | 10 M; 1 F |
| Sex of donor | 8 F3-151; 3 M3-152 |
| Marrow MNCs infused per kg, × 108 (median) | 2.1-9.5 (4.7) |
| Conditioning | 9 Bu, Cy; 1 Bu, Cy, TNI; 1 Bu, Cy, TT, ATG |
| Risk factors | 7 none; 4 Aspergillus pneumonia (history) |
| Outcome | 1 GVHD II; all alive and well |
| Myeloid engraftment, extent of hemopoiesis (median) | 0.3-4 y, all full (2 y) |
TNI indicates total node irradiation; and TT, thiothepa. For other abbreviations see Table 1 footnote.
Includes 2 heterozygous carriers of X-CGD.
Includes 2 matched unrelated donors (1 mixed leukocyte culture negative; one molecularly matched).
CGD was confirmed by the absence of NADPH-oxidase activity in stimulated neutrophils by one or more of the following tests: nitroblue tetrazolium (NBT) reduction, dihydrorhodamine oxidation, chemiluminescence, and superoxide generation. Twenty-two patients had X-CGD (by identification of a carrier mother and/or by gp91phox mutation analysis); 2 had autosomal recessive CGD (1 had p47phox deficiency; 1, p22phoxdeficiency); 2 were uncharacterized; and 1 was an X-CGD carrier with extreme lyonization (patient 4).
All 27 patients had had at least one invasive infection of lung, liver, blood, or bone, requiring intravenous antibiotic therapy. Nine of 27 patients had culture-proven, therapy-refractory, life-threatening infections (8 fungal, 1 mycobacterial) and received intravenous antibiotics as well as granulocyte transfusions (7 of 9) at the time of transplantation (Table1). Eighteen of 27 patients were free of infection at HSCT. Seven of the 18 patients without overt infection had signs of active ongoing inflammation (colitis) or organ sequelae, probably due to chronic inflammation (pulmonary restriction) (Table2); the remaining 11 had no inflammation (Table 3). The patients or their legal guardians gave informed consent for stem cell transplantation and collection of data. The informed consent process included advice on the beneficial effects of conventional antibacterial/antifungal prophylaxis/treatment and on the risks of allografting, especially in the presence of overt infection or inflammation.
Transplantation
Donor and recipient HLA matching was confirmed by serotyping and/or molecular typing of the HLA class I and II loci, respectively. Twenty-five of the 27 patients received transplants from a sibling with HLA-identical genotype (5 of the 25 donors were heterozygous carriers for CGD). Only 2 patients (with no overt infection or inflammation) received an HLA phenotypically identical graft from an unrelated donor (patients 19 and 25).
The majority of patients (23 of 27) received a busulphan-based myeloablative conditioning regimen, mostly combined with cyclophosphamide (21 of 27). Busulphan (Bu) was used at a total dose of 16 mg/kg or 20 mg/kg (in children younger than 5 years of age) at days −9 to −6 before transplantation, and cyclophosphamide (Cy) was used at a total dose of 200 mg/kg at days −5 to −2. Lower-intensity conditioning regimens were applied only in 4 debilitated patients, who were unable to tolerate a Bu/Cy regimen: 1 patient with active therapy-refractory infection (patient 4) and 2 patients with very compromised lung function (patients 13 and 16) were conditioned according to published myelosuppressive protocols (with the use of either low-dose busulphan [8 mg/kg]12 or low-dose total body irradiation [200 cGy]13); another patient with active infection (patient 5) was conditioned according to an immunosuppressive protocol (with the use of cyclophosphamide [120 mg/kg], fludarabine [125 mg/m2], and antithymocyte globulin14).
Twenty-four of the 27 patients received full, T-cell–replete marrow grafts with a median cell dose of 4.3 × 108/kg mononucleated cells (MNCs) (range, 1.1 to 9.5 × 108/kg). Three patients with a lower-intensity conditioning regimen obtained granulocyte colony-stimulating factor (G-CSF)–stimulated, T-cell–replete peripheral blood stem cells (PBSCs) with cell doses of 17.6 × 108 MNC/kg (patient 16); 27 × 108MNC/kg (patient 8); and 27.8 × 108 MNC/kg (patient 13), respectively. As prophylaxis for graft-versus-host disease (GVHD), all patients received cyclosporine A; 13 received short-term methotrexate; and 4, prednisone. All patients were nursed in a high-efficiency, particulate-air–filtered protected environment, and 24 were given oral gut decontamination. In addition, all patients received intravenous immunoglobulin therapy (except for 2) and Pneumocystis carinii prophylaxis by cotrimoxazole after HSCT (except for 1, given pentamidine). Chimerism was studied by karyotyping and/or analysis of informative microsatellite DNA sequences. The presence of oxidase-positive neutrophils was detected by cytochemical nitroblue tetrazolium (NBT) tests and/or by flow cytometry with the use of a dihydrorhodamine oxidation assay.
Disease-free survival was defined as survival with adequate neutrophil-killing function, reflected by (1) clearing of pre-existing (fungal) infection and/or pre-existing (pulmonary or intestinal) inflammation; (2) absence of new bacterial or fungal infection after withdrawal of antibiotic prophylaxis; and (3) demonstration of neutrophils with an NADPH-oxidase activity similar to the level of the respective stem cell donor.
Results
Engraftment
Full donor-derived hemopoietic chimerism was observed in 22 of 23 patients who received an HLA-identical unmodified stem cell graft (bone marrow for 22; PBSCs for 1) after myeloablative busulphan-based conditioning (1 patient [no. 1] died at day +9 [d+9] and could not be evaluated). Hemopoietic recovery in this group occurred with a median time of 18.5 days (range, 9 to 40 days) to neutrophil count greater than 500/μL. Donor-derived hemopoiesis was stable, with a median follow-up time of 2 years (range, 0.3 to 12 years). In 4 patients, who received an HLA-identical unmodified stem cell graft after lower-intensity conditioning (consisting of bone marrow for 2; PBSCs, for 2), full donor-derived hemopoiesis was achieved in only 2 patients (in no. 13 only at 9 months after a donor lymphocyte infusion), while the other 2 patients did not engraft. One patient (no. 4) received a very low CD34-cell number (0.9 × 106/kg) in a T-cell–replete graft and died of aspergillosis despite a stem cell boost and peritransplantation granulocyte transfusions. The other patient (no. 5) received an adequate number of CD34 cells (11 × 106/kg) in a T-cell–replete graft after an immunosuppressive conditioning protocol (120 mg/kg Cy, 125 mg/m2 fludarabine, and antithymocyte globulin [ATG]), but developed autologous reconstitution. He has not received granulocyte transfusions.
Neutrophil function
An NADPH-oxidase activity in the donor range could be documented in all 24 patients with donor-derived hemopoiesis. In the 5 patients who received transplants from heterozygous carriers of X-CGD, a mosaicism of oxidase-active/oxidase-nonactive neutrophils was demonstrated. The degree of lyonization of the X-linked gp91phox gene in the recipient was identical to the one in the carrier donor in all 5 cases (results not shown).
Clinical outcome and adverse events
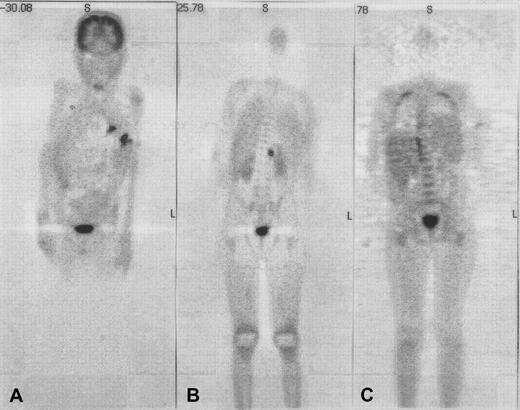
As a result of the development of oxidase activity, therapy-refractory pre-existing infections (3 episodes of life-threatening aspergillosis, 1 episode of severe gastritis due toUstilago) were eradicated in 4 of 4 evaluable patients. In patient 6, healing of a fistula over the rib and normalization of C-reactive protein was observed within the first month after HSCT; resolution of active (hypermetabolic) lesions was complete after 3 months as evidenced by repeat whole-body positron emission tomography (PET) with the use of F18-fluorodeoxyglucose (FDG) (Figure1). An exacerbation of the pre-existingAspergillus pneumonia during aplasia was seen in 3 of 9 patients (nos. 1, 4, and 7), and a diffuse inflammatory pulmonary reaction at the time of neutrophil engraftment in 2 of 6 evaluable patients (nos. 2 and 3). In 4 of the 5 patients, the pneumonia was bilateral, progressed to white lungs, required ventilation, and was not survived. Lung biopsies were considered too invasive to be performed.
Use of coronal emission fluorodeoxyglucose–positron emission tomography (FDG-PET) in patient 6 with multifocal aspergillosis.
Coronal emission FDG-PET before (panels A and B) and 3 months after (panel C) HSCT in patient 6 with multifocal aspergillosis. PET shows multiple lesions in the lungs and a focus in the upper-left psoas with intense FDG uptake before HSCT; these have disappeared at 3 months after HSCT. Physiologic high-FDG uptake is seen in the brain and bladder.
Use of coronal emission fluorodeoxyglucose–positron emission tomography (FDG-PET) in patient 6 with multifocal aspergillosis.
Coronal emission FDG-PET before (panels A and B) and 3 months after (panel C) HSCT in patient 6 with multifocal aspergillosis. PET shows multiple lesions in the lungs and a focus in the upper-left psoas with intense FDG uptake before HSCT; these have disappeared at 3 months after HSCT. Physiologic high-FDG uptake is seen in the brain and bladder.
The development of oxidase activity also led to the resolution of serious pre-existing inflammatory disease and the improvement of inflammatory sequelae. Two of 2 patients with severe biopsy-proven granulomatous colitis (1, steroid dependent) lost their symptoms within 2 months of HSCT and had no recurrence during a follow-up period of 1.2 and 1.3 years, respectively. Surprisingly, improvement of pre-existing pulmonary inflammatory sequelae was also seen, albeit at a slower pace. Before HSCT, 2 patients were carefully documented as having severe pulmonary restriction as evidenced by a forced vital capacity (FVC) of 34% (patient 12) and a diffusing capacity for CO (DLCO) of 40% (patient 14) as well as decreased oxygen saturations (SaO2 of 83% [patient 12] and 85% [patient 14]). After HSCT, there was normalization of oxygen transport within 1 year and near normalization of lung function within 2½ years (Figure 2).
Oxygen saturation (SaO2) and pulmonary function test results before and after HSCT in patients 12 and 14 with severe pulmonary restriction.
DLCO indicates diffusing capacity for CO; and FVC, forced vital capacity.
Oxygen saturation (SaO2) and pulmonary function test results before and after HSCT in patients 12 and 14 with severe pulmonary restriction.
DLCO indicates diffusing capacity for CO; and FVC, forced vital capacity.
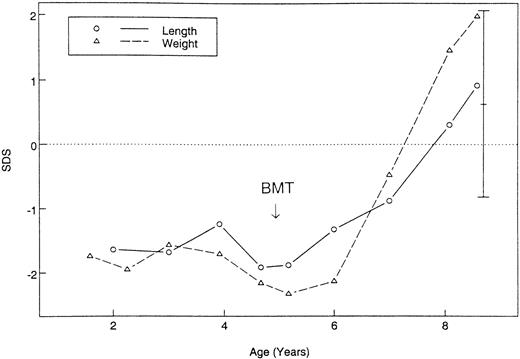
All together, improvement of pulmonary restriction was seen in 5 of 5 evaluable patients, with reversal of oxygen dependence in 3 of 3; disappearance of clubbing in 2 of 4; and in a wheelchair's becoming unnecessary in 1 out of 1. Pre-existing bronchiectasis and lung cysts, however, persisted on repeated computed tomography (CT) examinations. Catch-up growth was documented in 2 patients aged 4.4 and 5 years, respectively, with resolving pulmonary restriction (Figure 3).
Growth before and after HSCT of patient 14 with severe pulmonary restriction.
Standard deviation scores of weight and length are shown and compared with target height.
Growth before and after HSCT of patient 14 with severe pulmonary restriction.
Standard deviation scores of weight and length are shown and compared with target height.
Graft-versus-host disease
Acute GVHD grades 2 to 4 occurred in 7 of 27 patients; chronic GVHD, in 3 of 27 patients (limited in 2, extensive in 1). Acute GVHD of grade 3 or 4 occurred in 4 patients: 1 child aged 3 years, and 3 adolescents aged 14, 17, and 17 years, respectively. Of these, patients 2, 7, and 8 with life-threatening aspergillosis developed severe GVHD of the skin only, resembling Lyell syndrome. In the 2 survivors, GVHD became chronic and slowly resolved under prolonged immunosuppressive therapy. Patient 10 had overt granulomatous colitis and developed severe GVHD of the gut, liver, and skin. Again chronic GVHD developed and has gradually responded to treatment. In 16 patients without overt infection or active inflammation, only 3 cases of acute skin GVHD grade 2 developed: in children aged 1.2, 4.4, and 6 years, respectively.
Survival
Four of 27 (15%) CGD patients who had received transplants died, all in the group of patients with pre-existing therapy-refractory fungal infections. The causes of death were progression of aspergillosis before engraftment in 2 (patients 1 and 4); coincidence of inflammatory pulmonary reaction and skin GVHD grade 4 at neutrophil engraftment in 1 (patient 2); and uncontrolled hemorrhage through an eroded carotid artery in 1 (patient 3 with tracheostomy). In 1 of 23 surviving patients, CGD persists. This patient, a child, did not engraft after an immunosuppressive conditioning protocol and had autologous reconstitution (patient 5). In 22 of the 23 surviving patients, CGD has been cured (81% of all patients who received transplants). Nineteen of 22 cured patients are alive and well at last follow-up (median, 2 years; range, 0.3-12 years); in 3 of the 22 cured patients, quality of life has improved to normal activity without special care (2 with residual pulmonary sequelae of the lung and Karnofsky performance scores of 80 and 90 [patients 13 and 16], respectively; 1 with resolving GVHD grade 4 and a score of 80 [patient 7]).
Discussion
Despite data to show that good preventive treatment improves survival and quality of life in childhood,15 CGD is still associated with high morbidity and mortality.4 In spite of itraconazole and γ-interferon prophylaxis, which have been reported to reduce the number of Aspergillus infections, invasive aspergillosis is now by far the most common cause of death in CGD, accounting for one third of the mortality.4 Other invasive infections and inflammatory sequelae are not uncommon. Widespread granuloma formation in vital organs results in granulomatous colitis, lung fibrosis, and cor pulmonale.16 Patients surviving multiple Aspergillus pneumonias almost inevitably develop restrictive lung disease. Currently, there is no way to change this course.
Although overt infections refractory to antimicrobial treatment (eg, multifocal aspergillosis), acute inflammatory disease dependent on high-dose steroid therapy (eg, colitis), and inflammatory sequelae (eg, severe pulmonary restriction with oxygen dependence) may seem absolute contraindications to HSCT, this view must be challenged. In our study, a total of 16 CGD patients with these risk factors underwent transplantation, in addition to 11 patients without such risks. Despite this selective enrollment, 81% of all patients receiving transplants (22 of 27) are now cured of CGD and are alive and well (19 of 22) or have an improved quality of life (3 of 22). Infections and inflammatory lesions in these patients have all cleared. The 4 deaths occurred only in the group of patients with pre-existing fungal infections refractory to all conventional treatments. We can therefore conclude that HLA-genoidentical HSCT, hitherto reported only in individual CGD patients,6-11 17-22 is a valid alternative to conventional treatment.
To achieve maximal engraftment, we have (except for 4 debilitated patients) exclusively used myeloablative regimens, mostly 16 mg/kg Bu and 200 mg/kg Cy, and have refrained from T-cell depletion of the HLA-identical grafts. This technique has resulted in full and stable engraftment of donor-derived hemopoiesis after a median of 18.5 days in all 22 evaluable cases. This contrasts with the results of a recent National Institutes of Health (NIH) trial, using an immunosuppressive, nonmyeloablative conditioning followed by a T-cell–depleted HLA-genoidentical HSC transplant in 10 CGD patients without active infection.14 This procedure resulted in 2 cases of nonengraftment and necessitated donor lymphocyte infusions (DLIs) in all cases in order to convert to a more favorable donor chimerism. DLIs provoked GVHD in 3 patients: 2 of grade 2 and 1 of grade 4 (resulting in infectious death). Use of a T-cell–depleted graft was probably the main reason for the relatively high rejection rate with the use of the NIH approach. However, use of a T-cell–replete graft in patient 5 of our study, with an otherwise identical approach, did not prevent nonengraftment. For the present, we would therefore favor an unmodified transplant and a more myelosuppressive conditioning. The classical myeloablative protocol provides excellent disease-free survival and quality of life. Fears of secondary tumors following a single Bu/Cy course for conditioning remain theoretical and are not substantiated in our European registry of more than 1000 transplantations for immunodeficiency diseases since 1975 (A.F. et al, unpublished results). Despite the good outcome for the 18 noninfected, low-risk patients reported in this study, myeloablative conditioning still has several disadvantages compared with low-intensity regimens: for example, greater propensity to tissue injury, longer periods of neutropenia, and risk of permanent gonadal failure. Further search for an ideal low-intensity conditioning for transplantation of a nonmalignant disease such as CGD is warranted and would also benefit severely debilitated patients with active infection or pulmonary restriction who cannot tolerate a myeloablative regimen.
Most practitioners postpone HSCT until the CGD patient is chronically ill. We have used transplantation with 11 patients after recovery from one or more invasive infections of lung (includingAspergillus pneumonia), liver, blood, and/or bone before the manifestation of chronic illness. The transplantation was uneventful, without exacerbation of any occult infection, inflammatory reaction, or severe GVHD. All 11 children who received transplants are now cured of their CGD. The absence of transplantation-related deaths in this limited series is comparable to the excellent results in other nonmalignant hematologic disorders such as thalassemia (with a disease-free survival of up to 91%).
HLA-genoidentical HSCT in patients with active inflammation or organ disability due to chronic inflammation is also feasible, with excellent survival and increased quality of life. We were surprised by the gradual, but marked, regression of pulmonary restriction, previously considered to be irreversible owing to lung fibrosis. Although comparative biopsy specimens before and after HSCT were not available, it seems probable that restriction is reversible, because it is caused mainly by cellular infiltrates and granulomas that regress after HSCT. Such infiltrates may be the result of occult smoldering infections, overcome by the new phagocyte system with a normal microbial killing capacity. Alternatively, the infiltrates may be sterile and the result of an exaggerated inflammatory response directed against undigested microbial material,23 again overcome by the normal donor-derived phagocytes. Gross anatomic destruction, such as bronchiectases (patient 16) and lung cysts (patient 13), remained unchanged after HSCT. Active inflammatory disease, such as biopsy-proven granulomatous colitis, also responded well to HSCT; the short-term response was probably due to the massive immunosuppression by the conditioning regimen (eg, by cyclophosphamide) and the GvH prophylaxis (eg, cyclosporine A), and the long-term response probably occcured because of the new donor-derived immune system. Severe GVHD remains a risk, possibly because of tumor necrosis factor–α (TNF-α) levels raised in granulomatous colitis.24 Optimal GVHD prevention is thus imperative.
HSCT can even be successful in active infection that is refractory to conventional treatment, but is more risky. We have encountered 2 serious complications. One is a severe form of GVHD curiously limited to the skin and resembling Lyell syndrome. Since TNF-α is a critical mediator involved in the induction as well as the effector phase of acute GVHD,25 raised TNF-α levels in aspergillosis and the propensity of CGD phagocytes to increased TNF-α production might be responsible for this phenomenon.23 The severe skin GVHD is responsive to immunosuppression by steroids and ATG, but can become chronic before it finally resolves. TNF-antagonist therapy may be beneficial if given as early treatment for GVHD, but one would have to proceed cautiously in order not to compromise the anti-Aspergillus effect of granulocyte transfusions given during the aplasia period.26 The second complication encountered during transplantation in CGD patients with active infection is a severe inflammatory reaction at the infected site at the moment of neutrophil engraftment, manifesting as “white lungs.” Again, raised TNF-α levels may be responsible, resulting in neutrophil extravasation and stimulation.27 This possibility remains speculative, since no biopsy specimens were available to prove this sequence of events.
In future transplantations, the considerable risks of HLA-genoidentical HSCT in CGD patients with therapy-refractory fungal infections may be reduced by 3 precautions: first, all infectious foci must be detected and treated before and during HSCT. CT and combined PET/CT scans will reveal infectious foci,28 which should then be biopsied and cultured for identification of the organism(s) and resistance testing. Antimicrobials with intracellular action have to be combined with granulocyte transfusions, preferably G-CSF primed, since this protects the collected cells against apoptosis and prolongs their half life.5 Second, GVHD has to be prevented by increased immunosuppression, for example, by a cyclosporine/short-term methotrexate/steroid (1 mg/kg/d) regimen. New approaches might include the administration of TNF-α antagonists neutralizing circulating TNF-α or a selective allo–T-cell depletion of the graft.29 Finally, inflammatory reactions after HSCT should be dampened by omitting G-CSF in the recipient and administering heavy immunosuppression (eg, high-dose steroids), if needed.
The time of transplantation is of critical importance. Patients with completely absent NADPH-oxidase activity may follow different clinical courses for reasons not yet fully understood. Some reasons are probably genetic with polymorphisms of host defense molecules and proinflammatory cytokines acting as additional risk factors.16 Some are psychosocial, for example, involving the availability and adequacy of medical care and the daily compliance with lifelong antibiotic prophylaxis, even during periods of well-being, holidays, and puberty. If transplantation is delayed to adolescence or later, the chances of invasive fungal infections and of inflammatory sequelae increase,30 as does the probability of GVHD. Therefore, patients with CGD who have an HLA-identical sibling and a history of recurrent invasive infections and/or inflammatory, steroid-dependent disease, and/or inadequate medical care/compliance with antibiotic prophylaxis should be considered prime candidates for HSCT, before irreversible organ damage occurs. Patients with therapy-refractory infections or organ disability due to chronic inflammation may still be eligible, but run a higher risk of complications, especially of GVHD. Transplantations other than with perfectly matched donors are presently discouraged.
Prepublished online as Blood First Edition Paper, August 8, 2002; DOI 10.1182/blood-2002-02-0583.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Reinhard A. Seger, Division of Immunology/Hematology, University Children's Hospital, Steinwiesstrasse 75, CH-8032 Zurich, Switzerland; e-mail:reinhard.seger@kispi.unizh.ch.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal