We report the outcome of reduced-intensity allogeneic progenitor cell transplantation (alloPCT) for 188 patients with lymphoma from the Working Party Lymphoma of the European Group for Blood and Bone Marrow Transplantation (EBMT). The median age of the patients was 40 years, the median number of prior treatment courses was 3, and 48% of patients had undergone a prior autologous transplantation. Eighty-four percent of the patients received conditioning with fludarabine-based regimens and 10% with the BEAM (BCNU, etoposide, cytosine arabinoside, melphalan) protocol. Full donor chimerism was confirmed in 71% of 100 patients assessed. Acute graft-versus-host disease (GVHD) developed in 37% of patients and chronic GVHD in 17%. A disease response to donor leukocyte infusion (DLI) was seen in 10 of 14 patients. With a median follow-up of 283 days, the overall survival rates at 1 and 2 years were 62% and 50%, respectively. The 100-day and 1-year transplantation-related mortality (TRM) rates were 12.8% and 25.5%, respectively, and were significantly worse for older patients. The probability of disease progression at 1 year for patients with chemoresistant and chemosensitive disease were 75% and 25%, respectively (P = .001). The progression-free survival at 1 year was 46% and was significantly better for those with chemosensitive disease, Hodgkin disease (HD), and low-grade non-Hodgkin lymphoma (NHL). Patients with high-grade NHL, mantle cell lymphoma, or chemoresistant disease had a poor outcome. Reduced-intensity progenitor cell transplantation is associated with a reduced TRM and may control advanced HD and low-grade NHL. A longer period of follow-up is required to determine the benefit of DLI and the graft-versus-lymphoma effect.
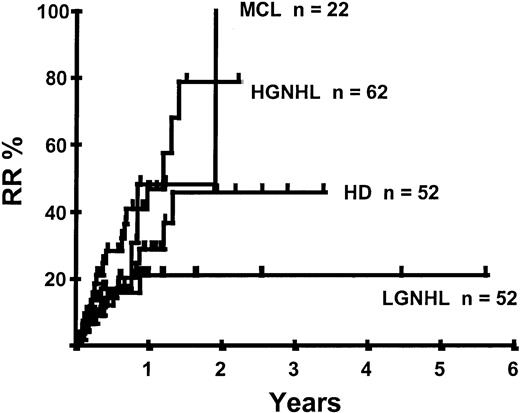
Introduction
Allogeneic progenitor cell transplantation (alloPCT) is a potentially curative treatment for patients in whom conventional therapy for lymphoma has failed, with up to 49% of patients achieving a long-term remission.1,2 The benefit of alloPCT was thought to be largely dependent on the intensity of the conditioning regimen prior to transplantation.3 However, the high doses of chemoradiotherapy used cause substantial toxicity and a high transplantation-related mortality (TRM) rate of up to 40%.1 For patients with comorbid conditions the TRM can be even higher and patients who have previously undergone a high-dose procedure have a reported TRM of 51% to 85%.4 5Consequently, the number of patients with lymphoma who have been offered an allogeneic transplant has been small.
An additional benefit of alloPCT is derived from an allogeneic graft-versus-malignancy effect that reduces the likelihood of disease relapse following transplantation.6 Although graft-versus-malignancy effect is best documented in chronic myeloid leukemia, there is evidence that an allogeneic graft-versus-lymphoma (GVL) effect also exists.2,7-9 To exploit the graft-versus-malignancy effect while reducing the toxicity of alloPCT, reduced-intensity progenitor cell transplants (RITs) have been developed. RITs use less toxic, reduced-dose conditioning that achieves sufficient immunosuppression, such that allogeneic donor cells may engraft.10-14 Consequently, patient eligibility for alloPCT may be extended, the graft-versus-malignancy effect exploited, and additional donor leukocyte infusions (DLIs) administered for treatment of persistent or relapsed disease. The degree of myelosuppression achieved with RITs is variable, ranging from minimally myelosuppressive regimens such as fludarabine plus cyclophosphamide, which are nonmyeloablative, to more intense regimens such as BEAM (BCNU, etoposide, cytosine arabinoside, melphalan), which are not truly myeloablative but cause prolonged pancytopenia and therefore require hematopoietic stem cell transplantation. The immunosuppression used with RIT is also heterogeneous and is dependent on the use of purine analogues, in vivo T-cell depletion (TCD), and the dose intensity of cytotoxic agents used. Several reports have documented that RITs are associated with both a low TRM and a high rate of donor cell engraftment.10-13However, there is limited experience with RIT for lymphoma15-17 and it remains to be established which types of lymphoma may respond to such therapy. We have therefore conducted a retrospective study of the outcome of RIT in 188 patients with lymphoma reported to the European Bone Marrow Transplant (EBMT) registry.
Patients, materials, and methods
The EBMT is a voluntary organization comprising 525 transplant centers mainly from Europe. All EBMT centers were invited to contribute data to the current study. Data from participating centres were derived from both the EBMT database and additionally from questionnaires distributed to each center. Additional follow-up questionnaires were sent to obtain missing data and to confirm the presence or absence of graft-versus-host disease (GVHD). Patients included in the study received conditioning with regimens of an intensity equivalent to or less than that of BEAM plus fludarabine. Minimum data required for the inclusion of a patient in the study were age, histologic diagnosis, prior treatment details, status at transplantation, conditioning regimen, date of transplantation, date of follow-up, disease status at follow-up, date of disease progression or death, and cause of death.
Definitions
Histologic diagnosis was based on local review. Low- and high-grade lymphomas were classified according to the International Working Formulation. Transformed low-grade lymphomas were classified as high-grade lymphomas. Those with chemosensitive disease included all patients who had shown a response to the last therapy prior to transplantation (partial remission [PR], complete remission [CR] unconfirmed, and CR); chemoresistant disease included those with primary refractory disease or refractory relapse prior to transplantation. Progression-free survival (PFS) was measured in months as the time from the day of transplantation until disease relapse/progression or death from any cause. Both relapse and progression were defined as disease progression with transplantation-related deaths being censored. TRM included all causes of death other than disease progression/relapse occurring at any time after RIT. Patients with progressive disease who died from transplantation-related causes were classified as TRM deaths. For the purposes of univariate and multivariate analysis the following groups were defined: no GVHD versus GVHD (acute GVHD grade I-IV, limited or severe chronic GVHD); regimen intensity low (cyclophosphamide < 60 mg/kg with or without fludarabine, fludarabine plus cytarabine with or without idarubicin, total body irradiation [TBI]) versus high (BEAM with or without fludarabine or Campath-1G) versus intermediate (all other regimens).
Statistical analysis
Probabilities of outcomes were calculated using the Kaplan-Meier product-limit estimate. Patient, disease, and transplant-related variables were studied for associations with outcomes by univariate analysis using the log-rank test and by multivariate analysis using Cox proportional hazards regression. All variables considered in the univariate analysis were studied in the multivariate analysis using a backward Cox multiple regression for each outcome. The final model was tested for interactions between variables. The proportional hazard assumption was tested for all variables in the selected models graphically and formally. The log of the baseline hazard rate was plotted against time for each variable level and the graph was examined for evidence of nonproportionality. Comparisons between models were done using the log likelihood test. Comparisons between coefficients were done using the Wald test. All statistical analysis were performed using Number Cruncher Statistical System (NCSS) software.
Results
Patient and disease details
Fifty-one transplant centers registered with the EBMT contributed data on 188 patients who underwent reduced-intensity conditioning with alloPCT between April 1996 and December 2000. Fifty-two patients had Hodgkin disease (HD), 52 had low-grade non-Hodgkin lymphoma (LGNHL; including follicular small cleaved cell, follicular mixed small cleaved cell, and large cell and small lymphocytic lymphoma), 62 had high-grade NHL (HGNHL; including the B-lineage lymphomas: diffuse large B cell, centroblastic, immunoblastic, anaplastic large cell, and pre-B lymphoblastic lymphoma, the T-cell lymphomas: peripheral T cell, angioimmunoblastic, and pre-T lymphoblastic lymphoma, and 7 transformed LGNHL), and 22 had mantle cell lymphomas (MCLs). For the whole group of patients the median number of prior lines of therapy was 3 (range, 1-6 lines), the median time from diagnosis to RIT was 30 months (range, 3-289 months), and the median age at transplantation was 40 years (range, 2-65 years). Forty-eight percent of the patients (90 of 188) had undergone a prior high-dose procedure with autologous stem cell support. Patient and disease characteristics for LGNHL, HGNHL, HD, and MCL are given in Table 1. At the time of RIT, 133 (71%) patients had chemosensitive disease, 40 (21%) had chemoresistant disease, and 15 (8%) were in untested relapse (Table1). Forty-nine patients (26%) were in CR at the time of transplantation including 12 patients with LGNHL, 19 with HGNHL, 11 with HD, and 7 with MCL.
Patient details and prior therapy
| . | LGNHL . | HGNHL . | HD . | MCL . |
|---|---|---|---|---|
| No. of patients | 52 | 62 | 52 | 22 |
| Age at RIT, median (range) | 46 (27-65) | 43 (2-61) | 30 (15-53) | 52 (44-57) |
| Months from diagnosis to NST, median (range) | 33 (6-289) | 21 (3-205) | 42 (7-223) | 35 (5-96) |
| No. of prior lines of therapy, median (range) | 3 (1-5) | 3 (1-5) | 3 (1-6) | 2 (1-5) |
| Prior autograft, % | 29 | 52 | 69 | 36 |
| Disease status at RIT, no. patients (%) | ||||
| Chemosensitive | 44 (85) | 45 (73) | 28 (54) | 16 (73) |
| Chemoresistant | 5 (10) | 13 (21) | 19 (37) | 3 (14) |
| Untested relapse | 3 (6) | 4 (6) | 5 (10) | 3 (14) |
| . | LGNHL . | HGNHL . | HD . | MCL . |
|---|---|---|---|---|
| No. of patients | 52 | 62 | 52 | 22 |
| Age at RIT, median (range) | 46 (27-65) | 43 (2-61) | 30 (15-53) | 52 (44-57) |
| Months from diagnosis to NST, median (range) | 33 (6-289) | 21 (3-205) | 42 (7-223) | 35 (5-96) |
| No. of prior lines of therapy, median (range) | 3 (1-5) | 3 (1-5) | 3 (1-6) | 2 (1-5) |
| Prior autograft, % | 29 | 52 | 69 | 36 |
| Disease status at RIT, no. patients (%) | ||||
| Chemosensitive | 44 (85) | 45 (73) | 28 (54) | 16 (73) |
| Chemoresistant | 5 (10) | 13 (21) | 19 (37) | 3 (14) |
| Untested relapse | 3 (6) | 4 (6) | 5 (10) | 3 (14) |
Conditioning and allograft details
All patients received conditioning with reduced-intensity chemotherapy. Eighty-four percent of patients received fludarabine-based regimens and 10% of patients received the BEAM protocol (Table 2). Fifty-five patients received alemtuzumab (Campath-1H) and 40 received antilymphocyte globulin (ALG) as pretransplantation immunosuppression. GVHD prophylaxis was achieved with cyclosporin with or without methotrexate in 182 patients and with additional ALG in 6.
Conditioning regimens
| Regimen . | No. of patients . |
|---|---|
| Fludarabine (90-150 mg/m2) + cyclophosphamide (20-120 mg/kg) | 41 |
| Fludarabine (150-300 mg/m2) + busulphan (4-10 mg/kg) | 31 |
| Fludarabine (90-150 mg/m2) + melphalan (140 mg/m2) +/− alemtuzumab (100 mg/m2) | 63 |
| Fludarabine (150 mg/m2) + thiotepa (300 mg/m2) | 1 |
| Fludarabine (100 mg/m2) + busulphan (8 mg/kg) + etoposide (30 mg/kg) | 3 |
| Fludarabine (100mg/m2) + cyclophosphamide (60 mg/kg) + thiotepa (300 mg/m2) | 1 |
| Fludarabine (150 mg/m2) + BCNU (300 mg/m2) + melphalan (140 mg/m2) | 8 |
| BCNU (300 mg/m2) + etoposide (800 mg/m2) + cytarabine (800 mg/m2) + melphalan (140 mg/m2) +/− Campath-IG antibody | 18 |
| BCNU (300 mg/m2) + etoposide (800 mg/m2) + cytarabine (800 mg/m2) + melphalan (140 mg/m2) + fludarabine (150 mg/m2) | 1 |
| Cyclophosphamide (60-120 mg/kg) +/− thiotepa (300-600 mg/m2) | 10 |
| Fludarabine (150 mg/m2) + cytarabine (800 mg/m2) +/− idarubicin (15 mg/m2) | 9 |
| Busulphan (4 mg/kg) | 1 |
| TBI (2 Gy) | 1 |
| Regimen . | No. of patients . |
|---|---|
| Fludarabine (90-150 mg/m2) + cyclophosphamide (20-120 mg/kg) | 41 |
| Fludarabine (150-300 mg/m2) + busulphan (4-10 mg/kg) | 31 |
| Fludarabine (90-150 mg/m2) + melphalan (140 mg/m2) +/− alemtuzumab (100 mg/m2) | 63 |
| Fludarabine (150 mg/m2) + thiotepa (300 mg/m2) | 1 |
| Fludarabine (100 mg/m2) + busulphan (8 mg/kg) + etoposide (30 mg/kg) | 3 |
| Fludarabine (100mg/m2) + cyclophosphamide (60 mg/kg) + thiotepa (300 mg/m2) | 1 |
| Fludarabine (150 mg/m2) + BCNU (300 mg/m2) + melphalan (140 mg/m2) | 8 |
| BCNU (300 mg/m2) + etoposide (800 mg/m2) + cytarabine (800 mg/m2) + melphalan (140 mg/m2) +/− Campath-IG antibody | 18 |
| BCNU (300 mg/m2) + etoposide (800 mg/m2) + cytarabine (800 mg/m2) + melphalan (140 mg/m2) + fludarabine (150 mg/m2) | 1 |
| Cyclophosphamide (60-120 mg/kg) +/− thiotepa (300-600 mg/m2) | 10 |
| Fludarabine (150 mg/m2) + cytarabine (800 mg/m2) +/− idarubicin (15 mg/m2) | 9 |
| Busulphan (4 mg/kg) | 1 |
| TBI (2 Gy) | 1 |
A total of 167 patients received a matched sibling allograft and 16 received a matched unrelated allograft. Five patients received a one-antigen mismatched allograft. Allogeneic stem cells were derived from granulocyte colony-stimulating factor (G-CSF)–mobilized blood in 158 patients and from bone marrow in 29 patients. One patient received both donor peripheral blood stem cells and bone marrow.
Engraftment and chimerism studies
The median time to both neutrophil recovery more than 0.5 × 109/L and platelet recovery more than 20 × 109/L was 13 days (ranges, 6-381 days and 11-1060 days, respectively). Ten patients died prior to neutrophil recovery. Chimerism studies were available on 100 patients of whom 71 were full donor chimeras (> 95% donor cells) at first testing (30-90 days after [RIT]), 28 were mixed chimeras, and 1 patient rejected the graft. On subsequent chimerism analysis one further patient had rejected the graft. Chimerism studies were available on 14 patients conditioned with BEAM of whom 9 were full donor chimeras, 4 were mixed chimeras, and 1 rejected the graft.
GVHD
Acute GVHD occurred in 69 of 188 patients (37%), being grade I in 24 (12%) patients and grade II to IV in 45 (24%) patients. Of 164 patients evaluable for chronic GVHD, 12 (7%) developed limited and 15 (9%) extensive chronic GVHD. GVHD was a contributory factor in the deaths of 11 patients. Of 95 patients receiving in vivo TCD with either alemtuzumab or antithymocyte globulin (ATG), 28 (29%) developed acute GVHD, whereas 54 of 93 (58%) nonrecipients of in vivo TCD developed acute GVHD (P = .03; Table4). In a multivariate analysis of risk factors for GVHD, TCD and sibling donor were both associated with a lower risk of GVHD (Table 5).
Disease response to transplantation and DLIs
Eighty-three of 133 patients with chemosensitive disease had measurable disease prior to transplantation and were therefore evaluable for disease response to conditioning therapy. At 100 days after NST, of the 70 surviving patients, 30 (43%) achieved a CR, 2 (3%) were in PR, 25 (36%) had stable disease, and 3 (4%) had progressive disease. Of 40 patients with resistant disease at transplantation, 30 were evaluable for disease response (10 died before assessment) of whom 3 (10%) had achieved a CR, 4 (13%) had achieved a PR, 8 (27%) had stable disease, and 12 (40%) had disease progression. A total of 22 patients have received DLIs for either disease progression or disease persistence following their transplantation. Of these 22 patients, 8 received chemotherapy prior to DLI and were therefore not evaluable for a GVL effect. Ten of the 14 patients receiving DLI alone showed evidence of disease response to DLI and 6 achieved a CR.
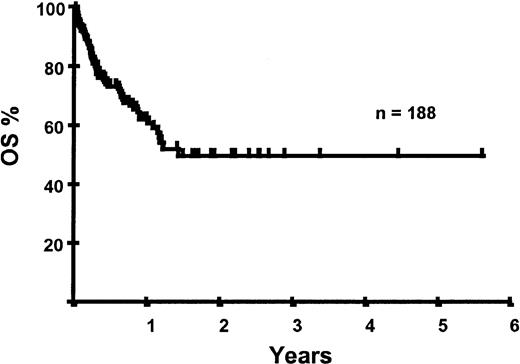
Survival following transplantation
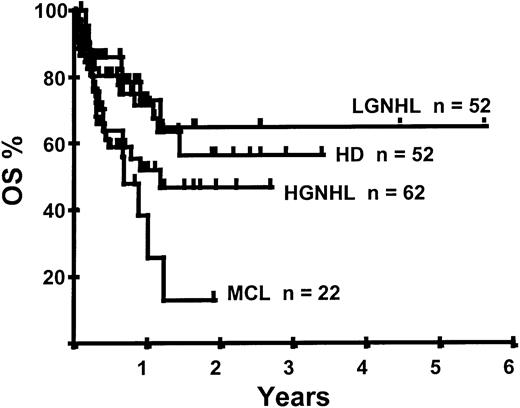
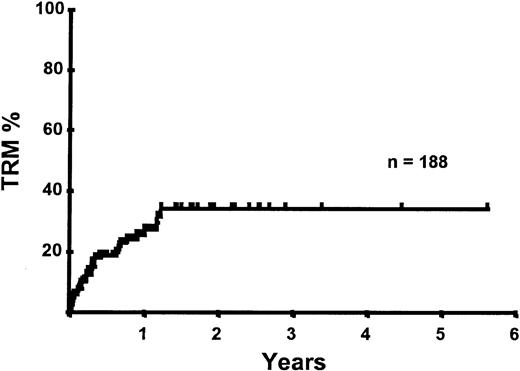
With a median follow-up of 283 days, 125 of the 188 patients were alive. The estimated overall survival (OS) at 1 and 2 years was 62% and 50%, respectively (Figure 1). In a univariate analysis, chemosensitive disease, remission status, and disease category were significantly associated with OS (Figures2 and 3, Tables 3 and 4). By multivariate analysis only chemosensitive disease was associated with a significantly better OS (Table 5). By 100 days following transplantation, 24 regimen-related deaths were recorded (12.8%) and a further 17 regimen-related deaths occurred thereafter. The causes of treatment-related deaths included infection (n = 20), idiopathic pneumonitis (n = 8), GVHD (n = 11), cardiac toxicity (n = 1), and hemorrhage (n = 1). There was no association between in vivo TCD and the risk of infection or death from infection (data not shown). A Kaplan-Meier estimate of the TRM at 1 year was 25.5% and 34.3% at 2 years (Figure 4). On univariate analysis there was a significantly higher TRM for older patients (1 year TRM > 50 years old versus < 50 years old, 39% versus 22%, P = .03) and those who had received more than 3 lines of prior therapy (1-year TRM ≥ 4 lines of therapy versus < 4 lines of therapy, 39% versus 16%, P = .04). There was a nonsignificant trend to a higher TRM in patients with MCL, patients receiving matched unrelated donor (MUD) allografts, and those with chemoresistant disease (Tables 4 and 5). In vivo TCD and the intensity of the conditioning regimen were not associated with TRM. By multivariate analysis only patient age over 50 was a significant predictor of TRM (Table 5).
Outcome by disease category
| . | LGNHL . | HGNHL . | HD . | MCL . |
|---|---|---|---|---|
| OS at 2 y, % | 65 | 46.7 | 56.3 | 12.8 |
| TRM | ||||
| At 100 d, % | 11.5 | 14.5 | 11.5 | 13.6 |
| At 1 y, % | 22.2 | 29.6 | 17.3 | 46 |
| At 2 y, % | 30.9 | 36.7 | 17.3 | 82 |
| Progression rate at 1 y, % | 21.1 | 47 | 28.8 | 48.2 |
| Progression rate at 2 y, % | 21.1 | 78.8 | 45.8 | 100 |
| PFS at 2 y, % | 54 | 12.9 | 42 | 0 |
| . | LGNHL . | HGNHL . | HD . | MCL . |
|---|---|---|---|---|
| OS at 2 y, % | 65 | 46.7 | 56.3 | 12.8 |
| TRM | ||||
| At 100 d, % | 11.5 | 14.5 | 11.5 | 13.6 |
| At 1 y, % | 22.2 | 29.6 | 17.3 | 46 |
| At 2 y, % | 30.9 | 36.7 | 17.3 | 82 |
| Progression rate at 1 y, % | 21.1 | 47 | 28.8 | 48.2 |
| Progression rate at 2 y, % | 21.1 | 78.8 | 45.8 | 100 |
| PFS at 2 y, % | 54 | 12.9 | 42 | 0 |
Results of univariate analysis
| Variable . | No. . | OS at 1 y (P) . | PFS at 1 y (P) . | TRM at 1 y (P) . | Prog at 1 y (P) . | GVHD (P) . |
|---|---|---|---|---|---|---|
| Age | (.33) | (.49) | (.03)‡ | (.91) | (.09) | |
| Younger than 50 y | 144 | 65 | 50 | 22 | 33 | 38 |
| 50 y or older | 44 | 51 | 34 | 39 | 44 | 64 |
| Disease | (.04)‡ | (.009)‡ | (.08) | (.04)‡ | (.12) | |
| LGNHL | 52 | 73 | 61 | 22 | 21 | 48 |
| HGNHL | 62 | 52 | 32 | 30 | 47 | 52 |
| HD | 52 | 72 | 55 | 17 | 29 | 27 |
| MCL | 22 | 38 | 31 | 46 | 48 | 60 |
| Disease duration | (.37) | (.25) | (.046)‡ | (.85) | (.02)‡ | |
| Less than 2 y | 71 | 62 | 58 | 17 | 25 | 36 |
| 2–4 y | 63 | 54 | 35 | 35 | 42 | 54 |
| More than 4 y | 54 | 73 | 51 | 21 | 34 | 41 |
| Prior HDT | (.19) | (.08) | (.32) | (.33) | (.12) | |
| Yes | 97 | 56 | 41 | 30 | 36 | 49 |
| No | 91 | 68 | 52 | 21 | 33 | 39 |
| Disease chemosensitivity | (.004)‡ | (.005)‡ | (.06) | (.001)‡ | (.68) | |
| Chemosensitive | 133 | 67 | 55 | 25 | 23 | 48 |
| Chemoresistant4-150 | 40 | 42 | 19 | 28 | 71 | 41 |
| Remission | (.006)‡ | (.049)‡ | (.11) | (.67) | (.02)‡ | |
| Yes | 49 | 86 | 61 | 12 | 30 | 24 |
| No | 139 | 53 | 41 | 31 | 36 | 53 |
| Relationship to donor | (.47) | (.99) | (.13) | (.23) | (.22) | |
| Related | 171 | 63 | 46 | 23 | 37 | 43 |
| Unrelated | 17 | 60 | 60 | 40 | 0 | 46 |
| Infusion | (.76) | (.72) | (.90) | (.88) | (.88) | |
| PBSCs | 158 | 64 | 47 | 25 | 34 | 44 |
| BM4-151 | 29 | 52 | 41 | 22 | 34 | 45 |
| TCD | (.56) | (.21) | (.94) | (.24) | (.03)‡ | |
| Yes | 95 | 73 | 60 | 18 | 27 | 29 |
| No | 93 | 64 | 46 | 23 | 39 | 58 |
| Regimen intensity | (.91) | (.95) | (.38) | (.47) | (.24) | |
| Low | 51 | 61 | 40 | 24 | 44 | 44 |
| Intermediate | 118 | 62 | 50 | 28 | 29 | 48 |
| High | 19 | 63 | 51 | 22 | 29 | 17 |
| Variable . | No. . | OS at 1 y (P) . | PFS at 1 y (P) . | TRM at 1 y (P) . | Prog at 1 y (P) . | GVHD (P) . |
|---|---|---|---|---|---|---|
| Age | (.33) | (.49) | (.03)‡ | (.91) | (.09) | |
| Younger than 50 y | 144 | 65 | 50 | 22 | 33 | 38 |
| 50 y or older | 44 | 51 | 34 | 39 | 44 | 64 |
| Disease | (.04)‡ | (.009)‡ | (.08) | (.04)‡ | (.12) | |
| LGNHL | 52 | 73 | 61 | 22 | 21 | 48 |
| HGNHL | 62 | 52 | 32 | 30 | 47 | 52 |
| HD | 52 | 72 | 55 | 17 | 29 | 27 |
| MCL | 22 | 38 | 31 | 46 | 48 | 60 |
| Disease duration | (.37) | (.25) | (.046)‡ | (.85) | (.02)‡ | |
| Less than 2 y | 71 | 62 | 58 | 17 | 25 | 36 |
| 2–4 y | 63 | 54 | 35 | 35 | 42 | 54 |
| More than 4 y | 54 | 73 | 51 | 21 | 34 | 41 |
| Prior HDT | (.19) | (.08) | (.32) | (.33) | (.12) | |
| Yes | 97 | 56 | 41 | 30 | 36 | 49 |
| No | 91 | 68 | 52 | 21 | 33 | 39 |
| Disease chemosensitivity | (.004)‡ | (.005)‡ | (.06) | (.001)‡ | (.68) | |
| Chemosensitive | 133 | 67 | 55 | 25 | 23 | 48 |
| Chemoresistant4-150 | 40 | 42 | 19 | 28 | 71 | 41 |
| Remission | (.006)‡ | (.049)‡ | (.11) | (.67) | (.02)‡ | |
| Yes | 49 | 86 | 61 | 12 | 30 | 24 |
| No | 139 | 53 | 41 | 31 | 36 | 53 |
| Relationship to donor | (.47) | (.99) | (.13) | (.23) | (.22) | |
| Related | 171 | 63 | 46 | 23 | 37 | 43 |
| Unrelated | 17 | 60 | 60 | 40 | 0 | 46 |
| Infusion | (.76) | (.72) | (.90) | (.88) | (.88) | |
| PBSCs | 158 | 64 | 47 | 25 | 34 | 44 |
| BM4-151 | 29 | 52 | 41 | 22 | 34 | 45 |
| TCD | (.56) | (.21) | (.94) | (.24) | (.03)‡ | |
| Yes | 95 | 73 | 60 | 18 | 27 | 29 |
| No | 93 | 64 | 46 | 23 | 39 | 58 |
| Regimen intensity | (.91) | (.95) | (.38) | (.47) | (.24) | |
| Low | 51 | 61 | 40 | 24 | 44 | 44 |
| Intermediate | 118 | 62 | 50 | 28 | 29 | 48 |
| High | 19 | 63 | 51 | 22 | 29 | 17 |
The outcomes of overall survival (OS), progression free survival (PFS), transplant-related mortality (TRM), and disease progression (Prog) are given in percentages for each variable at 1 year after transplant. P values for the univariate log-rank test are given in parentheses.
HDT indicates high-dose therapy; PBSCs, peripheral blood stem cells; BM, bone marrow.
Fifteen patients were in untested relapse at the time of transplantation.
One patient received both PBSCs and BM.
Statistically significant.
Results of multivariate analysis
| Outcome . | Variable . | Relative Risk . | Confidence interval . | P . |
|---|---|---|---|---|
| OS | Chemosensitive | 2.4 | 1.4-4.2 | .002 |
| PFS | Chemosensitive | 2.3 | 1.4-3.7 | .007 |
| TRM | Age >50 y | 2.02 | 1.03-4.0 | .041 |
| Progression | Chemoresistant | 3.3 | 1.69-6.35 | .0004 |
| GVHD | MUD | 3.7 | 1.37-9.9 | .0098 |
| TCD | 0.4 | 0.21-0.78 | .0071 |
| Outcome . | Variable . | Relative Risk . | Confidence interval . | P . |
|---|---|---|---|---|
| OS | Chemosensitive | 2.4 | 1.4-4.2 | .002 |
| PFS | Chemosensitive | 2.3 | 1.4-3.7 | .007 |
| TRM | Age >50 y | 2.02 | 1.03-4.0 | .041 |
| Progression | Chemoresistant | 3.3 | 1.69-6.35 | .0004 |
| GVHD | MUD | 3.7 | 1.37-9.9 | .0098 |
| TCD | 0.4 | 0.21-0.78 | .0071 |
Only variables that were statistically significant in the multivariate analysis are given.
Disease relapse and progression
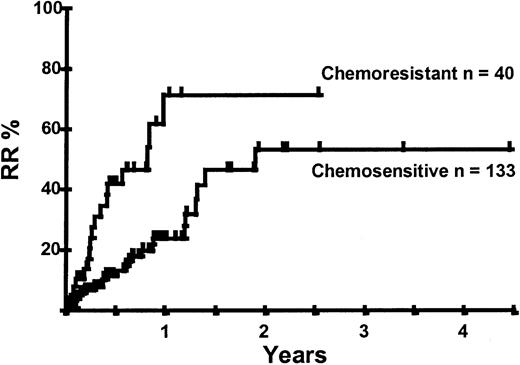
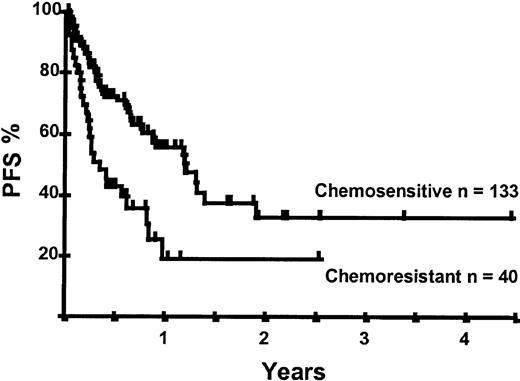
Disease relapse or progression occurred in 46 patients following transplantation and 20 patients have died from progressive disease. The median time to disease progression was 144 days for those with chemoresistant disease at the time of transplantation and 417 days for those with chemosensitive disease. The estimated risk of disease progression at 1 year for patients with chemoresistant disease was 71% and for those with chemosensitive disease 23% (P = .001; Figure 5 and Table 4). The 1-year probability of disease progression was 21%, 47%, 29%, and 48% for LGNHL, HGNHL, HD, and MCL, respectively (P = .03; Figure6 and Tables 3 and 4). The differences in progression rates between these disease categories were not significantly influenced by disease chemosensitivity prior to transplantation (data not shown). There were no cases of disease progression in patients receiving MUD allografts, whereas the 1-year probability of disease progression in recipients of sibling allografts was 37%. By multivariate analysis only disease chemosensitivity was associated with disease progression after transplantation (Table 5). One hundred patients remain alive and progression free at follow-up. The estimated PFS at 1 year was 46% and at 2 years was 30% (Figure7). Chemoresistant disease at the time of transplantation was associated with a significantly worse PFS at 1 year of 19% compared to 55% for those with chemosensitive disease (P = .005; Figure 8 and Table 4). The 1-year PFS rates for LGNHL, HGNHL, HD, and MCL were 61%, 32%, 55%, and 31%, respectively (P = .009; Figure9 and Table 4). By multivariate analysis only disease chemosensitivity was significantly associated with PFS (Table 5).
Kaplan-Meier plot of disease progression according to disease sensitivity.
Kaplan-Meier plot of disease progression according to disease category.
Kaplan-Meier plot of PFS probability according to disease chemosensitivity at the time of transplantation.
Kaplan-Meier plot of PFS probability according to disease chemosensitivity at the time of transplantation.
Discussion
This study reports the outcome of reduced intensity alloPCT for the largest cohort of patients with lymphoma reported to date. The patients had advanced disease and many had undergone a prior high-dose procedure with autologous transplantation. Despite these poor risk features the TRM was low relative to conventional allografts and good disease control was observed in patients with chemosensitive disease, HD, and LGNHL.
There have been several reports of RIT for lymphoma involving small numbers of patients.9,10,13,15-17 The cohort of patients reported here had relatively advanced disease compared to both patients reported in other studies of RIT10,15 and to those treated with conventional allogeneic transplantation.1 18 Many of the patients in the current report had received multiple lines of therapy or had undergone a prior high-dose procedure with an autologous stem cell transplantation and 21% of patients had refractory disease at the time of allografting. Consequently, this cohort of patients had a high risk for both transplantation-related toxicity and disease progression.
A variety of different regimens are currently used as conditioning for RIT.10-15 Although fludarabine-based regimens are most widely used, we have also included in this study patients who received the more intensive BEAM protocol. Autologous hemopoietic recovery may occur following BEAM19 and mixed chimerism develops in a substantial proportion of patients20 conditioned with this protocol. BEAM also offers the potential of greater disease control with acceptable toxicity as demonstrated in the autologous transplantation setting. However, conditioning with BEAM did not significantly affect outcome in terms of TRM, PFS, and OS in this study. Further, we were unable to demonstrate any influence of the intensity of the conditioning regimen on TRM or the relapse rate and it remains to be established which regimen is the optimal conditioning prior to RIT. Patients with more aggressive lymphomas may require intensive regimens to achieve adequate disease control prior to the development of any GVL effect, whereas patients with indolent lymphomas may benefit from less intensive regimens. However, prospective multicenter studies will be required to define the optimal conditioning regimens in these uncommon diseases. The extent of immunosuppression achieved with RIT is also variable and dependent on the chemoradiotherapy used and the use of in vivo TCD but must be sufficiently immunosuppressive to facilitate allogeneic engraftment. Donor engraftment was achieved in the majority of cases studied, confirming that allogeneic engraftment may be consistently achieved using RIT regimens in patients with lymphoma.10,12 13 In conventional allografts increasing the intensity of immunosuppression is associated with delayed immune reconstitution, a higher risk of infection, and a reduced GVL effect. In this study the use of in vivo TCD had no influence on the risk of infection, death from infection, or disease progression although it was associated with a lower risk of GVHD. Further research is required to characterize immune reconstitution following RIT and its relationship to both infection and disease progression.
Conventional allogeneic bone marrow transplantation is associated with a TRM of 40% to 50% in patients with HD,2 40% in patients with NHL,1,2 and up to 80% in patients who have undergone a prior autograft. The TRM of 34% at 2 years in this study was therefore lower than that observed with conventional allografting but in excess of that reported for RIT in other studies.10,11 13 In particular, the TRM for patients with HD undergoing RIT was low when compared to conventional allografts. Patient age, prior therapy, and donor relationship are significant determinants of TRM following conventional allografting and in this study the TRM was worse for patients over 50 years of age or those in receipt of more than 3 lines of prior therapy. However, the TRM was similar for patients undergoing an RIT as either a first or second transplantation and confirms that prior autografting should not exclude patients from RIT. In comparison to conventional alloPCT, RITs are generally well tolerated in the immediate posttransplantation period but late transplantation-related deaths are significant and suggest that prolonged and intense follow-up is required and that additional strategies to prevent infectious complications should be developed.
Acute and chronic GVHD occurred in 37% and 17% of patients, respectively, and was responsible for 10 deaths. The development of GVHD depends on several factors including the alloreactivity of the donor lymphocytes and tissue damage induced by the conditioning regimen and is reported to occur in 50% to 80% of conventional allograft recipients.18 The incidence of GVHD following NST remains significant with 38% to 60% of patients developing grade II to IV acute GVHD,10-12 but a low incidence of GVHD has been reported in patients undergoing RIT where alemtuzumab was incorporated in the conditioning regimen.13 The low incidence of GVHD in this study may therefore relate to the large number of patients who received in vivo TCD with either alemtuzumab or ATG.
Chemoresistant disease has been previously identified as a predictor of poor response to allografting1,2 and in this study the effectiveness of RIT in controlling disease was dependent on disease chemosensitivity at the time of transplantation. Thus, patients with chemoresistant disease responded poorly to the conditioning therapy and the majority of patients had evidence of disease progression by 1 year following transplantation. The reduced-intensity conditioning used in RIT places a greater emphasis on the graft-versus-malignancy effect. However, the beneficial effects of graft-versus-malignancy develop over several months and DLIs are generally avoided for at least 2 to 3 months following allografting because of the high risk of GVHD.21 22 Thus, with a median time to disease progression of 144 days in this study, most patients with chemoresistant disease are therefore unlikely to benefit from any GVL effect. Disease progression was also more marked in patients with MCL and HGNHL such that additional strategies may be required to achieve control of such aggressive diseases. However, the small number of patients receiving and responding to DLI included patients with HGNHL, LGNHL, and HD, suggesting that a beneficial GVL effect may exist in these diseases. The absence of any relapses in patients receiving MUD allografts is also suggestive of an allogeneic GVL effect. Whether GVL activity is significant for patients with chemosensitive disease will require a longer period of follow-up to determine the relapse rate and the effect of DLI. It is imperative to establish, through multicenter trials, the optimal conditioning regimens for disease control and to develop additional strategies to enhance any GVL effect.
The following are the centers and investigators who contributed data to this study.
Australia: A. P. Schwarer, Alfred Hospital Melbourne; Austria: G. Gastl, University Hospital Innsbruck, Innsbruck, H. Gadner, St Anna Kinderspital, Vienna, W. Linkesch, Karl-Franzens-University-Graz Graz; Belgium: J. M. Vossen, Leiden University Hospital, Leiden, M. A. Boogaerts, University Hospital Gasthuisberg Leuven, A. Ferrant, Cliniques Universitaires St Luc, Brussels; Czechoslovakia: J. Vorlicek, University Hospital Brno Brno; Finland: T. Ruutu, Helsinki University Central Hospital, Helsinki; France: S. Blanche, Hôpital Necker, Paris, G. Socié, Hopital St Louis, Paris, D. Blaise,Institut Paoli Calmettes, Marseille, C. Cordonnier, Hôpital Henri Mondor, Creteil, F. Guilhot, Hopital La Miletrie, Poitiers, J. Reiffers, Hôpital Haut-leveque, Pessac, J. P. Jouet, Hopital Claude Huriez Lille Cedex, M. Attal, Hopital de Purpan, Toulouse, M. Michallet, Hopital E. Herriot, Lyon Cedex, F. Oberling and B. Lioure, Strasbourg, P. Bordigoni, MD Vandoeuvre Les Nancy, H. Tilly, Center Rouen Becquerel Rouen CEDEX; Germany: D. Beelen, University of Saarland Homburg (Saar), J. Finke, University of Freiburg, Freiburg, N. Schmitz, Department of Internal Medicine II Kiel, F. Zintl, University of Jena, Jena; The Netherlands: H. Schouten, University Hospital Maastricht, Maastricht; Italy: A. Carella, Ospedale San Martino, Genoa, E. Alessandrino, Paolo Policlinico San Matteo, Pavia, T. Barbui, Ospedale Bergamo Bergamo, A. Zambelli and S. Fondazione, Maugeri Pavia, E. Volpe, Ematologia ‘Giovanni di Guglielmo,’ Avellino, F. Narni, Uni. Modena Policlinico, Modena; Spain: E. Carreras, Hospital Clinic, Barcelona, A. Iriondo, Hospital Universitario Santander, J. Sierra, Hospital Santa Creu i Sant Pau, Barcelona Maldonado, J. Eloy-Garcı́a, Hospital Regional de Malaga Málaga, A. Juliá, R. G. Vall d'Hebron, Barcelona, D. Caballero, Hospital Clı́nico Salamanca, J. L. Diez-Martin, Hospital G. U. Gregorio Maranon, Madrid; Switzerland: A. Gratwohl, Kantonsspital, Basel, B. Chapuis, Hopital Cantonal Universitaire, Geneva, T. Kovacsovics, Center Hospitalo Universitaire Vaudois, Lausanne; United Kingdom: H. G. Prentice, Royal Free Hospital and School of Medicine, London, I. Franklin, Glasgow Royal Infirmary, Glasgow, T. Littlewood, The Oxford Radcliffe Hospital, Oxford, D. W. Milligan, Birmingham Heartlands Hospital, Birmingham, J. E. Tighe, Grampian University Hospitals Trust, Aberdeen, R. Chasty, North Staffordshire Hospital Stoke-on-Trent, J. C. W. Marsh, St George's Hospital Medical School, London, N. H. Russell, Nottingham City Hospital, Nottingham, S. Mackinnon S, University College London, London.
Prepublished online as Blood First Edition Paper, August 15, 2002; DOI 10.1182/blood-2001-11-0107.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Stephen P. Robinson, Lymphoma Working Party, EBMT, 2nd Floor Macdonald Buchanan Wing, John Astor House, Foley St, London W1P 8AN, United Kingdom; e-mail:stephen@ifeoma.fsnet.co.uk.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal