We previously reported a benefit for all-trans retinoic acid (ATRA) in both induction and maintenance therapy in patients with acute promyelocytic leukemia (APL). To determine the durability of this benefit and identify important prognostic factors, long-term follow-up of the North American Intergroup APL trial is reported. A total of 350 patients with newly diagnosed APL were randomized to either daunorubicin and cytarabine (DA) or ATRA for induction and then either ATRA maintenance or observation following consolidation chemotherapy. The complete remission (CR) rates were not significantly different between the ATRA and DA groups (70% and 73%, respectively). However, the 5-year disease-free survival (DFS) and overall survival (OS) were longer with ATRA than with DA for induction (69% vs 29% and 69% vs 45%, respectively). Based on both induction and maintenance randomizations, the 5-year DFS was 16% for patients randomized to DA and observation, 47% for DA and ATRA, 55% for ATRA and observation, and 74% for ATRA and ATRA. There was no advantage of either induction regimen among any subgroups when CR alone was considered. However, female sex, classical M3 morphology (vs the microgranular variant [M3v]), and treatment–white blood cell count (WBC) interaction (ATRA/WBC below 2 × 109/L [2000/μL] best, DA/WBC above 2 × 109/L worst) were each significantly associated with improved DFS (P < .05). Treatment with ATRA, WBC below 2 × 109/L, and absence of bleeding disorder were each significantly associated with improved OS. Age more than 15 years, female sex, and treatment-morphology interaction (DA/M3v worst, ATRA best regardless of morphology) were each significantly associated with improved DFS based on maintenance randomization. The improvement in outcome with ATRA in APL was maintained with long-term follow-up.
Introduction
During the last decade a series of clinical trials has contributed to the development of remarkably effective therapeutic strategies for patients with acute promyelocytic leukemia (APL). The administration of all-trans retinoic acid (ATRA) for patients with newly diagnosed APL, either alone or combined with chemotherapy in induction, has improved the prognosis such that the event-free survival (EFS) at 2 to 4 years is 55% to 85% and the overall survival (OS) is 70% to 80%.1-9 The European APL Group has compared concurrent ATRA plus chemotherapy with sequential ATRA until complete remission (CR) followed by chemotherapy and reports a significantly reduced relapse rate at 2 years among patients receiving concurrent therapy (6% vs 16%,P = .04).10 Two randomized trials, including the first report of the North American Intergroup trial, with a median follow-up of 30 months show a benefit to maintenance therapy with ATRA.7,10 Furthermore, a combination of low-dose chemotherapy given together with ATRA as maintenance appears better than ATRA alone to prevent relapse, and either appears better than no maintenance.5 10
Although all of these studies suggest an important role for ATRA in both induction and maintenance, long-term follow-up is necessary to determine whether these early benefits are sustained. Despite the impact of ATRA in the treatment of APL, the induction mortality rate remains approximately 10% and acquired retinoid resistance contributes to relapse in approximately 20% to 30% of patients.1-10Therefore, an analysis of prognostic factors that predict for success or failure with contemporary treatment strategies may identify specific subsets of patients who require an alternative approach. This report provides long-term outcome results of the North American Intergroup protocol in which patients with APL were randomized to either ATRA or chemotherapy for induction and either ATRA maintenance or observation.7 An analysis of prognostic factors is also presented to identify patients at high risk of relapse.
Patients, materials, and methods
Patients
Briefly, 401 patients with previously untreated APL were registered from 6 cooperative oncology groups between April 1992 and February 1995.7 Randomization was stratified by age. Eligibility included (1) a diagnosis of APL based on bone marrow morphology, (2) no prior chemotherapy except hydroxyurea, (3) normal hepatic and renal function, and (4) Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 3. Although cytogenetic analysis for the t(15;17) translocation was mandatory, results did not affect eligibility. Diagnostic bone marrows were centrally reviewed within each participating cooperative group. Molecular analyses were performed according to individual cooperative group guidelines and have been previously reported.11
A total of 350 evaluable patients were randomized to ATRA 45 mg/m2/d orally until CR or 1 to 2 cycles of daunorubicin 45 mg/m2 by intravenous bolus on days 1 to 3 plus cytarabine 100 mg/m2 by continuous intravenous infusion on days 1 to 7. Patients younger than 3 years of age were randomized to receive either ATRA as just described or daunorubicin 1.5 mg/kg/d by intravenous infusion on days 1 to 3 plus cytarabine 3.3 mg/kg/d by continuous intravenous infusion on days 1 to 7. If the white blood cell count (WBC) was more than 10 × 109/L (10 000/μL), hydroxyurea was given prior to ATRA. Patients who failed to achieve CR after a maximum of 90 days of ATRA crossed over to chemotherapy. ATRA was supplied by the National Cancer Institute, Bethesda, MD. Patients who failed to achieve CR with 2 cycles of induction chemotherapy received no further protocol therapy and were treated at the physician's discretion. Patients who achieved CR with either chemotherapy or ATRA received 2 cycles of consolidation chemotherapy. The first cycle was identical to the chemotherapy administered in induction. The second cycle consisted of high-dose cytarabine 2.0 mg/m2 as a 1-hour intravenous infusion every 12 hours for 4 consecutive days with daunorubicin 45 mg/m2 by intravenous infusion on days 1 and 2. For patients less than 3 years of age, the second cycle included cytarabine 67 g/kg as a 1-hour intravenous infusion every 12 hours for 4 consecutive days and daunorubicin 1.5 mg/kg/d by intravenous infusion on days 1 and 2. Patients in CR after both cycles of consolidation chemotherapy, irrespective of induction therapy, underwent a second randomization to either maintenance ATRA 45 mg/m2/d orally given in divided doses every 12 hours for 1 year or observation. Patients intolerant of ATRA in induction were directly assigned to observation.
Fifty-one of the 401 patients registered were excluded because either they did not have APL after central pathology review (23), their medical condition deteriorated before any treatment was given (14), they did not fulfill entry criteria (7), or there was not adequate on-study data available (7). Four patients who were excluded from the initial analysis because of administrative reasons are now evaluable and included. Therefore, 350 patients are evaluable for this analysis. The WBCs used in the analyses here were the earliest recorded at diagnosis and prior to the introduction of hydroxyurea.
Response criteria
Complete remission and relapse were defined according to NCI criteria.12 Patients in durable clinical CR but lacking bone marrow confirmation (8 patients) were nonetheless considered nonresponders.
Statistical methods
Univariate associations between dichotomous variables were evaluated with the Fisher exact test. Associations involving ordered categoric variables were evaluated with a Wilcoxon rank sum test.13 Analyses of the primary induction end points were stratified by age. Analyses of the joint association of multiple variables with response were evaluated with logistic regression. Univariate and multivariate analyses of disease-free survival (DFS) and overall survival (OS) were evaluated with proportional hazards regression. Disease-free survival is defined as the time from achievement of CR to relapse or death from any cause. Overall survival is defined as the time from registration to death. Survival distributions were produced using the methods of Kaplan and Meier.14 Outcome analyses are based on the treatment the patient was intended to receive by the randomization.
Results
Patient characteristics
Patient characteristics have been previously published and are presented in Table 1.7 There were no significant differences between the 2 arms.
Patient characteristics
| Characteristic . | DA, n = 174 . | ATRA, n = 176 . |
|---|---|---|
| Sex, % | ||
| Male | 56 | 48 |
| Female | 44 | 52 |
| Age, y | ||
| Median | 38 | 37 |
| Range | 1-74 | 1-81 |
| % younger than 15 | 13 | 14 |
| % 15-55 | 66 | 66 |
| % 56-65 | 13 | 9 |
| % older than 65 | 9 | 10 |
| WBC, per μL | ||
| Median | 2300 | 2050 |
| Range | 0.6-162 000 | 0.3-69 000 |
| Morphology, % | ||
| Classical M3 | 76 | 82 |
| M3v | 23 | 18 |
| Characteristic . | DA, n = 174 . | ATRA, n = 176 . |
|---|---|---|
| Sex, % | ||
| Male | 56 | 48 |
| Female | 44 | 52 |
| Age, y | ||
| Median | 38 | 37 |
| Range | 1-74 | 1-81 |
| % younger than 15 | 13 | 14 |
| % 15-55 | 66 | 66 |
| % 56-65 | 13 | 9 |
| % older than 65 | 9 | 10 |
| WBC, per μL | ||
| Median | 2300 | 2050 |
| Range | 0.6-162 000 | 0.3-69 000 |
| Morphology, % | ||
| Classical M3 | 76 | 82 |
| M3v | 23 | 18 |
D indicates daunorubicin; A, cytarabine; ATRA, all-trans retinoic acid; WBC, white blood cell count; and M3v, microgranular variant.
Treatment outcome
Complete remission.
A total of 123 (70%) of the 176 patients randomized to chemotherapy achieved CR, as did 127 (73%) of the 174 patients randomized to ATRA (P = .56). The CR rates are presented in Table2 by age, WBC, morphology, and sex. Overall, there was no difference in CR rates between patients treated with ATRA or daunorubicin plus cytarabine (DA). Furthermore, there were no significant differences for any of the variables when CR alone was considered as the outcome (Table2).
CR rates for patients randomized to either DA or ATRA
| . | DA, % . | ATRA, % . |
|---|---|---|
| All patients | 70 | 73 |
| Age, y | ||
| Younger than 15 | 60 | 74 |
| 15 to 55 | 71 | 76 |
| 56 to 65 | 74 | 69 |
| Older than 65 | 69 | 53 |
| WBC, per μL | ||
| Less than 2000 | 69 | 84 |
| 2000 to less than 5000 | 71 | 68 |
| 5000 to less than 10 000 | 68 | 50 |
| 10 000 or more | 71 | 65 |
| Morphology | ||
| Classical M3 | 68 | 73 |
| M3v | 80 | 71 |
| Sex | ||
| Male | 73 | 74 |
| Female | 65 | 72 |
| . | DA, % . | ATRA, % . |
|---|---|---|
| All patients | 70 | 73 |
| Age, y | ||
| Younger than 15 | 60 | 74 |
| 15 to 55 | 71 | 76 |
| 56 to 65 | 74 | 69 |
| Older than 65 | 69 | 53 |
| WBC, per μL | ||
| Less than 2000 | 69 | 84 |
| 2000 to less than 5000 | 71 | 68 |
| 5000 to less than 10 000 | 68 | 50 |
| 10 000 or more | 71 | 65 |
| Morphology | ||
| Classical M3 | 68 | 73 |
| M3v | 80 | 71 |
| Sex | ||
| Male | 73 | 74 |
| Female | 65 | 72 |
CR indicates complete remission. Other abbreviations are explained in the footnote to Table 1.
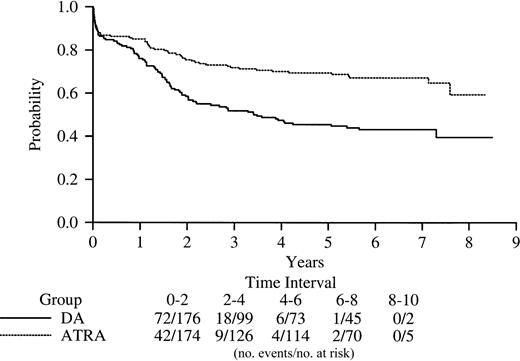
Disease-free survival based on induction randomization.
Among the 250 patients achieving CR, the 5-year DFS is 29% (95% confidence interval [CI], 20-38) and 69% (95% CI, 52-72) for patients randomized to chemotherapy or ATRA induction, respectively (P < .0001; Figure 1). The associations between DFS and a number of presenting characteristics (race, sex, platelet count, WBC, hemoglobin, percent marrow promyelocytes, percent of patients with extramedullary disease at entry, the presence of bleeding, and microgranular variant [M3v] morphology) and the corresponding treatment-factor interactions were evaluated. Previous reports in APL suggest a WBC of 10 × 109/L (10 000/μL) and above is an adverse prognostic factor.6 9 Analyses carried out here considered 10 × 109/L (10 000/μL) and above, 5 × 109/L (5000/μL), and 2 × 109/L (2000/μL) and found a WBC of 2 × 109/L to be a highly prognostic cut point. Female sex, classical morphology, and treatment-WBC interaction (where patients with a WBC below 2 × 109/L [2000/μL] who received ATRA did best; patients with a WBC above 2 × 109/L who received ATRA and those with a WBC below 2 × 109/L who received DA fared intermediately well; and those with a WBC above 2 × 109/L and received DA did worst) were each significantly associated with improved DFS when adjusted for the others based on a P ≤ .05 criterion for retention.
Kaplan-Meier product-limit estimate of disease-free survival from complete remission for patients randomly assigned to induction to either DA (daunorubicin plus cytarabine) or ATRA (all-trans retinoic acid) (P < .0001).
Kaplan-Meier product-limit estimate of disease-free survival from complete remission for patients randomly assigned to induction to either DA (daunorubicin plus cytarabine) or ATRA (all-trans retinoic acid) (P < .0001).
Overall survival based on induction randomization.
With a median follow-up for survival from study entry for all patients of 6.2 years (range, 0-8.5 years), the OS at 5 years was 45% (95% CI, 36-52) for patients randomized to induction chemotherapy and 69% (95% CI, 62-76) for patients randomized to ATRA (P = .0001; Figure 2). The associations between OS from induction randomization and the presenting characteristics listed above were evaluated. Treatment with ATRA for induction, WBC below 2 × 109/L [2000/μL], and absence of clinical bleeding at study entry were each significantly associated with improved OS when adjusted for the others.
Kaplan-Meier product-limit estimate of overall survival according to intention-to-treat analysis for patients receiving DA (daunorubicin plus cytarabine) and ATRA (all-transretinoic acid for induction) (P = .0001).
Kaplan-Meier product-limit estimate of overall survival according to intention-to-treat analysis for patients receiving DA (daunorubicin plus cytarabine) and ATRA (all-transretinoic acid for induction) (P = .0001).
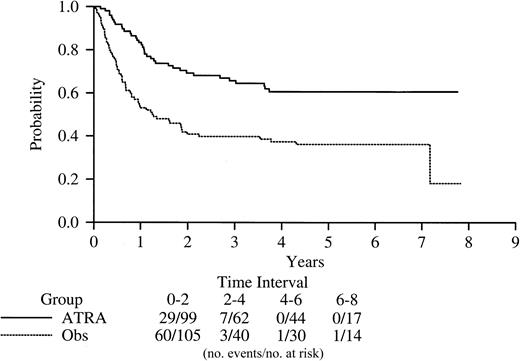
Disease-free survival based on maintenance randomization.
Five-year DFS based on maintenance randomization was 61% (95% CI, 49-70) for patients randomized to ATRA and 36% (95% CI, 23-44) for observation (P < .0001; Figure3). The associations between DFS from maintenance and presenting characteristics were evaluated. Age older than 15 years, female sex, and a treatment-morphology interaction (DA/M3v worst, ATRA best regardless of morphology) were each significant in a multicovariate analysis stratified by induction randomization.
Kaplan-Meier product-limit estimate of disease-free survival from the time of random assignment to maintenance with ATRA (all-trans retinoic acid) or to observation (P < .0001).
Kaplan-Meier product-limit estimate of disease-free survival from the time of random assignment to maintenance with ATRA (all-trans retinoic acid) or to observation (P < .0001).
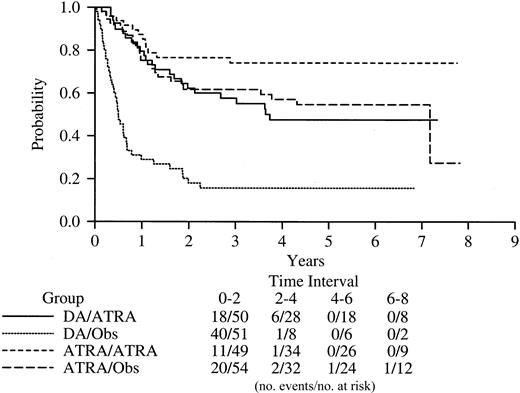
Disease-free survival based on both induction and maintenance randomization.
When both induction and maintenance randomizations were taken into account, the 5-year DFS was 16% (95% CI, 06-26) for patients randomized to chemotherapy and observation; 47% (95% CI, > 31-62) for patients randomized to chemotherapy for induction and ATRA for maintenance; 55% (95% CI, 35-67) for ATRA and observation; and 74% (95% CI, 61-87) for ATRA induction and ATRA maintenance (Figure4).
Kaplan-Meier product-limit estimates of disease-free survival based on both the induction and maintenance randomizations.
Kaplan-Meier product-limit estimates of disease-free survival based on both the induction and maintenance randomizations.
Late relapses.
Late relapses defined as relapse occurring after at least 2 years of continuous CR were documented in 23 patients (9% of patients achieving CR) primarily in the marrow, but 3 in the central nervous system (all of whom received DA for induction). Fourteen (64%) of these 23 patients were male, 2 (9%) had the M3v, and the median WBC at presentation was 1.6 × 109/L (1600/μL; range, 0.3 × 109/L to 29.9 × 109/L [300/μL to 29 900/μL]). The latest relapse occurred 7.5 years from CR. Thirteen (56%) of the 23 patients survived after relapse. Five of the patients relapsing late were randomized to chemotherapy for induction and then observation, 6 to ATRA and then observation, 8 to chemotherapy and then ATRA, and only 1 to ATRA for induction and ATRA for maintenance. Two of the 23 received ATRA for induction and were not randomized to maintenance.
Discussion
The most important finding of this long-term follow-up report is that among patients with newly diagnosed APL, ATRA therapy conferred a sustained significant DFS and OS benefit. Patients treated with ATRA for induction have an excellent outcome, with a 5-year DFS and OS of 69%, and may well be cured of their disease. The patients reported here have among the longest follow-up published, and the results for patients treated with ATRA during induction compare favorably with the best outcomes with similar therapy and follow-up.8-10 15Although in the study reported here there was a median follow-up of more than 6 years, we cite 5-year proportions surviving or disease free to facilitate comparisons with other studies.
We also found a durable benefit for maintenance with ATRA. The 5-year DFS for patients randomized to ATRA maintenance was 61% compared with 36% for patients observed after consolidation. Furthermore, the best outcome was observed for patients randomized to ATRA for both induction and maintenance who had a 5-year DFS of 74%. Additional support for the importance of maintenance comes from the Program a Espanol para el Tratamiento de las Hemopatias Malignas (PETHEMA) Cooperative Group in Spain, which has recently shown that cytarabine may be omitted from induction and consolidation in patients treated with ATRA and anthracyclines for induction plus maintenance therapy with ATRA and low-dose chemotherapy without compromising the outcome.15
It is noteworthy that similarly favorable results may also be observed with increased dose intensity of anthracyclines. In a trial conducted by the Medical Research Council (MRC), patients received considerably more anthracycline exposure (daunorubicin 50 mg/m2 for 3 days for 2 cycles in all patients followed by daunorubicin 50 mg/m2/d for 2 cycles in some patients and mitoxantrone 10 mg/m2 for 5 days in others) than in other trials.8 The excellent results reported by the MRC with ATRA in induction, but without ATRA maintenance, is consistent with the well-recognized unusual sensitivity of leukemic promyelocytes to anthracyclines.16-18 In a Southwest Oncology Group (SWOG) trial conducted prior to the availability of ATRA, daunorubicin was administered at a dose of 70 mg/m2 for 3 days during induction and consolidation, and the 9-year DFS was 72%.16 The present trial began prior to the availability of the SWOG results, and the dose of daunorubicin was the standard dose for induction in AML. This unusual sensitivity may be attributable to an inherent lack of expression of multidrug resistance proteins.19-21
We have identified certain subgroups of patients that particularly benefit from ATRA. In a multivariate analysis in the current study, the prognostic factors predictive of prolonged DFS stratified by age include female sex, classical morphology, and the interaction of WBC with therapy. Notably, the prognostic importance of the WBC is based on the presenting WBC before the introduction of hydroxyurea. Historically, patients with M3v treated have a less favorable prognosis than those with classical morphology.22,23 In the maintenance analysis, we found that ATRA improved the outlook for such patients to a degree similar to that achieved with ATRA for patients with classical morphology. High WBC at presentation, M3v morphology, and older age are factors reported in other trials to confer an unfavorable prognosis.6,8,10,24-26 Sanz and colleagues have identified patients who can be classified in risk groups based on presenting WBC and platelet counts. Those patients were treated with ATRA plus idarubicin for induction, noncytarabine-containing consolidation, and maintenance with ATRA plus 6-MP and methotrexate.27 Sanz et al have classified patients with an initial WBC above 10 × 109/L (10 000/μL) as high risk, and because the outcome for such patients is less favorable than that of patients with a WBC below 10 × 109/L,15,25,26 alternative strategies to improve the cure rate are necessary. The analysis reported here shows the prognostic importance of a presenting WBC of 2.0 × 109/L (2000/μL) or less. The expression of CD56 occurs in approximately 20% of patients with newly diagnosed AML.28 This antigen has been associated with extramedullary disease, megakaryocytic differentiation, and a poor outcome in certain subtypes of AML.29-32 Recently, 2 groups have reported that CD56 expression is associated with the S isoform of the PML-RARα fusion transcript and a poor outcome.33 34 Unfortunately, CD56 expression was not studied in the current analysis.
Although APL has become the most curable subtype of adult AML, approximately 20% of patients still die of the disease due to early death or relapse. The detection of minimal residual or recurrent disease by molecular techniques appears to be useful in guiding therapy. Treatment of molecular relapse is associated with a better outcome than if treatment is delayed until relapse is detected by morphology.35,36 The remarkable activity of arsenic trioxide in patients with relapsed and refractory APL suggests it may have a role in initial therapy or as consolidation.37-41In the current North American Intergroup study, newly diagnosed patients induced with ATRA, daunorubicin, and cytarabine are randomized to either 2 courses of arsenic trioxide followed by 2 courses of daunorubicin consolidation or just to the 2 courses of daunorubicin consolidation. Patients are then randomized to 1 of 2 maintenance regimens. This trial will determine if arsenic trioxide can improve the cure rate, particularly in high-risk patients. All-transretinoic acid with anthracycline-based chemotherapy is now the treatment of choice for patients with APL. With long-term follow-up, the advantages of ATRA in induction and maintenance are sustained.
Coordinated by the Eastern Cooperative Oncology Group (Robert L. Comis, group chair).
Prepublished online as Blood First Edition Paper, August 15, 2002; DOI 10.1182/blood-2002-02-0632.
Supported in part by Public Health Service grants from the National Cancer Institute, National Institutes of Health (grants CA17145, CA23318, CA11083, CA21115, and CA66636, ECOG; CA31983, CA31946, and CA37027, CALGB; CA20319 and CA32102, SWOG; CA03161, POG; and CA77658, CA16058, and CA14958); and the Department of Health and Human Services.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Martin S. Tallman, Northwestern University, Feinburg School of Medicine, Department of Medicine, Division of Hematology/Oncology, Robert H. Lurie Comprehensive Cancer Center, 676 N St Clair St, Suite 850, Chicago, IL 60611; e-mail:m-tallman@northwestern.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal