Human T-cell leukemia virus I is the etiologic agent of adult T-cell leukemia (ATL), an aggressive T-cell malignancy. The viral oncoprotein Tax, through the activation of nuclear factorκB (NF-κB), CCAAT-enhancer binding protein (CREB), and activated protein-1 (AP-1) pathways, is a transcriptional regulator of critical genes for T-cell homeostasis. In ATL cells, activated AP-1 complexes induce the production of transforming growth factor β1 (TGF-β1). TGF-β1 is an inhibitor of T-cell proliferation and cytotoxicity. Here we show that, in contrast to normal peripheral T cells, ATL cells are resistant to TGF-β1–induced growth inhibition. The retroviral transduction of the Tax protein in peripheral T cells resulted in the loss of TGF-β1 sensitivity. Transient transfection of Tax in HepG2 cells specifically inhibited Smad/TGF-β1 signaling in a dose-dependent manner. In the presence of Tax transfection, increasing amounts of Smad3 restored TGF-β1 signaling. Tax mutants unable to activate NF-κB or CREB pathways were also able to repress Smad3 transcriptional activity. Next we have demonstrated that Tax inhibits TGF-β1 signaling by reducing the Smad3 DNA binding activity. However, Tax did not decrease the expression and the nuclear translocation of Smad3 nor did it interact physically with Smad3. Rather, Tax induced c-Jun N-terminal kinase (JNK) activity and c-Jun phosphorylation, leading to the formation of Smad3/c-Jun complexes. Whereas c-Jun alone abrogates Smad3 DNA binding, cotransfection of Tax and of a dominant-negative form of JNK or a c-Jun antisense-restored Smad3 DNA binding activity and TGF-β1 responsiveness. In ATL and in normal T cells transduced by Tax, c-Jun was constitutively phosphorylated. Thus, we describe a new function of Tax, as a repressor of TGF-β1 signaling through JNK/c-Jun constitutive activation, which may play a critical role in ATL leukemogenesis.
Introduction
Human T-cell lymphotropic virus type I (HTLV-I) is the etiologic agent of an aggressive and fatal T-cell malignancy of activated CD4+CD45RO+ T lymphocytes termed adult T-cell leukemia/lymphoma (ATL).1,2 The mechanisms of leukemogenesis are not yet fully understood. Infection during infancy and a long clinical latency period of 20 to 30 years appear to be critical factors associated with the development of ATL. During this period, clonal expansion of HTLV-I–bearing T cells occurs, and, following a model of multistep oncogenesis, the accumulation of critical somatic mutations may contribute to the development of ATL. Viral protein expression from early infection to ATL may play a major role during all stages of the disease development.3
The HTLV-I Tax protein is a 40-kDa transcriptional transactivator of the HTLV-I gene via its interaction with activation transcription factor (ATF)/CCAAT-enhancer binding protein (CREB) proteins and the transcriptional coactivators CREB binding protein (CBP) and p300.4,5 Tax is also capable of increasing expression of other cellular genes by positively regulating nuclear factorκB (NF-κB) activity.3 There is strong evidence that Tax may also play a critical role in the cellular transformation of various in vitro models, including T cells, and is capable of inducing tumors in transgenic mice.6-8 In these models, Tax induction of transformation is also associated with cellular gene expression modulation via the NF-κB and/or ATF/CREB pathways.9
In ATL cells, activated protein-1 (AP-1) activity is constitutively activated10,11 and may play a critical role in cell proliferation and transformation. AP-1 is a transcription factor complex composed of members of Fos (c-fos, FosB, Fra-1, and Fra-2) and Jun (c-Jun, JunB, and JunD) families that play a major role in the positive regulation of proliferation and activation of T-cell and cytokine production.12,13 In nonstimulated normal T cells, the basal level of AP-1 proteins is low, but T-cell activation results in rapid induction of jun and fosgenes.14 AP-1 activity is also regulated at the posttranscriptional level by the activation of c-Jun N-terminal kinase (JNK).15 JNK phosphorylates c-Jun, thereby increasing its DNA binding activity.16 Tax contributes to this pathway by inducing the expression of various members of the AP-1 family, including c-Jun, and by constitutively activating JNK.10,13,17 18
Several reports have demonstrated that fresh ATL cells as well as ATL cell lines produce high levels of transforming growth factor β1 (TGF-β1) as a consequence of the activation of AP-1 sites located in the 5′ regulatory region of the TGF-β1 gene.19 20 However, the role of TGF-β1 production by ATL cells in HTLV-I leukemogenesis remains to be elucidated.
TGF-β1 controls various aspects of cell growth and differentiation by signaling through a heteromeric complex of type I (TGF-β1-RI) and II (TGF-β1-RII) serine/threonine kinase transmembrane receptors. TGF-β1 binds TGF-β1-RII, resulting in the recruitment and the activation of TGF-β1-RI.21 Then, TGF-β1-RI propagates the signal by phosphorylating the C-terminal region of transcription factors of the Smad family termed Smad2 and Smad3, resulting in the formation of heteromeric complexes with another Smad member termed Smad4.22 These heteromeric Smad2/3-Smad4 complexes are then translocated into the nucleus where they function as transcription factors, binding DNA directly on CAGAC sequences or associated with other proteins.23 Smad2/3-Smad4 complexes can activate transcription by recruiting the coactivators CBP/p300 or P/CAF (CBP associated factor), which may act through their histone acetyl transferase activity.22,24 Negative regulation of TGF-β1 signaling can occur at different levels. First, the phosphorylation of Smad2/3 by TGF-β-RI is prevented by an inhibitory Smad protein termed Smad7.25 Second, Smad2/3 phosphorylation in the linker region by the Ras pathway can inhibit its nuclear translocation.26 At the nuclear level, recruitment of corepressors with histone deacetylase activity by Smad proteins may regulate Smad transcriptional activity.27 TGF-β1 plays an essential role in the negative regulation of T-cell proliferation and activity.28 Mice expressing a T-cell–specific dominant-negative transforming TGF-β-RII receptor or with targeted disruption of Smad3 exhibit no or diminished T-cell responses to TGF-β1, respectively, whereas their T cells harbor an activated phenotype.29 30
Thus, the activated phenotype and the proliferation of T cells conflict with the fact that ATL cells produce high levels of TGF-β1 and suggest that ATL cells may have developed several mechanisms of resistance to escape the antiproliferative and inactivating signal mediated by TGF-β1. In this report we have tested this hypothesis, and we show that Tax inhibits Smad3 activity by impairing its DNA binding through activation of the JNK/ c-Jun pathway.
Materials and methods
Cell culture
Human hepatoma cell lines HepG2, HeLa, and Cos-7 cells were grown in a 5% CO2, 95% air atmosphere in Dulbecco modified Eagle medium (DMEM; Gibco BRL, Life Technologies, Cergy-Pontoise, France) supplemented with 10% fetal bovine serum (Gibco BRL, Life Technologies), 100 IU/mL penicillin (Gibco BRL, Life Technologies), 100 μg/mL streptomycin (Gibco BRL, Life Technologies), and 0.01% L-glutamine (Gibco BRL, Life Technologies). Human leukemic T cells (Jurkat cells) and HTLV-I–infected (MT2 and HUT 102) cell lines were grown in RPMI 1640 medium (Gibco BRL, Life Technologies) supplemented with 10% fetal bovine serum, 100 IU/mL penicillin, 100 μg/mL streptomycin, and 0.01% L-glutamine (complete medium). Peripheral blood mononuclear cells (PBMCs) and fresh ATL cells from patients (Champ, Sted, and Pabe) from the Hematology Department of Necker Hospital were isolated by Ficoll separation of blood samples. PBMCs were grown in RPMI complete medium (RPMI supplemented with 10% fetal bovine serum, 100 IU/mL penicillin, 100 μg/mL streptomycin, and 0.01% L-glutamine [Life Technology]). Fresh ATL cells were maintained in RPMI 1640 complete medium in the presence of phytohemagglutinin (PHA) (1 μg/mL) and interleukin 2 (IL-2) (10 IU/mL) for 1 week and then IL-2 alone for 2 weeks.
Plasmids and constructs
Wild-type Tax and mutants M22, M47 cDNA were subcloned in pCMV4 and G148V, K88A, V89A cDNA in pRcCMV. CBP/p300 and Rex expression vectors and HTLV-I long terminal repeat (LTR) reporter were constructed as described earlier.5 Flag- or Myc-tagged Smad expression vectors were provided by R. Derynck and C. H. Heldin (Ludwig Institute for Cancer Research, Uppsala, Sweden) and J. M. Blanchard (Institute of Molecular Genetic, Montpellier, France). CAGA12-luc reporter construct was provided by J. M. Gauthier (Glaxo-Wellcome, Les Ulis, France).23 The expression vector for the p15 reporter plasmid was obtained from X. F. Wang (Duke University, Durham, NC). Dominant-negative JNK expression vectors were provided by M. Kracht (Institute of Molecular Pharmacology, Medical School, Hanover, Germany).31Plasmid-encoding glutathione S-transferase (GST)–cjun1-79 fusion proteins were provided by F. Porteu (ICGM, INSERM U363 Hopital Cochin, Paris, France). Antisense c-Jun (ASc-Jun) expression vector was used as described.32
Construction of TRIP δU3-CMV-TAX vector
A 3-plasmid expression system was used to generate vector particles by transient transfection of 293 T cells by using the calcium phosphate coprecipitation method as previously described.33 Vector plasmids encode the HTLV-I Tax cDNA (TRIPδU3-CMV-Tax) under the transcriptional control of an hCMV promoter. The self-inactivating TRIP-δU3-CMV-Tax vector was constructed by replacing the EGFP gene of TRIP-δU3-CMV-EGFP34 with Tax cDNA. Briefly, Tax cDNA was further inserted by using BamH1 and Xho1 unique restriction sites of TRIP-δU3-CMV-EGFP. Vector particle concentration was assayed for p24 Gag antigen by enzyme-linked immunosorbent assay (ELISA; DuPont, Wilmington, DE).
Proliferation assays
Peripheral blood cells from healthy volunteers and from ATL patients were plated in 96-well plates in the presence of either anti-CD3 (Janssen-Cilag) 100 ng/mL or IL-2 (10 IU/mL) and PHA (Murex, Dartford, United Kingdom) (1 μg/mL). Cells were also cultured in the presence or absence of 2 ng/mL TGF-β1 (R&D Systems, Abington, United Kingdom). After 48 hours, cultures were pulsed for 18 hours with 1 μCi (0.037 MBq) [3H] (thymidine/well), and cells were subsequently harvested and analyzed by standard procedures. The magnitude of [3H] thymidine incorporation was used as a measure of cell proliferation. The results shown are representative of 3 experiments, each performed in triplicate.
Transfection and luciferase assays
HepG2, MT2, Jurkat, HUT102, and HeLa cells (105cells) were transiently transfected with the indicated constructs and the internal control PSV βgal by using LipofectAMINE PLUS (Gibco BRL, Life Technologies) according to the manufacturer's instructions. Cos-7 cells were transiently transfected with the indicated constructs and the internal control PSV βgal by using the DEAE-Dextran method. The amount of total DNA transfected with expression vectors was kept constant in all experiments by the addition of pcDNA3 plasmid. Twenty-four hours after transfection, cells were stimulated with 7 ng/mL human recombinant TGF-β1 (R&D Systems Europe, Lille, France) for 24 hours or with 10 μg/mL anisomycin (Sigma, St Quentin-Fallavier, France) for 30 minutes, when indicated, and luciferase activity was quantified by using Kit Luciferase Assay System (Promega, Charbonnières, France). Values were normalized with the β-galactosidase activity.
Assay of JNK activity
JNK was immunoprecipitated from cell lysates with polyclonal JNK antibody (Pharmingen BD, San Diego, CA) after transfection of an empty or a Tax-encoding vector. GST-cjun1-79 was used as substrate and added to 30 μL kinase assay buffer (25 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), pH 7.5, 20 mM MgCl2, 0.1 mM EGTA (ethyleneglycoltetraacetic acid), 50 mM sodium β-glycerophosphate, 0.1 mM sodium orthovanadate, 1 mM dithiothreitol, and 1 μM okadaic acid) supplemented with 20 μM adenosine triphosphate (ATP) and 5 μCi (0.185 MBq) [γ-32P] ATP at 30°C for 20 minutes. The reaction was stopped by addition of 2 × sodium dodecyl sulfate (SDS) sample buffer and then boiled for 5 minutes. The samples were analyzed by SDS–polyacrylamide gel electrophoresis (PAGE).
Preparation of whole cell, cytosolic, and nuclear extracts
Total extracts were prepared from transfected cells. Forty-eight hours after transfection, cells were washed with phosphate-buffered saline (PBS), scraped, and solubilized in the following buffer: 10 mM Tris (tris(hydroxymethyl)aminomethane) HCl, pH 7.4; 150 mM NaCl; 1% Nonidet P40 (NP40); 1 mM EDTA (ethylenediaminetetraacetic acid); 1 mM NA3VO4; 10 IU/mL aprotinin; 1 mM phenylmethylsulfonylfluoride (PMSF); and 5 μg/mL leupeptin. Lysates were cleared of debris by centrifugation at 15 000 rpm for 15 minutes at 4°C. Nuclear and cytosolic extracts were prepared from MT2-, HUT102-, or HepG2-transfected cells. Forty-eight hours after transfection, cells were washed with PBS, scraped, and suspended in cold buffer A (20 mM HEPES pH 7.9; 20 mM NaF; 20 mM Na3VO4; 1 mM Na4P2O7; 1 mM EDTA; 1 mM EGTA; 1 mM dithio-threitol (DTT); 0.1% NP40; 1 mM PMSF; 1 μg/μL leupeptin, aprotinin, and pepstatin). Cell lysates were centrifuged for 15 minutes at 15 000 rpm at 4°C. The cytosolic supernatant was removed. The pellet was resuspended in buffer C (20 mM HEPES, pH 7.9; 20 mM NaF; 20 mM Na3VO4; 1 mM Na4P2O7; 1 mM EDTA; 1 mM EGTA; 1 mM DTT; 1 mM PMSF; 1 μg/μL leupeptin, aprotinin, and pepstatin; 420 mM NaCl; 20% glycerol) and was mixed by pelleting up and down. After 30 minutes on ice, the nuclear extract was cleared at 15 000 rpm for 15 minutes at 4°C.
Antibodies
Mouse monoclonal anti-Tax antibody was provided by J. Brady (National Institutes of Health, Bethesda, MD). Rabbit polyclonal anti-Tax antibody was used as described by Bex et al.4Rabbit polyclonal anti-Smad3 and anti–Flag M2 antibodies were purchased from Upstate Biotechnology (Waltham, MA). Anti-Myc (9E10), anti-HA polyclonal antibody, anti–phospho-c-Jun and antiactin antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-JNK antibodies were purchased from Pharmingen BD (San Diego, CA).
Immunoblotting and immunoprecipitation
Protein (50 μg) from total extracts of transfected HepG2 cells were resolved by SDS-10% PAGE and were electrotransferred to a nitrocellulose membrane (Protran; Sleicher & Schuell, Strasbourg, France). The blots were blocked in 0.1% Tween-PBS containing 5% nonfat dry milk. Antibodies were added to the blocking solution at 1:1000 for 1 hour at room temperature. The blots were washed 5 times with 0.1% Tween-PBS, and the peroxydase-coupled second antibody was added at 1:10 000 for 30 minutes at room temperature. After 5 washes in Tween-PBS, bound antibodies were detected by using the Amersham enhanced chemiluminescence system (ECL plus; Amersham Pharmacia Biotech, Orsay, France), and blots were exposed on Hyperfilm ECL film (Amersham Pharmacia Biotech). For immunoprecipitation the cell lysates (nuclear or cytosolic extracts) were incubated with the appropriate antibody for 2 hours, followed by incubation with protein G-Sepharose beads (Santa Cruz Biotechnology) for 4 hours at 4°C. Beads were washed 4 times with the buffer used for cell solubilization. Immune complexes were then eluted by boiling for 3 minutes in 2 × Laemmli buffer, and then extracts were analyzed by immunoblotting as described above.
Electrophoresis mobility shift assays (EMSA)
Oligonucleotides were end-labeled with [γ-32P] dCTP using the T4 polynucleotide kinase (Gibco BRL, Life Technologies). Binding reactions containing 10 μg nuclear extracts and 2 ng labeled oligonucleotides were performed for 20 minutes at 37°C in 18 μL binding buffer (20 mM HEPES, pH 7.9; 30 mM KCl; 4 mM MgCl2; 0.1 mM EDTA; 20% glycerol; 0.2% NP40; 4 mM spermidin; 3 μg poly [dI-dC]). Protein-DNA complexes were resolved in 5% polyacrylamide gel containing 0.5 × Tris Borate EDTA (TBE). The sequences of the double-stranded oligonucleotides used as a probe were as follows: plasminogen activator inhibitor (PAI) probe, 5′-TCG AGA GCC AGA CAA GGA GCC AGA CAA GCA GCC AGA CAC-3′ and its complementary strand23; SBE probe, 5′-CTCTATCAATTGGTCTAGACTTAACCGGA-3′ and its complementary strand; AP-1 and NF-κB probe, 5′-CCGGGGATGACTCAGCC-3′ and 5′-ACAAGGGACTTTCCGCTGGGGACTTTCC-3′, respectively, and their complementary strands.
Immunofluorescence and confocal analysis
Cells were cultured on coverslip slides and transfected with a combination of Flag-Smad3 and/or Tax expression vectors. Twenty hours after transfection cells were treated with TGF-β1 (R&D Systems Europe) for 30 minutes and fixed in 4% paraformaldehyde and permeabilized with 0.5% Triton-X100 for 15 minutes. Preparations were incubated for 1 hour with primary antibodies (diluted 1:50 to 1:1000) in PBS and 0.2% bovine serum albumin (BSA). After 3 washes with PBS/BSA 0.2%, samples were incubated with secondary antibodies consisting of Cy3 antimouse, fluorescein isothiocyanate (FITC) antirabbit (Jackson Immunologicals). Images were obtained by using a confocal microscope (Zeiss Axiovert 100M, Oberkochen, Germany).
Results
HTLV-I oncoprotein Tax confers resistance to the antiproliferative effect of TGF-β1 on HTLV-I–transformed and –activated peripheral T cells
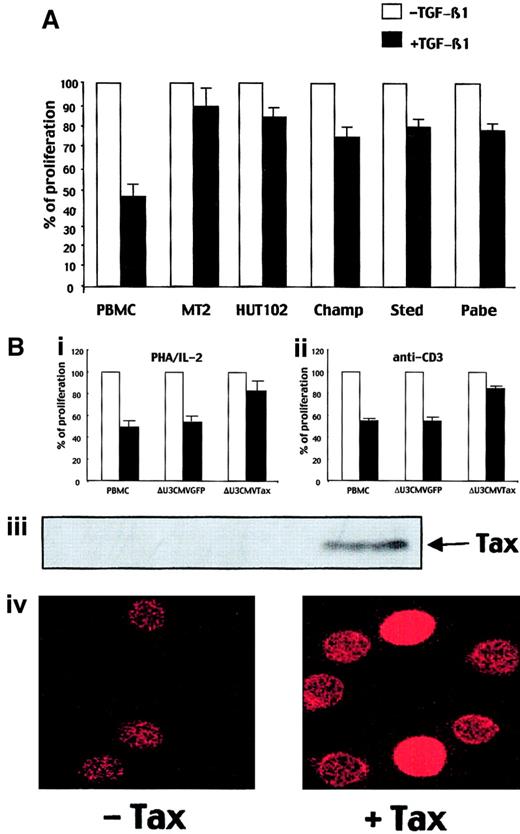
TGF-β1 plays a role in the negative regulation of the immune response in part by inhibiting proliferation of normal T cells after stimulation. ATL cells, which are proliferative activated T cells, produce high levels of TGF-β1.19 Thus, we have investigated the effect of TGF-β1 on ATL cell proliferation. During the first 48 hours, a weak inhibition of normal T-cell proliferation was observed (data not shown). However, at 72 hours, TGF-β1 (2 ng/mL) markedly inhibited the proliferation of normal T cells stimulated with PHA/IL-2 (55% inhibition) (Figure 1A). This inhibition was even greater at 96 hours (> 80% inhibition) (data not shown). In contrast, TGF-β1 did not inhibit the proliferation of either ATL cell lines MT2 and HUT102 or IL-2–dependent ATL cells derived from patients (Champ, Sted, Pabe), even after 5 days of culture (data not shown). These results indicate that HTLV-1–transformed cells have developed a mechanism of resistance to the growth inhibitory effect of TGF-β1. Then, we investigated whether or not Tax could play a role in this TGF-β1 resistance. We transduced normal T cells with a triplex retroviral construct encoding the Tax gene directed by the CMV promoter (δU3CMVTax). Twelve hours after transduction, T cells were stimulated through the CD3/TCR complex or with PHA/IL-2 in the presence or absence of TGF-β1 (2 ng/mL). As expected, at 72 hours, proliferation of nontransduced T cells or T cells transduced with a control construct were inhibited by TGF-β1 by approximately 50%. In contrast, the proliferation of Tax-transduced T cells (65% transduction efficiency) was only weakly inhibited in the presence of the same amount of TGF-β1 (Figure 1B). These data indicated that Tax impairs TGF-β1 growth inhibitory effect in normal T cells.
IL-2–dependent ATL cells and HTLV-1–transformed cell lines are resistant to the TGF-β1–induced growth inhibition.
(A) PBMCs, MT2 and HUT102 cell lines, and IL-2–dependent fresh ATL cells from patients (Champ, Sted, Pabe) were stimulated with PHA (1 μg/mL) and IL-2 (10 IU/mL) in the presence or in the absence of TGF-β1 (2 ng/mL) for 72 hours, and their proliferation was determined as described in “Materials and methods.” The results are representative of 3 independent experiments, each conducted in triplicate. (B) PBMCs and ΔU3CMVGFP- or ΔU3CMVTax-transduced PBMCs were stimulated with either PHA/IL-2 (i) or anti-CD3 (100 ng/mL; ii) in the presence or in the absence of TGF-β1 (2 ng/mL) for 72 hours and their proliferation was determined as described in “Materials and methods.” The results are representative of 3 independent experiments, each conducted in triplicate. Tax expression in ΔU3CMVTax-transduced PBMCs, as compared with ΔU3CMVGFP or untransduced PBMCs, is detected with an anti-Tax antibody by immunoblot assay (iii) or immunofluorescence (iv). Original magnification Biv, × 40.
IL-2–dependent ATL cells and HTLV-1–transformed cell lines are resistant to the TGF-β1–induced growth inhibition.
(A) PBMCs, MT2 and HUT102 cell lines, and IL-2–dependent fresh ATL cells from patients (Champ, Sted, Pabe) were stimulated with PHA (1 μg/mL) and IL-2 (10 IU/mL) in the presence or in the absence of TGF-β1 (2 ng/mL) for 72 hours, and their proliferation was determined as described in “Materials and methods.” The results are representative of 3 independent experiments, each conducted in triplicate. (B) PBMCs and ΔU3CMVGFP- or ΔU3CMVTax-transduced PBMCs were stimulated with either PHA/IL-2 (i) or anti-CD3 (100 ng/mL; ii) in the presence or in the absence of TGF-β1 (2 ng/mL) for 72 hours and their proliferation was determined as described in “Materials and methods.” The results are representative of 3 independent experiments, each conducted in triplicate. Tax expression in ΔU3CMVTax-transduced PBMCs, as compared with ΔU3CMVGFP or untransduced PBMCs, is detected with an anti-Tax antibody by immunoblot assay (iii) or immunofluorescence (iv). Original magnification Biv, × 40.
Tax represses TGF-β1–mediated Smad transcriptional responses through the Smad pathway
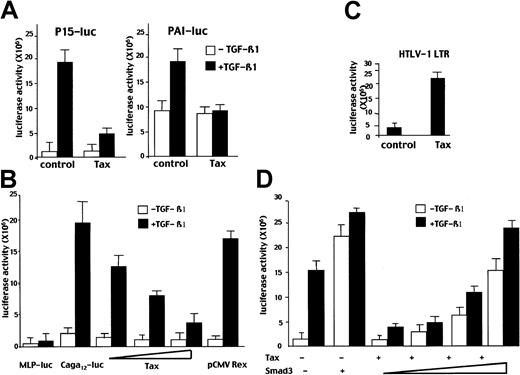
We then investigated whether Tax, the main viral oncoprotein involved in ATL leukemogenesis, played a role in TGF-β 1 resistance by repressing TGF-β1–mediated transcriptional responses. In the first set of experiments we used cotransfection assays in the TGF-β1–responsive cell line HepG2 with a luciferase reporter construct containing the natural promoter of the TGF-β1 target gene p15, a cyclin-dependent kinase inhibitor or the PAI-1 promoter (PAI-luc). Cotransfection of a Tax-expressing vector led to the repression of p15-luc as well as of PAI-luc induction by TGF-β1 (Figure 2A).
Tax represses TGF-β1–mediated transcriptional responses in a dose-dependent manner.
TGF-β1 responsive HepG2 cells were cotransfected with (A) p15-luc (5 μg) or PAI-luc (2 μg) and an expression vector encoding for Tax (2 μg) or an empty vector (control); (B) HTLV-I LTR Luc (2 μg) and the Tax expression vector (2 μg) (Tax) or an empty vector (control) were cotransfected; (C) 2 μg of an empty vector containing the minimal adenovirus MLP promoter (MLP-luc) or a vector containing 12 copies of the CAGA box upstream from the MLP promoter (CAGA12-luc) and with an expression vector encoding for various levels of Tax construct (0.5, 2, or 5 μg) or a Rex vector expression (pCMV Rex) used as control; (D) the (CAGA)12-Luc (2 μg) and a Tax construct (2 μg) when indicated (+) and increasing amounts of Smad3 construct (0.2, 0.5, and 2 μg). Basal and TGF-β1–induced luciferase activities are indicated. The results are representative of at least 3 independent experiments in which each assay was conducted in triplicate.
Tax represses TGF-β1–mediated transcriptional responses in a dose-dependent manner.
TGF-β1 responsive HepG2 cells were cotransfected with (A) p15-luc (5 μg) or PAI-luc (2 μg) and an expression vector encoding for Tax (2 μg) or an empty vector (control); (B) HTLV-I LTR Luc (2 μg) and the Tax expression vector (2 μg) (Tax) or an empty vector (control) were cotransfected; (C) 2 μg of an empty vector containing the minimal adenovirus MLP promoter (MLP-luc) or a vector containing 12 copies of the CAGA box upstream from the MLP promoter (CAGA12-luc) and with an expression vector encoding for various levels of Tax construct (0.5, 2, or 5 μg) or a Rex vector expression (pCMV Rex) used as control; (D) the (CAGA)12-Luc (2 μg) and a Tax construct (2 μg) when indicated (+) and increasing amounts of Smad3 construct (0.2, 0.5, and 2 μg). Basal and TGF-β1–induced luciferase activities are indicated. The results are representative of at least 3 independent experiments in which each assay was conducted in triplicate.
TGF-β1–mediated transcriptional responses result from the interplay between Smad3/4 proteins and other transcription factors. To test whether Tax specifically impaired Smad3/4 activity we used a concatemerized CAGA (CAGA)12 construct derived from the PAI-1 promoter that is known to specifically explore Smad3 and Smad4 transcriptional activity.23 As expected, when (CAGA)12-luc alone was transfected, a substantial increase (× 20) in luciferase activity was observed in the presence of TGF-β1. This transactivation was repressed in a dose-dependent manner when a Tax encoding vector was cotransfected with (CAGA)12-luc (Figure 2B). This effect appeared to be specific for Tax because Rex, another HTLV-I protein, had no substantial effect on the TGF-β1 responsive reporter. As a positive control of Tax activity in the HepG2 cell line, the same amount of Tax-expressing plasmid strongly activated transcription from the HTLV-I LTR, indicating that the Tax expression plasmid was functioning properly and that Tax protein was not toxic to the cells and did not act as a general transcription repressor (Figure 2C). To confirm the specific effect of Tax on Smad3/4 signaling, we investigated whether or not the overexpression of Smad3 or Smad4 could reverse TGF-β1–signaling repression by Tax. We found that in the presence of Tax, cotransfection of increasing amounts of Smad3 but not of Smad4 (data not shown) could reverse the repression of TGF-β1 response by Tax (Figure 2D). Taken together, these results demonstrate that Tax specifically inhibits TGF-β1 response through the Smad pathway and specifically inhibits Smad3 transcriptional activity.
Tax inhibition of Smad3 transcriptional activity is neither linked to its ability to bind the coactivators CBP/p300 nor to the activation of the NF-κB pathway
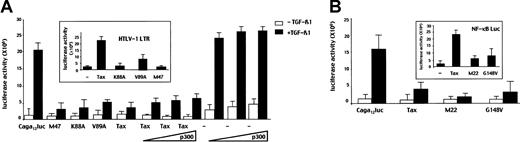
Next, we investigated the mechanisms of repression of Smad3 transcriptional activity by Tax. First, we tested whether Tax could disrupt the association between CBP/p300 and Smad3, thereby providing a squelching effect on Smad3 transcriptional activity. We used cotransfection assays with the well-characterized Tax mutants K88A, V89A, and M47, which fail to bind p300, CBP, and p/CAF, respectively.5 In transfection assays in HepG2 cells, Tax mutant K88A, V89A, and M47 resulted in the inhibition of TGF-β1–induced Smad3 transcriptional activity to the same extend as wild- type Tax did (Figure 3A), suggesting that the Tax effect was independent of CBP/p300 or p/CAF. To emphasize this finding, increasing amounts of p300 or CBP expression vectors were transfected with Tax. As shown in Figure 3A, neither p300 nor CBP (data not shown) allowed the recovery of the TGF-β1 response. As a control in our system, in the absence of Tax cotransfection of p300 or CBP (data not shown) increased TGF-β1–induced Smad3/4 transcriptional activity. These results demonstrate that Tax inhibition of Smad3 function is independent of CBP/p300 level and is not due to squelching of either CBP/p300 or p/CAF.
Tax represses TGF-β1 signaling independently of NF-κB activation or recruitment of CBP/p300.
HepG2 cells were cotransfected with (A) the (CAGA)12-Luc reporter construct (5 μg) and the wild-type Tax (5 μg) or the K88A (5 μg), V89A (5 μg), or M47 (5 μg) mutant expression vectors encoding proteins unable to bind CBP/p300 and p/CAF, respectively. In inset, Tax (5 μg), K88A (5 μg), V89A (5 μg), or M47 (5 μg) constructs were cotransfected with HTLV-I LTR Luc (2 μg) to assess their functional capacities. When indicated, increasing amounts (0.2, 0.5, or 2 μg) of a p300 expression vector alone or in combination with a Tax construct (5 μg) were cotransfected. (B) the (CAGA)12-Luc reporter (2 μg) and wild-type Tax (5 μg), M22 (5 μg), or G148V (5 μg) mutant expression vectors. In inset, Tax (5 μg), M22 (5 μg), or G148V (5 μg) constructs were cotransfected with an NF-κB–responsive reporter gene.
Tax represses TGF-β1 signaling independently of NF-κB activation or recruitment of CBP/p300.
HepG2 cells were cotransfected with (A) the (CAGA)12-Luc reporter construct (5 μg) and the wild-type Tax (5 μg) or the K88A (5 μg), V89A (5 μg), or M47 (5 μg) mutant expression vectors encoding proteins unable to bind CBP/p300 and p/CAF, respectively. In inset, Tax (5 μg), K88A (5 μg), V89A (5 μg), or M47 (5 μg) constructs were cotransfected with HTLV-I LTR Luc (2 μg) to assess their functional capacities. When indicated, increasing amounts (0.2, 0.5, or 2 μg) of a p300 expression vector alone or in combination with a Tax construct (5 μg) were cotransfected. (B) the (CAGA)12-Luc reporter (2 μg) and wild-type Tax (5 μg), M22 (5 μg), or G148V (5 μg) mutant expression vectors. In inset, Tax (5 μg), M22 (5 μg), or G148V (5 μg) constructs were cotransfected with an NF-κB–responsive reporter gene.
Second, we examined whether or not the NF-κB pathway is involved in the repression of Smad3 transcriptional activity by performing similar experiments with 2 Tax mutants, M22 and G148V, that are unable to activate NF-κB but have conserved their ability to transactivate the HTLV-I-LTR through the CREB/ATF pathway (data not shown). As shown in Figure 3B, these mutants repressed Smad3 transcriptional activity to the same extent as wild- type Tax but were unable to transactivate a NF-κB responsive promoter. This finding suggests that Tax did not inhibit TGF-β1 signaling through NF-κB induction. Taken together, these results indicate that Tax inhibits Smad3/TGF-β1 signaling independently of CREB/ATF or NF-κB pathways.
Tax impairs Smad3 DNA binding activity
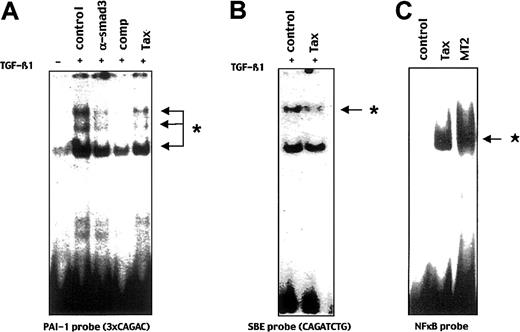
TGF-β1–activated Smad3/4 complexes specifically recognize a binding site CAGAC within the PAI-1 promoter. Thus, we investigated whether Tax may affect the Smad3/4 DNA binding activity by using an electrophoretic mobility shift assay with a probe containing 3 CAGA box, derived from the PAI-1 promoter. As previously described,23 TGF-β1 stimulation induced the formation of specific Smad complexes in HepG2 cells. As shown in Figure4A, levels of Smad3/4-DNA complexes were substantially decreased in the presence of Tax. To further confirm that the decrease of the Smad-DNA complexes occurred at the level of Smad3/4 DNA binding activity, we used a synthetic probe (SBE) that contains a palindromic Smad3/4-specific sequence CAGATCTG. As shown in Figure 4B, Smad3/4 complexes were also substantially decreased in the presence of Tax. As a control, to rule out a general negative effect of Tax on DNA binding activity of transcription factors, we next used a probe specific for NF-κB DNA binding activity. As previously described, Tax could induce a NF-κB promoter (Figure 3B) and DNA binding activities (Figure 4C). These results indicate that through decreased Smad3-DNA binding activity, Tax inhibits TGF-β1 signaling.
Tax impairs TGF-β1–stimulated Smad3 DNA binding activity.
(A) An EMSA was performed by using a 32P-labeled probe derived from the PAI-1 promoter containing 3 CAGAC sequences and 10 μg of nuclear extracts from HepG2 cells transfected, with the Tax (Tax) or an empty expression vector (control), and induced (+) or not (−) for 30 minutes by TGF-β1. TGF-β1–induced complexes are indicated by arrows. Fifty molar excess of non–radio-labeled CAGAC sequence was added as competitor in 50 × molar excess (comp). Specific anti-Smad3 antibody (α-Smad3) was incubated before mixing with the CAGA probe. * indicates Smad3/4 complex. (B) HepG2 nuclear extracts used in (A) were mixed with a synthetic and palindromic CAGATCTG sequence. * indicates Smad3/4 complex. (C) A specific NF-κB probe derived from the IL-8 promoter was used with nuclear extract from MT2 cell line (MT2) or HepG2 cells transfected with a Tax expression vector (Tax) or an empty vector (control). ★ indicates NF-κB.
Tax impairs TGF-β1–stimulated Smad3 DNA binding activity.
(A) An EMSA was performed by using a 32P-labeled probe derived from the PAI-1 promoter containing 3 CAGAC sequences and 10 μg of nuclear extracts from HepG2 cells transfected, with the Tax (Tax) or an empty expression vector (control), and induced (+) or not (−) for 30 minutes by TGF-β1. TGF-β1–induced complexes are indicated by arrows. Fifty molar excess of non–radio-labeled CAGAC sequence was added as competitor in 50 × molar excess (comp). Specific anti-Smad3 antibody (α-Smad3) was incubated before mixing with the CAGA probe. * indicates Smad3/4 complex. (B) HepG2 nuclear extracts used in (A) were mixed with a synthetic and palindromic CAGATCTG sequence. * indicates Smad3/4 complex. (C) A specific NF-κB probe derived from the IL-8 promoter was used with nuclear extract from MT2 cell line (MT2) or HepG2 cells transfected with a Tax expression vector (Tax) or an empty vector (control). ★ indicates NF-κB.
Tax-induced decrease of Smad3-DNA binding activity is not linked to impairment of Smad3 nuclear translocation, decrease of Smad3 expression, or Tax/Smad3 interaction
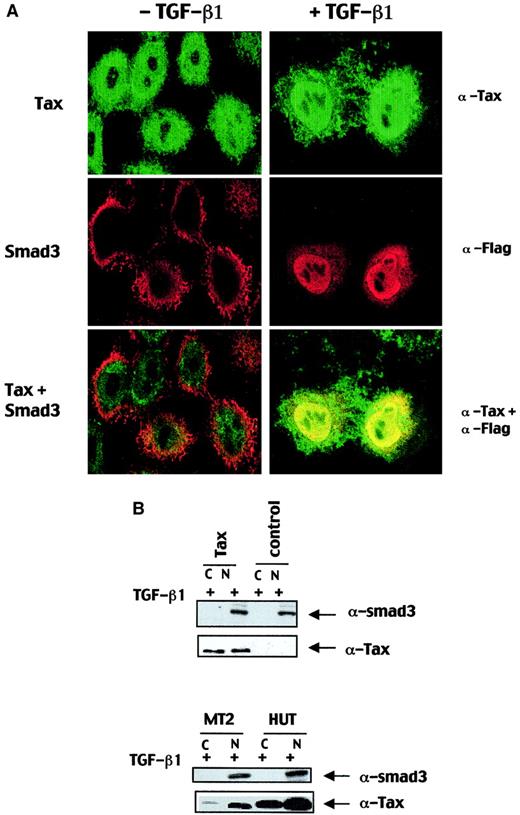
To explain the mechanism of decrease of Smad-DNA complexes, we tested whether expression of Smad3- or TGF-β1–induced nuclear translocation of Smad3 could be impaired by Tax localization of Smad3. We used immunofluorescence confocal microscopy analysis of cells cotransfected with a Tax- and Flag-tagged Smad3 expression vectors to study the subcellular. Smad3 and Tax localizations were analyzed before and after stimulation with TGF-β1. As expected, with or without TGF-β1 stimulation Tax was predominantly localized in the nucleus, and no substantial change in the TGF-β1–induced nuclear translocation of Smad3 was observed in the presence of Tax (Figure5A). In immunoblot assays, cells transfected with a Tax construct and stimulated with TGF-β1 expressed endogenous nuclear Smad3 proteins to a similar extent as in untransfected cells (Figure 5B). Interestingly, Smad3 was highly expressed and was found constitutively in the nucleus of HTLV-I–transformed cell lines MT2 and HUT102 expressing high level of Tax (Figure 5B). Taken together, these results indicate that Tax neither impairs endogenous Smad3 expression nor modifies nuclear localization of Smad3 in the presence of TGF-β1.
Tax neither impairs TGF-β1–induced Smad3 nuclear accumulation nor modifies Smad3 expression.
(A) HeLa cells were transfected with Flag-Smad3 and Tax expression vectors and were incubated either in the absence or in the presence of TGF-β1 for 30 minutes. Flag-Smad3 was visualized with an anti-Flag antibody, and Tax was detected with a rabbit anti-Tax antibody. Localization of the indicated proteins was analyzed by confocal immunofluorescence microscopy. Original magnification × 100. (B) HepG2 cells were transfected with Tax expression vector (Tax) or an empty expression vector (control) and treated with TGF-β1 for 1 hour. HepG2, MT2, and HUT nuclear (N) and cytoplasmic (C) lysates were subjected to immunoblot analysis with a polyclonal rabbit anti-Smad3 antibody and a mouse anti-Tax monoclonal antibody.
Tax neither impairs TGF-β1–induced Smad3 nuclear accumulation nor modifies Smad3 expression.
(A) HeLa cells were transfected with Flag-Smad3 and Tax expression vectors and were incubated either in the absence or in the presence of TGF-β1 for 30 minutes. Flag-Smad3 was visualized with an anti-Flag antibody, and Tax was detected with a rabbit anti-Tax antibody. Localization of the indicated proteins was analyzed by confocal immunofluorescence microscopy. Original magnification × 100. (B) HepG2 cells were transfected with Tax expression vector (Tax) or an empty expression vector (control) and treated with TGF-β1 for 1 hour. HepG2, MT2, and HUT nuclear (N) and cytoplasmic (C) lysates were subjected to immunoblot analysis with a polyclonal rabbit anti-Smad3 antibody and a mouse anti-Tax monoclonal antibody.
Next, we asked whether Tax interacts directly with Smad3. The immunoprecipitation analysis and GST pull-down assay did not demonstrate the presence of Smad in the immune complex (data not shown). This same experiment also indicated that Tax did not affect the interaction between Smad3 and Smad4 on TGF-β1 receptor activation (data not shown). These data suggest that Tax affects Smad3 DNA binding activity by an indirect mechanism.
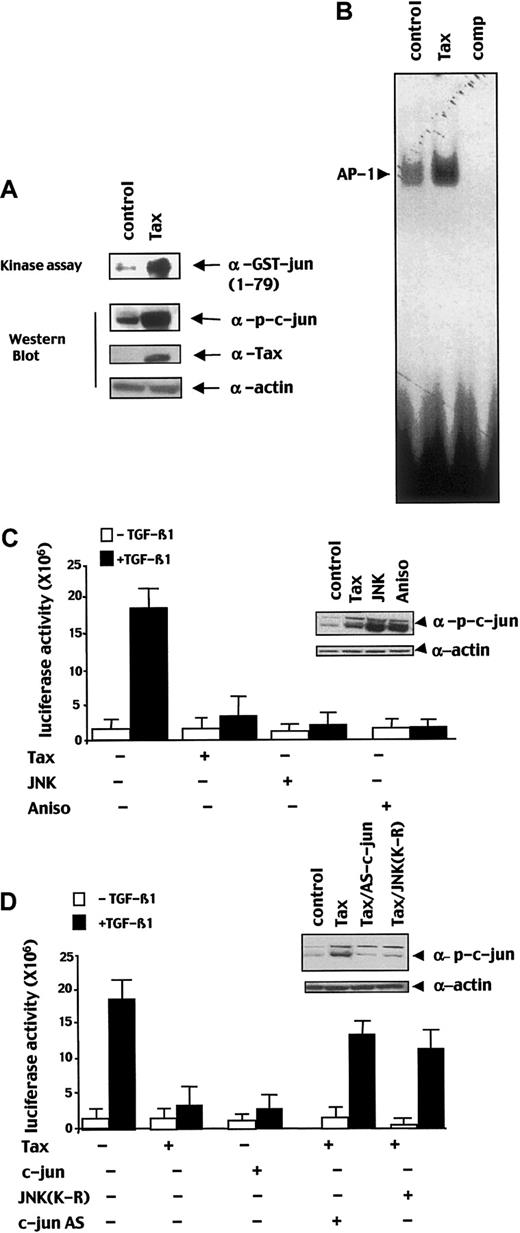
Tax induces constitutive JNK activation and c-Jun phosphorylation that prevent TGF-β1–mediated transcriptional response
Then, we have investigated whether the constitutive AP-1 activity observed in ATL cells could be due to Tax on and responsible of TGF-β1 signaling inhibition. First, we confirmed that Tax induces JNK activity, leading to a high level of phosphorylated c-Jun (p-c-Jun) and AP-1 activity. As shown in Figure 6A, in kinase assay, Tax induced JNK activity. As a consequence, in immunoblot, the amount of p-c-Jun was increased in HepG2 cells transfected with Tax as compared with untransfected cells (Figure 6A). Furthermore, Tax induced AP-1 activity in a gelshift experiment (Figure6B). To investigate the feasibility of the Tax-induced constitutive JNK pathway activation in TGF-β1 signaling repression, we performed transient transfection by using the (CAGA)12-luc construct in various conditions of JNK/c-Jun pathway stimulation. Cotransfection of a JNK encoding vector or treatment of the cells with anisomycin that induce JNK activity led to substantial repression of the TGF-β1–induced transcriptional response (Figure 6C). To attribute the inhibitory role of JNK to c-Jun activity, we transfected a c-Jun–encoding vector and found that c-Jun repressed TGF-β1 signal transduction (Figure 6D). In addition, cotransfection of Tax with a dominant-negative JNK protein (JNK-K-R) or a c-Jun antisense construct reversed Tax-mediated transcriptional repression (Figure 6D). Taken together these results indicate that Tax-induced activation of the JNK/c-Jun pathway represses TGF-β1–mediated transcriptional response.
Tax induces JNK activity and subsequent phosphorylation of c-Jun.
(A) Total lysate of HepG2 cells transfected with a Tax expression vector (Tax) or an empty expression vector (control) was subjected to JNK kinase assay by using the GST-c-Jun1-79 and to an immunoblot analysis probed with an anti–p-c-Jun, an anti-Tax, or an anti–α-actin antibody. (B) Nuclear extract from Tax expression vector (Tax) or an empty expression vector (control) transfected HepG2 cells were used for EMSA with an AP-1–specific probe, 50 M excess of non–radio-labeled AP-1 probe was added as competitor in 50 × molar excess (comp). (C,D) HepG2 cells were cotransfected with the (CAGA)12-Luc reporter construct (2 μg) and the indicated combinations of Tax (5 μg) and/or c-Jun (5 μg), JNK (5 μg), dominant-negative JNK (JNK-(K-R); 5 μg), antisense c-Jun (c-Jun AS; 5 μg) expression vectors and were treated with or without TGF-β1. In the indicated condition, anisomycin (Aniso) was added 24 hours after transfection for 30 minutes before lysis. Error bars represent the variability of one of the experiments performed 3 times in duplicates. For each condition, a part of the lysate was subjected to immunoblot with a p-c-Jun antibody to assess the level of p-c-Jun.
Tax induces JNK activity and subsequent phosphorylation of c-Jun.
(A) Total lysate of HepG2 cells transfected with a Tax expression vector (Tax) or an empty expression vector (control) was subjected to JNK kinase assay by using the GST-c-Jun1-79 and to an immunoblot analysis probed with an anti–p-c-Jun, an anti-Tax, or an anti–α-actin antibody. (B) Nuclear extract from Tax expression vector (Tax) or an empty expression vector (control) transfected HepG2 cells were used for EMSA with an AP-1–specific probe, 50 M excess of non–radio-labeled AP-1 probe was added as competitor in 50 × molar excess (comp). (C,D) HepG2 cells were cotransfected with the (CAGA)12-Luc reporter construct (2 μg) and the indicated combinations of Tax (5 μg) and/or c-Jun (5 μg), JNK (5 μg), dominant-negative JNK (JNK-(K-R); 5 μg), antisense c-Jun (c-Jun AS; 5 μg) expression vectors and were treated with or without TGF-β1. In the indicated condition, anisomycin (Aniso) was added 24 hours after transfection for 30 minutes before lysis. Error bars represent the variability of one of the experiments performed 3 times in duplicates. For each condition, a part of the lysate was subjected to immunoblot with a p-c-Jun antibody to assess the level of p-c-Jun.
c-Jun inhibits TGF-β1 signaling by interacting with Smad3 and by preventing its DNA binding activity
Then we investigated whether c-Jun inhibition of Smad3 transcriptional activity was related to the impairment of Smad3 DNA binding activity. In gelshift experiments, we found that c-Jun impaired Smad3 DNA binding in a dose-dependent manner (Figure7A). To assess the mechanism of this inhibition, we performed an immunoprecipitation assay and found that c-Jun interacted directly with Smad3 and that this interaction was increased in the presence of Tax (Figure 7B). Further, emphasizing the role of the JNK/c-Jun pathway in Tax-induced inhibition of Smad3 DNA binding, we have shown that cotransfection of JNK-K-R or c-Jun antisense restored Smad3 DNA binding activity in Tax-transfected HepG2 cells (Figure 7C).
Tax-induced c-Jun interacts with Smad3 and abrogates Smad3 DNA binding activity.
(A) Nuclear extracts from TGF-β1–stimulated HepG2 cells transfected with the Tax expression vector, empty vector (control), or increasing doses of c-Jun expression vector (c-Jun 0.5, 4) were used with the32P-labeled probe containing 3 CAGA boxes for EMSA. (B) Cos-7 cells were transfected with the indicated combination of HA-c-Jun, Myc-Smad3 (top panel), and Tax expression vectors (bottom panel) and were subjected to immunoprecipitation by using an anti-Myc antibody. The expression of Tax or Smad3 was detected by immunoblot by using an anti-Tax or an anti-Myc antibody before immunoprecipitation. (C) Nuclear extracts, from TGF-β1–stimulated (+) or not (−) HepG2 cells transfected with the indicated expression vectors (empty vector [control]), Tax alone (Tax), or associated with an antisense c-Jun (Tax/AS c-Jun) or a dominant-negative JNK (Tax/JNK-(K-R)) expression vectors were used with the 32P-labeled probe containing 3 CAGA boxes for EMSA.
Tax-induced c-Jun interacts with Smad3 and abrogates Smad3 DNA binding activity.
(A) Nuclear extracts from TGF-β1–stimulated HepG2 cells transfected with the Tax expression vector, empty vector (control), or increasing doses of c-Jun expression vector (c-Jun 0.5, 4) were used with the32P-labeled probe containing 3 CAGA boxes for EMSA. (B) Cos-7 cells were transfected with the indicated combination of HA-c-Jun, Myc-Smad3 (top panel), and Tax expression vectors (bottom panel) and were subjected to immunoprecipitation by using an anti-Myc antibody. The expression of Tax or Smad3 was detected by immunoblot by using an anti-Tax or an anti-Myc antibody before immunoprecipitation. (C) Nuclear extracts, from TGF-β1–stimulated (+) or not (−) HepG2 cells transfected with the indicated expression vectors (empty vector [control]), Tax alone (Tax), or associated with an antisense c-Jun (Tax/AS c-Jun) or a dominant-negative JNK (Tax/JNK-(K-R)) expression vectors were used with the 32P-labeled probe containing 3 CAGA boxes for EMSA.
Thus, these results demonstrate that Tax exerts its inhibitory effect on TGF-β1 signal transduction by activating the JNK/c-Jun pathway, resulting in impairment of Smad3 DNA binding activity by the direct interaction between Smad3 and c-Jun.
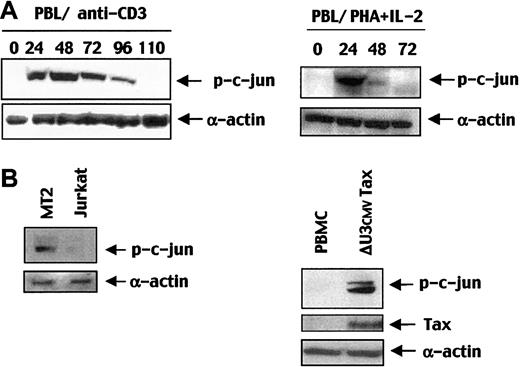
JNK activation is transient in stimulated normal T cells, whereas it is constitutive in Tax-expressing T cells
To assess the pathophysiologic relevance of these results, we studied the ability of Tax to induce JNK activity in T cells. We first investigated the kinetics of JNK activation in normal T cells stimulated through the CD3/TCR complex or with PHA/IL-2. In immunoblot, using a p-c-Jun antibody we found that JNK activity was transiently induced and decreased 72 (PHA/IL-2) to 96 (anti-CD3) hours after stimulation, depending on the type of stimulation (Figure8A). As shown in Figure 8B, high levels of p-c-Jun were detected in the Tax-expressing HTLV-I–transformed cell line MT2 and in ΔU3CMVTax-transduced T cells compared with Tax-negative Jurkat T cells and untransduced normal PBMCs. In contrast to normal T cells, JNK activity was constitutively induced in ATL cell line and in Tax-expressing T cells. Therefore, these results indicate that Tax induces constitutive c-Jun activity and thereby permanently inhibits TGF-β1 signaling in T cells and in HTLV-1–transformed T-cell lines.
Tax induces constitutive JNK activation and p-c-Jun up-regulation in peripheral T cells and in HTLV-1–transformed cell line MT2.
(A) Peripheral T cells were stimulated with anti-CD3 (100 ng/mL), and PBMCs were stimulated with PHA/IL-2 and were harvested at the indicated times. Lysates were subjected to immunoblot probed with an anti–p-c-Jun antibody. (B) p-c-Jun and Tax expression were detected by an anti–p-c-Jun and anti-Tax antibodies using immunoblot analysis with total lysates of Jurkat or MT2 cell lines, ΔU3CMVTax-transduced or -untransduced PBMCs.
Tax induces constitutive JNK activation and p-c-Jun up-regulation in peripheral T cells and in HTLV-1–transformed cell line MT2.
(A) Peripheral T cells were stimulated with anti-CD3 (100 ng/mL), and PBMCs were stimulated with PHA/IL-2 and were harvested at the indicated times. Lysates were subjected to immunoblot probed with an anti–p-c-Jun antibody. (B) p-c-Jun and Tax expression were detected by an anti–p-c-Jun and anti-Tax antibodies using immunoblot analysis with total lysates of Jurkat or MT2 cell lines, ΔU3CMVTax-transduced or -untransduced PBMCs.
Discussion
TGF-β1 is a family of pleiotropic cytokines that regulate the survival, proliferation, and differentiation fate of various cell types.35 In most epithelial, endothelial, and hematopoietic cells, including T lymphocytes, TGF-β1 is a potent inhibitor of cell proliferation; hence, TGF-β1 may suppress tumor progression in early steps of tumorigenesis. Tumor cells, however, generally evolve various mechanisms to escape TGF-β1 inhibitory signals for tumor progression, and it has been estimated that most tumor cells have mutations disabling a component of the TGF-β1 signaling pathway. Some of these mutations may occur in the TGF-β1 receptors, as in the case of the progression of cutaneous T-cell lymphoma.36-38 Downstream TGF-β-RI and TGF-β-RII, Smad2, or Smad4 mutations frequently occur in pancreatic and metastatic colon cancers.35,39 Although Smad3 mutations have not yet been described in human cancer, Smad3 transcriptional activity suppression by an oncogenic process may also contribute to cell transformation. In this respect, it has been demonstrated that Ras overactivity impairs Smad3 translocation to the nucleus.26Furthermore, some oncogenes may directly interact with and block Smad3 activity as exemplified by the Evi-1 oncogene in myelogenous leukemia.40
We have shown that in ATL cells, HTLV-I oncoprotein Tax abrogates TGF-β1 signaling by interfering with Smad3 within the nucleus. In the process of understanding the mechanisms of Tax inhibition, we have systematically investigated which pathway activated by Tax might be responsible for this resistance.
The ability of some viral proteins, such as adenovirus E1A, to transform cells is closely associated with their ability to interact with CBP/p300.41 E1A has been shown to block TGF-β1 responses through its interactions with p300, thereby preventing Smads transcriptional activity.42 Tax interaction with coactivators CBP/p300 or p/CAF also contribute to its oncogenic activity.4,5 However, we show here that in HepG2 hepatic cells as well as in the Jurkat T-cell line, despite the fact that these cells exhibited various levels of CBP/p300 (data not shown), Tax mutants defective for CBP/p300 or p/CAF recruitment also blocks the TGF-β1 response. Furthermore, this inhibition was not recovered by overexpression of the coactivators CBP/p300. Similar results were found with either a synthetic Smad-specific or the natural TGF-β1–responsive promoter of the cell cycle inhibitor p15 (data not shown). Thus, our present results show that the sequestering ability of CBP/p300 by Tax is unlikely to be the main mechanism of the Tax inhibitory effect on the Smad pathway. These results are in contrast to those recently published by Mori et al.43 In that paper, coexpression of CBP/p300 allowed Tax-induced recovery of Smad3-mediated transcriptional activity. Furthermore, the Tax mutant K88A that does not bind p300 failed to repress Smad3-mediated transactivation. These conflicting results may be explained by differences in experimental procedures. Indeed, Mori et al43 have performed their experiments with the K88A Tax mutant or their cotransfections with CBP/p300 by directly cotransfecting Smad3 rather than using TGF-β1 as an activator of the Smad pathway. In their experiments, the amount of transfected Smad3 was not assessed and because Smad3 can dose dependently reverse the inhibitory effect of Tax (as shown by Mori et al43 as well as by us), reduced amount of transfected Smad3, or activation of endogenous Smad3 by TGF-β1, may have resulted in similar findings than ours.
The activation of NF-κB by Tax could have also explained our findings because activation of NF-κB may result in the induction of the Smad2/3 antagonist Smad7.44 In our experience, however, Tax mutants defective for NF-κB activation were still able to block TGF-β1 transduction.
In fact, the Tax repressor effect is mediated by JNK activation and c-Jun phosphorylation. It has been demonstrated previously that in ATL cells, AP-1 activity is elevated but did not always correlate with Tax expression.18,45 However, more recently in the Jurkat T-cell line, Tax was shown to induce JNK activity and c-Jun activation.10,11 Similarly, we show here that increased phosphorylated c-Jun levels are detected in Tax-expressing cells, including normal transduced T cells. Tax activation of JNK and sustained activation of c-Jun in the context of T cells and HTLV-I infection may play a role in viral transformation and pathogenesis and may explain the activated T-cell phenotype observed in ATL. This mechanism of viral transformation seems to be a common feature of viral oncogenesis. The JNK pathway has been shown to be activated by the E1B/19K protein of adenovirus, the Tat protein of HIV, the LMP1 protein of Epstein-Barr virus (EBV), the angiogenic G protein receptor of the Kaposi sarcoma virus, and more recently by the HbX protein of hepatitis B virus (HBV).46 An antiapoptotic role of this enhanced JNK activity has been suggested.47 Our findings, however, may extend the role of JNK activation as an inhibitor of TGF-β1 signaling, allowing host T cells infected with various oncogenic viruses to escape the negative growth regulation of TGF-β1. In the context of HTLV-1 lymphomagenesis, however, the mechanism of JNK activation by Tax remains to be elucidated. Tax could act either directly on the signal transduction pathway or by inducing synthesis of a secreted factor that may induce by an autocrine loop JNK/c-jun activity.
In ATL cells, induction of JNK activity and subsequent activation/phosphorylation of the nuclear factor c-Jun disrupt DNA binding of the Smad3 complexes. Several studies have suggested that the activation of the SAPK/JNK pathway may repress Smad signaling.48-50 The mechanism of DNA binding repression is likely to be explained by a squelching effect by c-Jun on Smad3, resulting in Smad3 recruitment inhibition to specific DNA binding sites. In our model, this mechanism explains the reversion of Tax inhibitory effect by Smad3 overexpression.
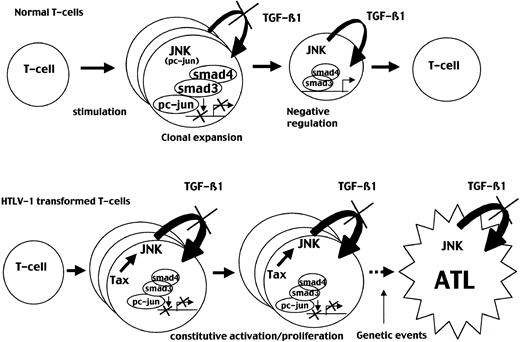
Our findings may be relevant to the understanding of physiologic immune homeostasis as well as ATL leukemogenesis and can be summarized as follows and as shown in Figure 9. During the immune response, TGF-β1 plays a critical role as a negative regulator of T-cell proliferation.28 Stimulated T cells exhibit increased TGF-β1 receptor expression while progressively producing TGF-β1.51 In this context, the JNK/AP-1 pathway plays a major role in T-cell activation and proliferation as recently illustrated in JNK knockout mice.52-54 The balance between the Smad and the JNK pathways may explain physiologically how stimulated T cells are allowed to proliferate at the beginning of stimulation, while producing TGF-β1, and then are negatively autoregulated when p-c-Jun level decreases, thereby limiting T-lymphocyte clonal expansion. Our data on the kinetics of the TGF-β1 effects on T-cell proliferation after stimulation as well as mice deficient for Smad3 support this hypothesis.
JNK activation is transient in normal T cells but constitutive in ATL cells leading to permanent TGF-β1 resistance.
(Top) Stimulation of normal peripheral T cells induces JNK activity, leading to TGF-β1 production and preventing TGF-β1 inhibition through the induction of a Smad3/c-Jun complexes. This period may allow clonal expansion and triggering of the immune response. In normal T cells, JNK activation is transient and decreases after 72 to 96 hours, allowing TGF-β1 antiproliferative effect and restoration of a resting state. (Bottom) In contrast, in HTLV-1–transformed cells, Tax induces constitutive JNK activity that may lead to a continuous TGF-β1 resistance, allowing clonal expansion and a constitutively activated state observed in patients infected with HTLV-1. Subsequent oncogenic events associated with TGF-β1 resistance may result in ATL development.
JNK activation is transient in normal T cells but constitutive in ATL cells leading to permanent TGF-β1 resistance.
(Top) Stimulation of normal peripheral T cells induces JNK activity, leading to TGF-β1 production and preventing TGF-β1 inhibition through the induction of a Smad3/c-Jun complexes. This period may allow clonal expansion and triggering of the immune response. In normal T cells, JNK activation is transient and decreases after 72 to 96 hours, allowing TGF-β1 antiproliferative effect and restoration of a resting state. (Bottom) In contrast, in HTLV-1–transformed cells, Tax induces constitutive JNK activity that may lead to a continuous TGF-β1 resistance, allowing clonal expansion and a constitutively activated state observed in patients infected with HTLV-1. Subsequent oncogenic events associated with TGF-β1 resistance may result in ATL development.
Molecular mechanisms leading to the development of ATL in patients infected with HTLV-I remain enigmatic. Particularly unclear is the latency period from 20 to 30 years, which is thought to be necessary to accumulate secondary mutations leading to the development of ATL.3 In the natural history of the disease, early stages of HTLV-I infection are associated with a high replication state and with a high level of expression of viral proteins, including Tax. This viral replication is associated with clonal expansion of mature peripheral blood T cells. In ATL patients this period is crucial for the development of an antitumoral immune response. At this step, Tax may induce high levels of TGF-β1 production and may mediate the repression of TGF-β1 signaling that may help future tumor cells to escape from negative regulation of proliferation and also from cytotoxic T cells. This high proliferation state, in addition to the inhibitory effect of Tax on DNA repair, may result in the development of ATL. In later stages of HTLV-I infection, Tax is rarely detected in fresh peripheral ATL cells. A possible explanation could be that immortalized T cells, by accumulating genomic mutations, no longer require Tax expression and are selected during the development of ATL. In agreement with this hypothesis it has been demonstrated that in some ATL cells the JNK/c-jun pathway might be activated independently of Tax.45
Our findings have several clinical and therapeutic applications. Despite advances in therapeutic drugs consisting of a combination of antiretroviral and interferon α (IFNα), the cases of cure are rare, and ATL prognosis remains poor with an overall median survival of 6 months.55 56 Thus, new therapeutic approaches are needed. In this regard, it could be interesting to develop new drugs that allow the restoration of TGF-β1 responsiveness by blocking the JNK pathway.
In conclusion, in this report we have demonstrated a new function of Tax in T-cell transformation as an inhibitor of TGF-β1 signaling. The repression of Smad3 activity by the JNK/AP-1 pathway represents a new role for viral oncoproteins and further extends the targeting of Smad3 in oncogenesis by inhibition of nuclear translocation, squelching of CBP/p300 or by direct interaction.26,40 42
We are indebted to M. Kracht for providing the dominant-negative form of JNK, JNK(K-R); we thank J. M. Gauthier for providing the CAGA12-luc reporter construct and C. H. Heldin and R. Derynck for the Smad3 construct.
Prepublished online as Blood First Edition Paper, July 25, 2002; DOI 10.1182/blood-2001-12-0372.
Supported by grants from Fondation de France contre la leucémie, Association de Recherche contre le Cancer (ARC), and Ligue Nationale contre le Cancer. B.A. is a recipient of Poste d'accueil Centre National de Recherche Scientifique/Assistance Publique-Hopitaux de Paris (CNRS/AP-HP) grant.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Olivier Hermine, CNRS UMR 8603, Hopital Necker, Batiment Sèvres porte 584, 149-161 rue de Sèvres, 75743 Paris cedex 15, France; e-mail:hermine@necker.fr.

![Fig. 7. Tax-induced c-Jun interacts with Smad3 and abrogates Smad3 DNA binding activity. / (A) Nuclear extracts from TGF-β1–stimulated HepG2 cells transfected with the Tax expression vector, empty vector (control), or increasing doses of c-Jun expression vector (c-Jun 0.5, 4) were used with the32P-labeled probe containing 3 CAGA boxes for EMSA. (B) Cos-7 cells were transfected with the indicated combination of HA-c-Jun, Myc-Smad3 (top panel), and Tax expression vectors (bottom panel) and were subjected to immunoprecipitation by using an anti-Myc antibody. The expression of Tax or Smad3 was detected by immunoblot by using an anti-Tax or an anti-Myc antibody before immunoprecipitation. (C) Nuclear extracts, from TGF-β1–stimulated (+) or not (−) HepG2 cells transfected with the indicated expression vectors (empty vector [control]), Tax alone (Tax), or associated with an antisense c-Jun (Tax/AS c-Jun) or a dominant-negative JNK (Tax/JNK-(K-R)) expression vectors were used with the 32P-labeled probe containing 3 CAGA boxes for EMSA.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/12/10.1182_blood-2001-12-0372/5/m_h82323486007.jpeg?Expires=1769101035&Signature=PEFmpGNQkfPn0CdpD0ewHrjSnBEIF2yO8Cp51yAPbX7GOEVQCRcWbQqbVz37vhbTFQR4xvQvWDjEYNMcJxA1dWtqMMVJMf81zG5ABqGwOFrRgoQkZ~0YUFrjeAkUIGH~wWjPUSZDWZZzphF1T~N3nxVrmFDnl8wkWvi64n4gjF86b6U6nOtDWxF1yA6Ls86gCFLTWSiL-rZK3InoknMeIJR0UAsZsq4viS4Gr8X4vL8HSkpM3C-DJFJFqqwOM07TbFZu7eRAJpmh6xCzg4EjCRGsqGv3IceXCTmYFgUMfRQDamd2aegkjGvYAS3pySPM8KoFa51vQdtNuPp6s~fnVA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal