Leptin promotes the growth and viability of hematopoietic cells, and it also stimulates microvessel formation, indicating a role for leptin in angiogenesis. Acute myelocytic leukemia (AML) remains a disease with poor prognosis. Similar to solid tumors, it probably requires angiogenesis to ensure adequate supplies of nutrients. We studied rats with transplanted AML to test if a neutralizing anti–leptin receptor monoclonal antibody (mAb) (anti–OB-R) could inhibit leukemogenesis. At 4 weeks after transplantation, the bone marrow contained about 80% leukemic cells as assayed with a specific mAb and flow cytometry. Microscopic examination of bone marrow sections stained with an anti–von Willebrand mAb revealed a marked increase in microvessel density in the leukemic rats compared with controls. Treatment with anti–OB-R for 3 weeks more than halved the content of bone marrow leukemic cells with a concomitant, substantial decrease in angiogenesis. A parallel experiment using an irrelevant anticasein mAb showed no effect on either leukemic cell growth or angiogenesis. We could not detect surface expression of the leptin receptor on the leukemic cells, but on mononuclear cells from healthy rats. The anti–OB-R did not affect in vitro proliferation of leukemic cells whereas proliferation of the mononuclear cells was markedly impaired. The anti–OB-R had no effect on either leukemic cell growth or angiogenesis in leukemic fa/fa rats with a mutated leptin receptor. We conclude that leptin stimulates leukemic cell growth in vivo by promoting angiogenesis. Inhibition of binding of leptin to its receptor might be a new adjunct therapy in AML.
Introduction
Leptin was originally identified as a key hormone in the regulation of energy expenditure by reducing food intake and controlling adipose tissue metabolism.1,2 Subsequent studies revealed that leptin also can modulate hematopoiesis by stimulating proliferation and inhibiting apoptosis of leukocyte subgroups.3-5 Moreover, leptin does not only promote normal hematopoiesis, but it also stimulates the growth and viability of leukemic cells, suggesting a role for leptin in the pathogenesis of hematologic malignancies.6-8
Several studies have shown that the growth of both a primary tumor and its metastasis to other tissues depend on angiogenesis.9-11 More recent data have indicated that the development and progression of nonsolid tumors such as leukemias also may depend on generation of new blood vessels from the pre-existing vasculature.12-15 Inhibition of angiogenesis reportedly retarded leukemic growth in mice.16,17 Among its pleiotropic actions, leptin may function as an angiogenic factor in various in vitro systems as well as in rodent models of angiogenesis.18-21 Inhibition of the angiogenic process in hematopoietic tissues by targeting leptin activity might therefore represent a novel therapeutic option in leukemia.
Human acute myelocytic leukemia in the adult has a poor prognosis with a reported 5-year-survival rate usually less than 50%.22,23 Cure can only be achieved with either intensive cytostatic regimens or bone marrow transplantation, but both treatment modalities are associated with substantial morbidity and mortality. The Brown Norway (BN) leukemic rat (BNML) is an established model of human acute promyelocytic leukemia regarding growth and dissemination of the leukemic cells, their response to various therapeutic regimens, and clinical outcome.24-26 Untreated BNML rats die after approximately 25 to 28 days. We have gained considerable experience with the BNML rats, and used it to study the bone marrow microenvironment during development and progression of acute myelocytic leukemia.27-29 In the present study we used this model to examine the effect on leukemogenesis of selectively inhibiting leptin binding to its cognate receptor with a specific monoclonal antibody (mAb), anti–leptin receptor (anti–OB-R).
Materials and methods
Animals and induction of leukemia
Rats were purchased from Møllegaard (Copenhagen, Denmark). Leukemia was induced in the rats by injection of splenic-derived leukemic cells (20 million intraperitoneally). This leukemic cell line was originally established by injection of the carcinogenic compound 9,10-dimethyl-1,2-benzanthracene into BN rats.26 In a separate set of experiments we injected this compound into adult Zucker fatty (fa/fa) rats to induce leukemia. These rats harbor a missense mutation in the leptin receptor gene, thus encoding a receptor deficient in generating intracellular signaling upon leptin binding.30 Approximately 4 weeks after injection of the carcinogen into the fa/fa rats, they were killed with an overdose of barbiturate (administered intraperitoneally) before the spleen was removed and a suspension of cells was prepared. These cells were then reinjected into other fa/fa rats, and this cycle was repeated for 4 generations of rats. The last harvest of splenic-derived leukemic cells was used to establish leukemicfa/fa rats for the present study. The local ethics committees approved the protocol.
Identification of leukemic cells
To determine the fraction of leukemic cells within the rat femoral bone marrow, we collected the marrow content and stained it with the mAb RM124 as previously described.31,32This mAb specifically recognizes the leukemic cells even at very low frequencies, such as those occurring in minimal residual disease.33 The mAb RM124 was linked to a secondary fluorescent antibody before the samples (10 000 cells) were analyzed with flow cytometry (FACScan; Becton Dickinson, Mountain View, CA). In separate experiments we found that mAb RM124 specifically also bound to the leukemic cells derived from the fa/fa rats (data not shown).
Treatment protocol
Overt leukemia had developed both in the BN and in thefa/fa rats 4 weeks after injection of leukemic cells. At that time some animals were put to death with an overdose of barbiturate (administered intraperitoneally), whereas others were slightly sedated with barbiturate (25 mg/kg intraperitoneally). The rats were randomized to one of 3 treatments: (1) a neutralizing anti–rat leptin receptor mAb (anti–OB-R; 0.5 μg in 0.5 mL saline; Research Diagnostics, Flanders, NJ); (2) an irrelevant anti–human casein mAb (0.5 μg in 0.5 mL saline; Biogenesis, Poole, United Kingdom); or (3) saline (0.5 mL). They were treated with subcutaneous injections every other day for the next 3 weeks, and killed with an overdose of barbiturate (administered intraperitoneally).
In vitro growth of bone marrow progenitor cells
To test the proliferative capacity of progenitor cells we separated mononuclear cells from crude bone marrow cell suspensions by erythrocyte sedimentation followed by density gradient centrifugation (Isopaque-Ficoll; Nycomed, Oslo, Norway). Leukemic cells from the leukemic BN rats and the leukemic fa/fa rats were isolated using the mAb RM124 and flow cytometry. Then, the leukemic and normal mononuclear cells were grown in a humidified atmosphere (37°C, 5% CO2) in liquid culture with supplemented (antibiotics and glutamine) RPMI 1640 medium and added tritiated thymidine (3.7 × 104 Bq/mL [1 μCi/mL]). To the cultures (50 000 cells per mL) we added either anti–OB-R (10 μg/mL), leptin (10 ng/mL; Research Diagnostics), or cyclophosphamide (30 μg/mL; Sendoxan; Astra Medica, Frankfurt, Germany). After 24 hours the cells were harvested and thoroughly washed 3 times before incorporation of the tritiated thymidine was measured with a β-counter.
Counting of microvessel density
The density of microvessels in femoral bone marrow was used as a measure of angiogenesis, and was manually counted using light microscopy and sections stained with a mAb raised against von Willebrand factor (VWF), a constituent of the endothelial lining of the bone marrow microvessels, known to be a reliable marker of rat endothelial cells.34 In some experiments we also included measurements of microvessel density based on staining of bone marrow sections with a mAb raised against platelet-endothelial cell adhesion molecule (PECAM).
Determination of plasma concentration of leptin and vascular endothelial growth factor
The plasma concentration of leptin was measured by a competitive radioimmunoassay (Linco Research, St Charles, MO) with recombinant125I-leptin as a tracer.35 Intra-assay coefficient of variation was 1.1%. We used an enzyme-linked immunosorbent assay (ELISA) kit (detection limit > 20 pg/mL; R&D Systems, Minneapolis, MN) to determine the plasma concentration of vascular endothelial growth factor (VEGF).
Detection of antibodies against anti–OB-R
We examined if the animals produced antibodies against the injected antibodies using a dot-plot method. The neutralizing anti–OB-R as well as the irrelevant anti–human casein mAb were diluted 5-fold from the injected concentration of 1 μg/mL to 0.16 ng/mL in tris-buffered saline (TBS). Rat plasma (5 μL), as positive control, and each dilution of antibody were applied on nitrocellulose blotting membrane. Excess binding capacity of nitrocellulose membranes was blocked by incubation in TBS containing 0.02% Tween 20 for 30 minutes at room temperature before the test solutions were added. The membrane was incubated for 2 hours at room temperature in plasma samples diluted 1:10 in TBS. The membranes were washed 3 times in TBS containing 0.002% Tween prior to another 1-hour incubation with a 1:4000 dilution of horseradish peroxidase–labeled goat anti–rat immunoglobulin (Southern Biotechnology, Birmingham, AL). Moreover, the membranes were washed 3 times in TBS containing 0.002% Tween, then twice in TBS buffer, and finally in distilled water prior to visualization.36
Statistics
The measurements were performed in triplicate and the corresponding median values were used to calculate the means and SEM for each experimental group of 8 to 10 animals. Differences were evaluated with Wilcoxon rank sum test or Kruskal-Wallis test with the Bonferroni correction when appropriate. 2-tailed tests were used.P < .05 was considered statistically significant.
Results
Inhibition of leptin binding impairs growth of bone marrow leukemic cells by reducing angiogenesis
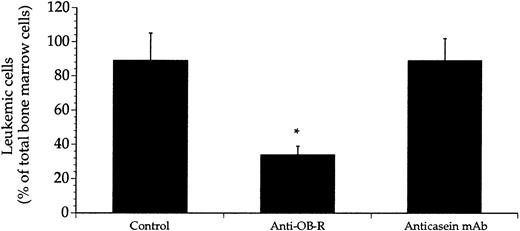
More than 80% of the nucleated cells within the femoral bone marrow were of leukemic origin 4 weeks after the BN rats received transplants (Figure 1). This leukemic cell content was markedly reduced in rats receiving injections of anti–OB-R on alternate days for another 3 weeks. Treatment of the leukemic rats with the irrelevant anticasein mAb had no effect on leukemic growth indicating the specificity of targeting the leptin receptor. Concomitant with the decreased leukemic cell mass was the profound decrease in the density of microvessels within the bone marrow of rats treated with anti–OB-R, but not in rats treated with the anticasein mAb (Figure 2A-B). The data obtained with the anti-VWF mAb correlated closely with data obtained from bone marrow sections stained with the anti-PECAM mAb (Figure 2B). On average the leukemic rats treated with anti–OB-R survived 4.9 ± 1.1 days (P < .05, n = 8) longer than the untreated leukemic rats. The percentages of leukemic cells were reduced to 37 ± 6 and 36 ± 7 (n = 5) upon increasing the dose of anti–OB-R to 0.75 μg and 1.0 μg, respectively. The corresponding reductions in microvessel densities were to 387 ± 21 and 392 ± 26 vessels per 1.5 mm2, that is, not significantly different from the results obtained with 0.5 μg of anti–OB-R (Figures 1 and 2). Daily injections of anti–OB-R did not further inhibit either leukemic cell growth or angiogenesis (data not shown).
Treatment with anti–OB-R, but not with anticasein mAb, reduced leukemic cell mass in the bone marrow.
Leukemic rats were either injected with saline (control) or with anti–OB-R or the anticasein mAb. Bone marrow leukemic cells were retrieved from the femoral bone post mortem and enumerated using staining with the mAb RM124 and flow cytometry. Data represent the percentage of RM124-positive cells among the total number of nucleated bone marrow cells, and are presented as means + SEM; n = 8 rats; * P < .05.
Treatment with anti–OB-R, but not with anticasein mAb, reduced leukemic cell mass in the bone marrow.
Leukemic rats were either injected with saline (control) or with anti–OB-R or the anticasein mAb. Bone marrow leukemic cells were retrieved from the femoral bone post mortem and enumerated using staining with the mAb RM124 and flow cytometry. Data represent the percentage of RM124-positive cells among the total number of nucleated bone marrow cells, and are presented as means + SEM; n = 8 rats; * P < .05.
Reduced bone marrow angiogenesis in leukemic rats treated with anti–OB-R, but not with anticasein mAb.
(A) Representative fields of histologic cross sections from bone marrow of a healthy (i) and a leukemic (ii) rat. The microvessels were identified with an anti–von Willebrand mAb (red stain). Note the higher microvessel density in leukemic versus normal marrow. Magnification × 500. (B) Pooled data of leukemic rats treated with saline (control) or with anti–OB-R or anticasein mAb. Values are based on staining with an anti-VWF mAb and an anti-PECAM mAb, and presented as means + SEM; n = 8 rats; *P < .05 compared with control.
Reduced bone marrow angiogenesis in leukemic rats treated with anti–OB-R, but not with anticasein mAb.
(A) Representative fields of histologic cross sections from bone marrow of a healthy (i) and a leukemic (ii) rat. The microvessels were identified with an anti–von Willebrand mAb (red stain). Note the higher microvessel density in leukemic versus normal marrow. Magnification × 500. (B) Pooled data of leukemic rats treated with saline (control) or with anti–OB-R or anticasein mAb. Values are based on staining with an anti-VWF mAb and an anti-PECAM mAb, and presented as means + SEM; n = 8 rats; *P < .05 compared with control.
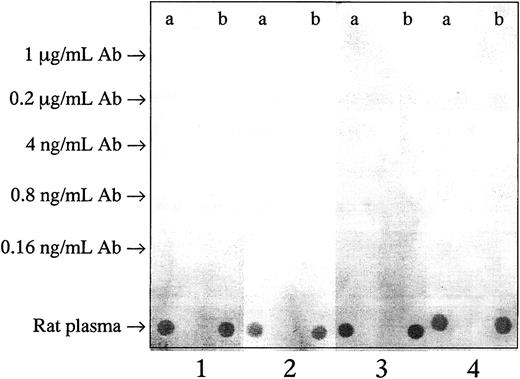
In search of possible antibodies generated against the exogeneously administered anti–OB-R and anticasein mAbs, we tested plasma samples from treated rats. We were unable to detect any such antibodies (Figure3), indicating that neutralizing antibodies against the treatment molecules had apparently not been made in the rats.
No immune reaction against the injected antibodies was observed.
Anti-OB-R (a) and irrelevant (anti–human casein) mAbs (b) were diluted 5-fold from the treatment concentration of 1 μg/mL, and 5 μL of the different dilutions were applied on nitrocellulose membranes. Rat plasma was used as positive control. The membranes were incubated with pooled plasma from 3 BN rats given the irrelevant mAb (1), or from 3 BNML rats given anti–OB-R (2), or from 3 leukemic fa/farats given anti-Ob-R (3), or buffer as negative control (4).
No immune reaction against the injected antibodies was observed.
Anti-OB-R (a) and irrelevant (anti–human casein) mAbs (b) were diluted 5-fold from the treatment concentration of 1 μg/mL, and 5 μL of the different dilutions were applied on nitrocellulose membranes. Rat plasma was used as positive control. The membranes were incubated with pooled plasma from 3 BN rats given the irrelevant mAb (1), or from 3 BNML rats given anti–OB-R (2), or from 3 leukemic fa/farats given anti-Ob-R (3), or buffer as negative control (4).
In the healthy BN rats the plasma concentration of leptin was 12.4 ± 2.1 ng/mL (n = 8) and the body weight was 371 ± 29 g. No significant effects on either the plasma concentrations of leptin (10.6 ± 1.6 ng/mL and 13.7 ± 2.6 ng/mL) or the body weights (367 ± 32 g and 359 ± 29 g) were detected in leukemic rats with (n = 9) or without injection of anti–OB-R (n = 10), respectively.
The plasma concentrations of VEGF in untreated and treated healthy BN rats were 37 ± 8 pg/mL and 39 ± 11 pg /mL (n = 8;P > .05), respectively. Similarly, there were no differences (P > .05) in the VEGF levels in untreated and treated leukemic rats: 35 ± 9 pg/mL and 39 ± 7 pg/mL (n = 8).
Anti-OB-R does not affect in vitro proliferation of the leukemic cells
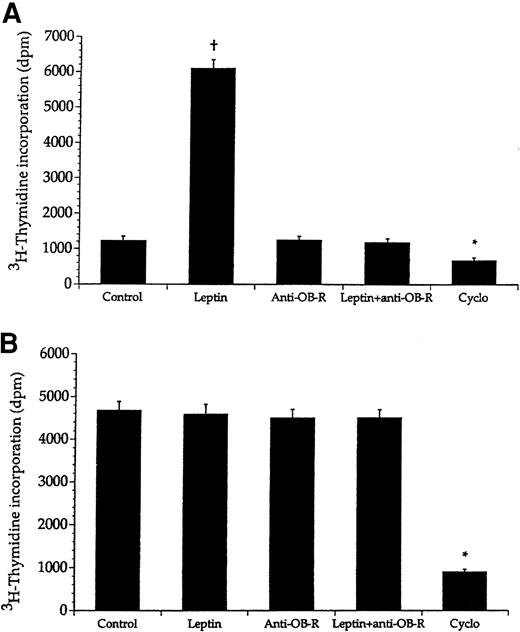
To investigate whether anti–OB-R exerted its antileukemic effects by direct interaction with the leukemic cells, we studied proliferation in vitro of these cells and of mononuclear cells sampled from healthy BN rats. Leptin (10 ng/mL) markedly stimulated proliferation of the mononuclear cells whereas the leukemic cells did not respond (Figure4). Moreover, addition of anti–OB-R (10 μg/mL) to mononuclear cells prestimulated with leptin (10 ng/mL) completely abolished leptin-induced proliferation. Furthermore, addition of only anti–OB-R (10 μg/mL) apparently did not affect growth of either the leukemic cells or mononuclear cells. A 10-fold increase of anti–OB-R concentration also had no effect (data not shown). In contrast, the proliferative capacity of both cell types was reduced upon addition of the cytostatic drug cyclophosphamide as an assay control.
Leptin promotes in vitro growth of bone marrow cells from healthy BN rats, but not from leukemic rats.
Bone marrow cells from healthy BN rats (A) and leukemic rats (B) were given either leptin (10 ng/mL), anti–OB-R (10 μg/mL), a combination of leptin (10 ng/mL) and anti–OB-R (10 μg/mL), or cyclophosphamide (Cyclo; 30 μg/mL). Values represent means + SEM; n = 8. The asterisk denotes significantly lower values compared with the control cells (no compound added), whereas the plus sign denotes a significantly higher value.
Leptin promotes in vitro growth of bone marrow cells from healthy BN rats, but not from leukemic rats.
Bone marrow cells from healthy BN rats (A) and leukemic rats (B) were given either leptin (10 ng/mL), anti–OB-R (10 μg/mL), a combination of leptin (10 ng/mL) and anti–OB-R (10 μg/mL), or cyclophosphamide (Cyclo; 30 μg/mL). Values represent means + SEM; n = 8. The asterisk denotes significantly lower values compared with the control cells (no compound added), whereas the plus sign denotes a significantly higher value.
Binding of anti–OB-R to the leukemic cells was assayed using flow cytometry. This antibody bound to a high proportion of the mononuclear cells from healthy BN rats whereas no such binding was detectable on the leukemic cells (Figure 5).
Anti-OB-R binds to normal mononuclear cells, but not to leukemic cells.
Bone marrow cells from healthy BN rats (A) and leukemic rats (B) were incubated with anti–OB-R linked to a secondary fluorescent antibody before analysis with flow cytometry. An irrelevant antibody was used as a negative control. Data are from one experiment and representative of 7 other experiments.
Anti-OB-R binds to normal mononuclear cells, but not to leukemic cells.
Bone marrow cells from healthy BN rats (A) and leukemic rats (B) were incubated with anti–OB-R linked to a secondary fluorescent antibody before analysis with flow cytometry. An irrelevant antibody was used as a negative control. Data are from one experiment and representative of 7 other experiments.
No effect of inhibiting leptin binding in leukemic fa/farats
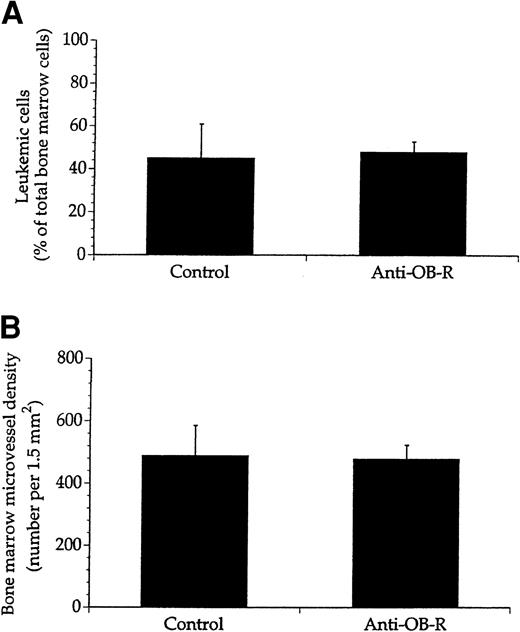
To further explore whether anti–OB-R exerted its antileukemic effects by interfering with the leukemic cells, we studied leukemicfa/fa rats expressing a mutated leptin receptor rendering it nonfunctional. The leukemic cells homed to the bone marrow in thefa/fa rats, and 4 weeks after transplantation they accounted for almost 50% of the total number of nucleated cells in the recipient bone marrows (Figure 6A). Treatment with anti–OB-R did not affect either the leukemic cell mass or the microvessel density (Figure 6A-B). Moreover, the in vitro growth patterns of mononuclear cells from healthy fa/fa rats as well as leukemic cells sampled from fa/fa rats, were unaltered upon treatment with leptin (10 ng/mL) as well as anti–OB-R (10 μg/mL), whereas cyclophosphamide inhibited proliferation of both cell types (Figure 7).
No effect of anti–OB-R on leukemic cell growth or angiogenesis in bone marrow of rats with deficient leptin receptors.
Leukemic fa/fa rats were either not injected (control) or injected with anti–OB-R. Nucleated cells and histologic sections from bone marrows of these rats were then examined for the number of leukemic cells (A) and microvessel density (B). Values represent means + SEM; n = 8 rats.
No effect of anti–OB-R on leukemic cell growth or angiogenesis in bone marrow of rats with deficient leptin receptors.
Leukemic fa/fa rats were either not injected (control) or injected with anti–OB-R. Nucleated cells and histologic sections from bone marrows of these rats were then examined for the number of leukemic cells (A) and microvessel density (B). Values represent means + SEM; n = 8 rats.
No effect of leptin on in vitro growth of bone marrow cells from healthy and leukemic fa/fa rats.
Bone marrow cells from healthy fa/fa rats (A) and leukemicfa/fa rats (B) were injected with either leptin (10 ng/mL), anti–OB-R (10 μg/mL), or cyclophosphamide (Cyclo; 30 μg/mL). The asterisk denotes significantly lower values compared with control cells (no compound added). Values represent means + SEM; n = 8.
No effect of leptin on in vitro growth of bone marrow cells from healthy and leukemic fa/fa rats.
Bone marrow cells from healthy fa/fa rats (A) and leukemicfa/fa rats (B) were injected with either leptin (10 ng/mL), anti–OB-R (10 μg/mL), or cyclophosphamide (Cyclo; 30 μg/mL). The asterisk denotes significantly lower values compared with control cells (no compound added). Values represent means + SEM; n = 8.
Discussion
Several independent studies have demonstrated increased microvessel density within the bone marrow of patients with acute leukemia.12,13,15 Whether this process of neovascularization significantly modulates development and progression of acute leukemia, or if antiangiogenic compounds can retard or inhibit leukemogenesis, has not been clarified. Recent evidence indicates that the lipostatic hormone leptin can induce formation of new microvessels in addition to its growth-promoting effects on normal and malignant hematopoietic cells.18-20 Targeting leptin signaling might therefore be a potential therapeutic strategy in acute leukemia.
In the present study we have shown that a neutralizing mAb (anti–OB-R) competing with leptin binding to its receptor substantially reduced the leukemic cell mass in a rat model mimicking human acute promyelocytic leukemia. This antileukemic effect coincided with a marked decrease in microvessel density within the bone marrow of the leukemic rats. In contrast, treatment of the leukemic rats with an irrelevant anticasein mAb did not affect the leukemic process or angiogenesis within the bone marrow microenvironment. Previously it has been shown that the central action of leptin on food intake can be prevented by blocking its binding to the leptin receptor by injections of specific anti–leptin receptor antibodies in rats, indicating that specific inhibition of leptin signaling may hamper leptin-induced biologic effects.37
The selectivity of this antileptin treatment was underscored by (1) the lack of effect of an irrelevant mAb (anti–human casein), and (2) the inability of anti–OB-R to inhibit leukemic cell growth and angiogenesis in the leukemic fa/fa rats. These rats with a deficient leptin receptor exhibit clinical features and leukemic cell kinetics similar to those reported for the BNML rats.24 25Furthermore, anti–OB-R most likely exerted its effect on the angiogenic process rather than on the leukemic cells per se because the leptin receptor was not detectable on these cells in contrast to normal mononuclear cells. Moreover, neither leptin itself nor anti–OB-R affected growth of the leukemic cells in vitro, whereas the mononuclear cells responded. The present study was not designed to examine whether anti–OB-R also could retard dissemination of the leukemia, but treated leukemic rats survived longer compared with untreated ones, suggesting an inhibitory effect also on extramedullary leukemic deposits.
An increase in dose or frequency of antibody injections did not further inhibit leukemic cell growth or angiogenesis. Our focus has been on observing effects, and we did not study lower dosages or dose-dependent effects of antibodies or data on half-life of circulating antibodies. Thus, we cannot detail any saturation of the binding of antibodies to the leptin receptor or a steady-state competition with leptin on binding to its receptor.
Theoretically, injected antibodies might induce an immune response generating antibodies against anti–OB-R or anti–human casein mAb, but we could not detect such a neutralizing activity using dot plots. In addition, our treatment protocol does not favor such responses, because we used saline as solvent and a short period (3 weeks) of treatment.
The association between leptin and leukemia is unclear. Total leukocyte counts in healthy humans are positively correlated with both body fat and leptin.38 Moreover, increasing body mass index reportedly correlated with the diagnosis of acute promyelocytic leukemia.39 Furthermore, leukemic blast cells can express a functional leptin receptor.6-8 In our study we failed to detect differences in the plasma concentrations of leptin among healthy and leukemic rats treated with anti–OB-R and rats without treatment. Blocking leptin from binding to its receptor did not induce detectable feedback regulation of leptin secretion, or in any other way induce alteration in adipose tissue causing changes in circulating levels of leptin. A negative feedback response on leptin expression is centrally regulated in rats.40 A positive feedback response on leptin expression due to decreased levels of circulating leptin, or blocking of leptin binding to its receptor, has apparently not been previously detected.
The mechanisms governing microvessel formation and how the angiogenic process may support the development of leukemia are not well defined. Like normal hematopoietic cells, the function and survival of leukemic cells relies on sufficient oxygen and nutrient supplies as well as efficient removal of waste products, hence their dependence on an adequate blood supply. We have previously shown that a declining perfusion of the bone marrow with anoxia and acidity does not occur until the animals are terminally ill.28,29,41 Whereas decreased local oxygen tension is a powerful enhancer of proangiogenic factors like VEGF, and leptin reportedly synergizes with VEGF in stimulating angiogenesis,19 we could not detect any change in the plasma concentration of VEGF between treated healthy BN rats and leukemic rats. Whether other proangiogenic factors are up-regulated in the leukemic rats remains to be tested.
During leukemia the malignant cell clone competes with normal progenitor cells for available substrates and growth space.42 Increased angiogenesis may support leukemogenesis by creating new niches for leukemic expansion similar to those reported for normal hematopoietic stem cells.43 Alternatively, increased microvessel formation may facilitate the postulated interplay between leukemic cells, stroma cells, and vascular endothelial cells; for example, by increasing the expression of integrins, heparans, and matrixmetalloproteinases necessary for leukemic dissemination.44 45 Regardless of how the angiogenic process is controlled during leukemia, the present study suggests that reducing angiogenesis might hold promise in the treatment of hematologic malignancies.
The mAb RM124 was a kind gift from Jørgen Larsen (Finsen Institute, Copenhagen, Denmark).
Prepublished online as Blood First Edition Paper, July 5, 2002; DOI 10.1182/blood-2001-11-0134.
Supported by the Norwegian Cancer Society, the Freia Chocolade Fabriks Foundation, and the Throne Holst Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Per Ole Iversen, Institute for Nutrition Research, University of Oslo, PO Box 1046 Blindern, 0316 Oslo, Norway; e-mail: poiversen@hotmail.com.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal