Patients with mutations of either RAG-1 or RAG-2 genes suffer from severe combined immunodeficiency (SCID) characterized by the lack of T and B lymphocytes. The only curative treatment today consists of hematopoietic stem cell (HSC) transplantation, which is only partially successful in the absence of an HLA genoidentical donor, thus justifying research to find an alternative therapeutic approach. To this end, RAG-2–deficient mice were used to test whether retrovirally mediated ex vivo gene transfer into HSCs could provide long-term correction of the immunologic deficiency. Murine RAG-2−/−Sca-1+ selected bone marrow cells were transduced with a modified Moloney leukemia virus (MLV)–based MND (myeloproliferative sarcoma virus enhancer, negative control region deleted, dl587rev primer-binding site substituted) retroviral vector containing the RAG-2 cDNA and transplanted into RAG-2−/− sublethally irradiated mice (3Gy). Two months later, T- and B-cell development was achieved in all mice. Diverse repertoire of T cells as well as proliferative capacity in the presence of mitogens, allogeneic cells, and keyhole limpet hemocyanin (KLH) were shown. B-cell function as shown by serum Ig levels and antibody response to a challenge by KLH also developed. Lymphoid subsets and function were shown to be stable over a one-year period without evidence of any detectable toxicity. Noteworthy, a selective advantage for transduced lymphoid cells was evidenced by comparative provirus quantification in lymphoid and myeloid lineages. Altogether, this study demonstrates the efficiency of ex vivo RAG-2 gene transfer in HSCs to correct the immune deficiency of RAG-2−/− mice, constituting a significant step toward clinical application.
Introduction
Severe combined immunodeficiencies (SCID), the most severe form of primary immunodeficiencies, consist of a group of diseases characterized by an early block in T-cell differentiation. At least 9 different forms of human SCID have now been recognized and can be grouped according to inheritance, phenotype, and genes involved1 (ie, γc, ADA, Artemis, ZAP-70, JAK-3, IL7Rα, CD45, RAG-1, and RAG-2). Two main subgroups can be identified, distinguished on the basis of the presence (+) or the absence (−) of B lymphocytes, while other hematopoietic lineages can be variably affected. These disorders are lethal within the first year of life, and the treatment of choice is allogeneic hematopoietic stem cell transplantation (HSCT). However, in the absence of an HLA-identical donor, HSCT provides partially unsatisfying results, with a survival curve ranging from 40% to 80%, depending on the SCID condition and the magnitude of HLA disparity.2,3 Hence, gene therapy could provide a useful alternative to allogeneic HSCT. Expression of the RAG-2 gene in RAG-2−/− hematopoietic progenitor cells could relieve the early block in the T- and B-cell differentiation pathways and confer a selective advantage to gene-corrected cells over uncorrected progenitor cells. The long life span of T cells further justifies this therapeutic approach, endorsed by results obtained in gene therapy of X-linked SCID caused by γc-deficiency.4 5
Approximately 20% of SCID are characterized by a deficiency in both T- and B-cell lineages (T−B−), while natural killer cells are present and functional (NK+). Schwarz et al have demonstrated that in some of these patients, recombination-activating genes RAG-1 or RAG-2 were mutated.6 These observations were later confirmed by Corneo et al7,8 as well as by others.9
The RAG-1 and RAG-2 proteins initiate the somatic recombination process of the gene elements encoding the variable (V), diversity (D), and joining (J) segments that lead to the generation of a diverse repertoire of antigen-specific receptors at the surface of T and B lymphocytes, essential for adaptative immune responses. The RAG-1/2 complex introduces a DNA double-strand break (DSB) in the recombination signal sequences (RSS) composed of conserved heptamer and nonamer sequences separated by either 12 or 23 base pairs (bp) flanking all V, D, and J segments. During the subsequent step, a non–lymphoid-specific process is responsible for the repair of this DSB.10 RAG-1 and RAG-2 gene knock-out murine models exhibit an identical phenotype to that of the human condition, with a severe and early blockade of both T-cell development (at the triple-negative CD3−CD4−CD8−, CD25+stage) and B-cell development (at the B220loCD43+IgM− proB stage).11,12 This mouse model is an extremely useful model for verifying whether a gene transfer therapeutic approach can result in the correction of defects in lymphoid development and B- and T-cell function. Taking into consideration that the blockade conferred by RAG-2 deficiency in the T-cell differentiation pathway occurs later than the one conferred by γc-deficiency, one of the potential concerns was whether selective growth and differentiation advantage of RAG-2–transduced hematopoietic progenitor cells were important enough to restore a functional immunologic system, as RAG-2–transduced precursor cells might compete with endogenous RAG-2−/− precursor cells during early lymphoid differentiation. The second question is the potential toxicity in peripheral lymphocytes of constitutive RAG-2 expression driven by a retroviral long terminal repeat (LTR)–controlled gene expression system. Indeed, RAG proteins can induce chromosome translocations in lymphoid malignancies.13 In consequence, any possible toxicity caused by the constitutive expression of the RAG-2 gene in the lymphohematopoietic system in the absence of constitutive coexpression of RAG-1 has to be excluded. To address these questions, RAG-2 gene transfer experiments were performed. A Moloney leukemia virus (MLV)–based vector, MND (myeloproliferative sarcoma virus enhancer, negative control region deleted, dl587rev primer-binding site substituted), was used to transduce ex vivo RAG-2−/− marrow progenitors14 with a retrovirus-bearing RAG-2 transgene. The retroviral infection protocol used in this work is derived from the one that was proven effective in a clinical setting.15 The replacement of interleukin 3 (IL-3) by IL-6 was based on the extensive work published by Ogawa and colleagues showing the negative role of IL-3 in murine B-cell development.16 IL-7 was added to increase cell cycling of the common lymphoid precursors (CLP), which, when transduced, should lead to lymphocyte development.17 Following transplantation of the transduced cells into 3-Gy irradiated RAG-2−/−mice, lymphocyte development characteristics, gene integration, and potential toxicity were assessed.
Materials and methods
Mice
C57BL/6 RAG-2–deficient mice were obtained from the Centre de Développement des Techniques Avancées (CDTA-CNRS; Orléans, France) and maintained in a specific pathogen-free animal facility (Laboratoire d'expérimentation animale et de transgénèse, Faculté de Necker, Paris, France). C57BL/6 and C3H wild-type mice were provided by IFFA-CREDO (l'Arbresles, France). All mice used, donors as well as recipients, were from both sexes and from 3 to 8 weeks of age.
Retroviral supernatant
pMND-RAG2 plasmid was obtained by ligation of the whole human RAG-2 cDNA polymerase chain reaction (PCR) product (forward primer: 5′-GAAAACTGTCTCTGCAGATGGTA-3′, reverse primer: 5′-CAAAAGTCGACCTAATCAAACAACC-3′). The human RAG-1 and RAG-2 genes both share 86% to 88% identity with murine RAG-1 and RAG-2 genes, respectively. Such a degree of identity between the 2 proteins should be sufficient for human RAG-2 and murine RAG-1 genes to cross-react efficiently. The MND vector, designed by Kohn and colleagues,14 bears 4 mutations eliminating previously described repressor sequences in the MLV enhancer region. Amongst these, a 66-bp sequence in the 5′ flanking region including the YY1 box was deleted, preventing the binding of the YY1 factor, which can act as a transcription repressor, thus improving gene expression in murine hematopoietic cells. The neo gene of the MND-neo plasmid was replaced with the RAG-2 cDNA PCR product. Retroviral supernatant was produced in Stemspan medium (Stem Cell Technologies, BC, Canada) supplemented with 5% fetal bovine serum (FBS; Stem Cell Technologies) 48 hours after calcium-phosphate–mediated transient transfection of pMND-RAG2 plasmid into ecotropic Phoenix producer cells (protocol published online athttp://www.stanford.edu/group/nolan/NL-phnxr.html).
Transduction of bone marrow cells
Bone marrow cells were flushed from both tibias and femora of donor mice and treated with a 0.75% NH4Cl (Sigma-Aldrich, St Louis, MO) solution in Tris (tris(hydroxymethyl)aminomethane buffer) (Sigma-Aldrich) to remove red blood cells (RBCs). Cells were marked with phycoerythrin (PE)–conjugated Sca1 Ly6A/E (D7) antibody (Becton Dickinson, San Jose, CA). Sca-1–positive cells were then purified using an anti-PE magnetic selection kit (Miltenyi Biotech, Auburn, CA).
Cells were cultured in serum-free Stemspan medium (Stem Cell Technologies) for 24 hours at 1 × 106 cells/mL in flat-bottomed P96 wells coated with 25 μg/mL recombinant human fibronectin fragment (RetroNectin CH-296; Takara Biomedicals, Shiga, Japan) in the presence of the following recombinant cytokines: murine stem cell factor (mSCF) 300 ng/mL (Stem Cell Technologies), human FMS-like tyrosine kinase 3 ligand (hFlt3-L) 300 ng/mL, and human megakaryocyte growth and development factor (hMGDF) 100 ng/mL (Amgen, Thousand Oaks, CA), mIL-6 50 ng/mL, mIL-11 50 ng/mL (R&D, Minneapolis, MN), and hIL-7 100 ng/mL (kindly provided by Biotech Inflection Point Company, Paris, France). Then 3 transduction cycles were performed at 24-hour intervals by replacing the medium with retroviral supernatant supplemented with the same cytokines and protamine sulfate (3 μg/mL) (Choay, Gentilly, France). One million cells were injected intravenously under halothane anesthesia to each RAG-2–deficient mouse less than 2 hours after 3-Gy total body irradiation (described in text as “RAG-2–transduced mice”). Three positive control groups were obtained by transplanting 1 × 106, 1 × 105, or 1 × 104 mock-transduced wild-type C57BL/6 cells into 3 Gy irradiated RAG-2–deficient mice. Secondary grafts were performed 6 months after initial RAG-2–transduced bone marrow transplantation as follows: bone marrow cells were treated with an NH4Cl solution, and a total of 5 × 106 bone marrow cells were reinjected intravenously to 3 Gy irradiated RAG-2−/−mice.
Flow cytometry analysis
Cells for flow cytometry analysis were obtained from blood, thymus, bone marrow, spleen, and lymph nodes. All axillary, cervical, abdominal, and mesenteric lymph node cells were taken. Cells were counted and stained with the following rat antimouse monoclonal antibodies (Pharmingen, San Diego, CA): fluorescein isothiocyanate (FITC)–conjugated CD3ε (145-2C11), biotin-conjugated CD4 (GK1.5), PE-conjugated CD8β (53-5.8), biotin-conjugated T-cell receptor αβ (TCRαβ) (H57-597), PE-conjugated CD45R/B220 (RA3-6B2), and goat anti–mouse FITC–conjugated IgM (Jackson ImmunoResearch Laboratory, West Grove, PA). When necessary, streptavidin-conjugated tricolor (CALTAG, Burlingame, CA) was added. To prevent possible binding to Fc receptors, peripheral blood cells were preincubated with antimouse CD16/CD32 monoclonal antibodies (MoAbs) (2,4G2; Becton Dickinson) and after staining were treated with fluorescence-activated cell-sorter (FACS)–lysing solution (Becton Dickinson) to remove RBCs. Analyses were performed on a FACScalibur (Becton Dickinson) using Cellquest software (Becton Dickinson).
Serum immunoglobulin quantification and T-cell–dependent responses
IgM and IgG immunoglobulin levels were obtained by quantitating serial dilutions of serum samples with an enzyme-linked immunosorbent assay (ELISA) kit according to the manufacturer's instructions (Bethyl Laboratories, Montgomery, TX). T-cell–dependent responses were obtained by immunizing mice intraperitoneally with 50 μg of a keyhole limpet hemocyanin (KLH) (Sigma-Aldrich) solution in Alum (Sigma-Aldrich). Mice were boosted with a 50 μg KLH intraperitoneal injection 21 days after immunization, and serum samples were drawn 28 days after immunization. For local immunization, 50 μg KLH was mixed with 30 μL complete Freund adjuvant (Sigma-Aldrich) and injected in the front footpads of the mice under halothane anesthesia. Mice were killed 8 days after injection, and lymph node cells were isolated and cultured at a concentration of 1 × 106cells/mL in culture medium supplemented with increasing levels of soluble KLH (0, 0.125, 0.4, 2, 10, 50 ng/mL). Anti-KLH–specific immunoglobulins were detected by precoating the wells with KLH at a concentration of 50 μg/mL before ELISA detection.
Lymphocyte proliferation assays
Spleen or lymph node cells were cultured at 1 × 106 cells/mL in supplemented Dulbecco modified Eagle medium (DMEM) (GibcoBRL, Renfrewshire, Scotland) with 10% FBS (GibcoBRL) for 3 days in the presence of concanavalin A (conA, 5 μg/mL; Sigma-Aldrich) or for 4 days in the presence of lipopolysaccharide (LPS, 50 μg/mL; Sigma-Aldrich). Mixed lymphocytes reactions were performed with 2 × 106 cells/mL spleen cells for 5 days in the presence of 4.106 cells/mL fully allogenic C3H 20-Gy irradiated spleen cells. Proliferation was assayed by incorporation of [3H] thymidine (2 μCi/mL [0.074 MBq]; Amersham, Saclay, France). After overnight incubation, cells were transferred onto filters and placed in 1-mL scintillation liquid (Packard Biosciences, Groningen, The Netherlands). Uptake of [3H] thymidine was determined with a scintillation β-counter (Packard Biosciences).
Provirus integration study
Integration study was performed on RAG-2–transduced Sca1+ cells and on 2 different lineage subsets: lymphoid and myeloid. Briefly, 10 months after transplantation, splenocytes from 3 RAG-2–transduced mice were marked with FITC-conjugated CD3ε (145-2C11; Pharmingen) and PE-conjugated CD11b (Mac1a, M1/70; Pharmingen). CD3+ and CD11b+ cells were then sorted with a FACStar (Becton Dickinson). Genomic DNA was extracted using a High Pure DNA PCR Template kit (Roche Diagnostics, Indianapolis, IN). Transgene was detected with a real-time quantitative PCR system (ABI Prism 7700 Sequence Detector Systems, Applied Biosystems, Foster City, CA) by using the following primers: 1870MNDRAG2.F (AAGTAGACGGCATCGCAGCT), which is specific for the MND retroviral sequence, 2014MNDRAG2.R (CAGTGAGAAGCCTGG), located inside the RAG-2 transgene, and the fluorescent probe: 1903MNDRAG2.P (CCCACGTGAAGGCTGCCGACC). Total DNA quantification was performed by using the following primers located in the fifth exon of the murine Titine gene: M261MEX5.M (TTCAGTCATGCTGCTAGCGC), M139MEX5.A (AAAACGAGCAGTGACGTGAGC) and probe: M161MEX5.P (TGCACGGAAGCGTCTCGTCTCAGTC). The results obtained from Titine quantification were used to normalize the number of copies back to the number of cells.
Histology
For histologic studies, gut, spleen, and thymus tissues were fixed in formaldehyde, embedded in paraffin, and sections were stained with periodic acid–Schiff. For immunohistochemical studies, tissues were frozen in liquid nitrogen and fixed in acetone. Cryostat sections were then stained with rat anti–CD8α supernatant (T1B105; American Type Culture Collection [ATCC], Manassas, VA) or rat anti–CD4 supernatant (GK1.5; ATCC) followed by biotinylated mouse anti–rat IgG mAb (Jackson ImmunoResearch Laboratory, West Grove, PA), streptavidin-biotinylated horseradish peroxidase complex (Amersham), and diaminonobenzidine reagent (Enzo Diagnostics, New York, NY). Slides were finally counterstained with methyl green.
Analysis of TCRβ repertoire
Total RNA was prepared from splenocytes using the Rneasy kit (Qiagen, Valencia, CA). cDNA was synthesized using M-MLV superscript reverse transcriptase (GibcoBRL). cDNA was amplified using a Cβ-specific primer along with 24 Vβ-specific primers. PCR products were then subjected to a run-off reaction using a fluorescent Cβ-specific primer.18 Labeled products were resolved on an automated 373A sequencer (Perkin Elmer, Foster City, CA). The fluorescent intensity of each band was recorded and analyzed using Immunoscope software (developed by C. Pannetier, Paris, France).19
Statistical analysis
Results were analyzed by Mann-Whitney tests, using InStat (GraphPad Software, San Diego, CA). Results were considered significant when P < .05.
Results
Immunological development of RAG-2–transduced mice
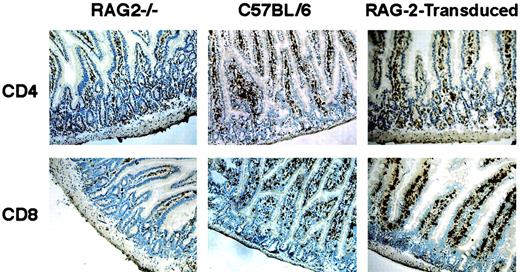
White blood cells were analyzed every month during the first 3 months after transplantation in RAG-2–transduced mice as well as in control mock-transduced mice. In all RAG-2–transduced mice (n = 21), mature T and B cells could be detected 4 to 8 weeks after transplantation and remained detectable at stable levels for more than one year (Figure 1). The kinetics of T- and B-lymphocyte development observed in RAG-2–transduced mice were compared to those observed following transplantation of 1 × 106, 1 × 105, or 1 × 104 mock-transduced C57BL/6 RAG-2+/+ bone marrow Sca1+ cells to 3 Gy-irradiated RAG-2−/−mice. Briefly, 2 to 6 months after bone marrow transplantation, peripheral B220+IgM+ cells were found in significantly higher counts (P < .01) in the blood of mice transplanted with 1 × 106 mock-transduced cells (more than 1400 cells/mm3, data not shown) than in that of RAG-2–transduced mice. During the same posttransplantation period, peripheral T CD4+ and CD8+ cells were in significantly lower numbers (P < .02) in the blood of mice transplanted with 1 × 104 mock-transduced cells than in that of RAG-2–transduced mice. Overall (Figure 1), after transplantation of RAG-2–transduced cells, peripheral B220+IgM+, CD4+, and CD8+ cell counts were not significantly different after 1 × 105 mock-transduced cell transplantation (further simply referred to as “mock-transduced” mice). Peripheral organs of both RAG-2–transduced and mock-transduced mice were tested for periods ranging from 6 months to one year after transplantation. Cellularity of peripheral organs of RAG-2–transduced mice was of lower magnitude than that of mock-transduced controls (Table1), while the distributions of CD3+CD4+ and CD3+CD8+subpopulations in lymph nodes and spleen were similar to those of mock-transduced controls (Figure 2 and Table 2). In 3 of 11 RAG-2–transduced mice tested, the thymus contained a normal percentage of CD4+CD8+ double-positive cells (Figure 2), while CD4+CD8+ double-positive cells were detectable but in low percentage in the thymus of the other mice. To assess the development of intraepithelial lymphocytes (IEL) in RAG-2–transduced mice, gut sections were stained with CD4 and CD8 antibodies. This experiment showed the presence of CD4+ and CD8+ IEL after gene transfer, which are absent in RAG-2−/− mice (Figure 3).
T/B lymphocyte peripheral reconstitution after gene transfer.
Peripheral blood was tested for T- and B-cell counts throughout the experiment. Nucleated blood cells were counted and stained for immunocytofluorometry (black: B220+IgM+; dotted: CD3+TCRαβ+; white: CD3+CD4+; gray: CD3+CD8+). (A) Number of nucleated cells in peripheral blood of RAG-2–transduced mice (n = 11). (B) Number of nucleated cells in peripheral blood of mock-transduced mice (n = 7). (C) Number of nucleated cells in peripheral blood of C57BL/6 mice (n = 4). RAG2−/− mice consistently lacked any expression of B220+IgM+, CD3+TCRαβ+, CD3+CD4+, or CD3+CD8+cells throughout their lives. Data are expressed as means ± SEM.
T/B lymphocyte peripheral reconstitution after gene transfer.
Peripheral blood was tested for T- and B-cell counts throughout the experiment. Nucleated blood cells were counted and stained for immunocytofluorometry (black: B220+IgM+; dotted: CD3+TCRαβ+; white: CD3+CD4+; gray: CD3+CD8+). (A) Number of nucleated cells in peripheral blood of RAG-2–transduced mice (n = 11). (B) Number of nucleated cells in peripheral blood of mock-transduced mice (n = 7). (C) Number of nucleated cells in peripheral blood of C57BL/6 mice (n = 4). RAG2−/− mice consistently lacked any expression of B220+IgM+, CD3+TCRαβ+, CD3+CD4+, or CD3+CD8+cells throughout their lives. Data are expressed as means ± SEM.
Cell counts of organs from RAG-2-transduced, mock-transduced (WT-BMT), wild-type, and RAG-2-deficient mice 6 to 8 months after gene transfer*
| . | Thymus, × 106 cells . | Spleen, × 106 cells . | Lymph nodes, × 106cells . | Bone marrow, × 106 cells . |
|---|---|---|---|---|
| RAG-2-transduced, n = 6 | 2.2 ± 0.7 | 11.8 ± 3.2 | 7.1 ± 2.8 | 38.4 ± 18 |
| WT-BMT, n = 3 | 17 ± 9 | 13 ± 5.4 | 15 ± 6.2 | 35 ± 13 |
| C57BL/6, n = 5 | 69.3 ± 20 | 36.7 ± 14 | 25.8 ± 10 | 64 ± 12 |
| RAG-2, n = 3 | 0.32 ± 09 | 4.3 ± 8 | 0.46 ± 0.3 | 65.2 ± 11 |
| . | Thymus, × 106 cells . | Spleen, × 106 cells . | Lymph nodes, × 106cells . | Bone marrow, × 106 cells . |
|---|---|---|---|---|
| RAG-2-transduced, n = 6 | 2.2 ± 0.7 | 11.8 ± 3.2 | 7.1 ± 2.8 | 38.4 ± 18 |
| WT-BMT, n = 3 | 17 ± 9 | 13 ± 5.4 | 15 ± 6.2 | 35 ± 13 |
| C57BL/6, n = 5 | 69.3 ± 20 | 36.7 ± 14 | 25.8 ± 10 | 64 ± 12 |
| RAG-2, n = 3 | 0.32 ± 09 | 4.3 ± 8 | 0.46 ± 0.3 | 65.2 ± 11 |
Data are expressed as means ± SD.
Lymphoid reconstitution after gene transfer.
Flow cytometric analyses and cellularity of lymphoid organs from a mock-transduced mouse, a RAG-2–transduced mouse 6 months after transplantation, and a mouse 3 months after secondary transplantation.
Lymphoid reconstitution after gene transfer.
Flow cytometric analyses and cellularity of lymphoid organs from a mock-transduced mouse, a RAG-2–transduced mouse 6 months after transplantation, and a mouse 3 months after secondary transplantation.
Lymphocyte subset determination in lymphoid organs of RAG-2-transduced (RAG-2-T) or mock-transduced mice (WT-BMT) 6 to 8 months after transplantation*
| . | Spleen . | Lymph nodes . | Bone marrow . | |||
|---|---|---|---|---|---|---|
| RAG-2-T n = 6 . | WT-BMT n = 3 . | RAG-2-T n = 6 . | WT-BMT n = 3 . | RAG-2-T n = 6 . | WT-BMT n = 3 . | |
| B220+IgM+, % | 21 ± 8 | 18 ± 5 | 6 ± 3 | 16 ± 10 | 1 ± 0.6 | 2 ± 0.4 |
| CD3+TCRαβ+, % | 52 ± 12 | 56 ± 18 | 76 ± 11 | 65 ± 12 | 13 ± 5 | 4 ± 0.5 |
| CD3+CD4+, % | 19 ± 6 | 21 ± 9 | 30 ± 9 | 30 ± 8 | 2 ± 1 | 1 ± 0.2 |
| CD3+CD8+, % | 18 ± 4 | 31 ± 15 | 41 ± 12 | 39 ± 12 | 8 ± 3 | 2 ± 0.7 |
| . | Spleen . | Lymph nodes . | Bone marrow . | |||
|---|---|---|---|---|---|---|
| RAG-2-T n = 6 . | WT-BMT n = 3 . | RAG-2-T n = 6 . | WT-BMT n = 3 . | RAG-2-T n = 6 . | WT-BMT n = 3 . | |
| B220+IgM+, % | 21 ± 8 | 18 ± 5 | 6 ± 3 | 16 ± 10 | 1 ± 0.6 | 2 ± 0.4 |
| CD3+TCRαβ+, % | 52 ± 12 | 56 ± 18 | 76 ± 11 | 65 ± 12 | 13 ± 5 | 4 ± 0.5 |
| CD3+CD4+, % | 19 ± 6 | 21 ± 9 | 30 ± 9 | 30 ± 8 | 2 ± 1 | 1 ± 0.2 |
| CD3+CD8+, % | 18 ± 4 | 31 ± 15 | 41 ± 12 | 39 ± 12 | 8 ± 3 | 2 ± 0.7 |
Data are expressed as mean percentages ± SD.
Intraepithelial lymphocyte development after gene transfer.
CD4 and CD8 immunohistochemical stainings (n = 3) were used to phenotype gut-specific intraepithelial lymphocytes (IEL) from frozen intestine sections of RAG-2−/−, C57BL/6, and RAG-2–transduced mice (6 months after transplantation). CD4+ and CD8+IELs were detected in RAG-2–transduced mice as well as in wild-type C57BL/6 controls. Original magnifications × 200.
Intraepithelial lymphocyte development after gene transfer.
CD4 and CD8 immunohistochemical stainings (n = 3) were used to phenotype gut-specific intraepithelial lymphocytes (IEL) from frozen intestine sections of RAG-2−/−, C57BL/6, and RAG-2–transduced mice (6 months after transplantation). CD4+ and CD8+IELs were detected in RAG-2–transduced mice as well as in wild-type C57BL/6 controls. Original magnifications × 200.
Study of provirus integration
Provirus copies were quantified in Sca1+ cells before injection. RAG-2–transduced Sca1+ cells contained a mean of 0.6 copy/cell, indicating an overall low transduction rate. To assess the possible existence of a selective advantage of RAG-2–transduced cells over nontransduced RAG-2–deficient cells, provirus copies were quantified by real-time quantitative PCR in both lymphoid (CD3+) and nonlymphoid (Mac1+) lineages 9 months after gene transfer. The CD3+ fraction of RAG-2–transduced mice spleen cells contained a mean of 2.4 copies/cell, while the Mac1+ fraction contained a mean of 0.02 copy/cell. The ratio of transduced cells is thus many-fold greater in the lymphoid fraction than in the myeloid fraction, indicating that a selective advantage was conferred to RAG-2–transduced lymphoid cells. Provirus copies were also quantified in Sca1+ cells before injection.
TCR repertoire study and T-cell function
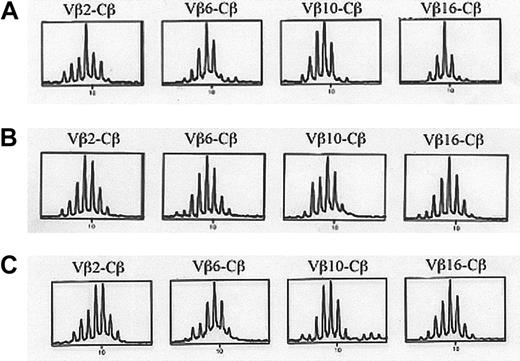
Immunoscope analyses of splenocytes from C57BL/6 wild-type mice, mock-transduced mice, and RAG-2–transduced mice were performed to analyze the TCR Vβ diversity of peripheral T cells. No significative difference in Vβ diversity was observed, showing that RAG-2 gene transfer had restored a broad T-cell differentiation capacity with a diverse T-cell repertoire (Figure 4). Recovered T lymphocytes were functional, as proven by the observed proliferative response of splenocytes stimulated with conA (Figure 5A) or with allogeneic cells (Figure 5B) when tested 4 to 6 months after gene transfer. As expected, RAG-2−/− lymphocytes failed to proliferate with any of these stimuli. After subcutaneous KLH immunization, lymphocytes isolated from draining lymph nodes of RAG-2–transduced mice were shown to proliferate in the presence of KLH (Figure 6). Although the cellularity of the lymphoid organs of RAG-2–transduced mice was less than that of mock-transduced mice (Table 1), these results provide evidence for restored T-lymphocyte function and a diverse TCR repertoire of RAG-2−/− mice after RAG-2 gene transfer.
TCR Vβ repertoire analysis.
Eleven months after gene transfer, 24 Vβ-specific amplifications were performed to test Vβ diversity and analyzed following the immunoscope technique. Two mice were tested. Representative Vβ2, Vβ6, Vβ10, and Vβ16-Cβ amplifications from a RAG-2–transduced mouse (A), a mock-transduced mouse (B), and a C57BL/6 wild-type mouse (C) are represented.
TCR Vβ repertoire analysis.
Eleven months after gene transfer, 24 Vβ-specific amplifications were performed to test Vβ diversity and analyzed following the immunoscope technique. Two mice were tested. Representative Vβ2, Vβ6, Vβ10, and Vβ16-Cβ amplifications from a RAG-2–transduced mouse (A), a mock-transduced mouse (B), and a C57BL/6 wild-type mouse (C) are represented.
T-cell function after gene transfer.
(A) Splenocyte proliferation of RAG-2−/−, C57BL6, and RAG-2–transduced mice in the absence (■) or presence (▪) of concanavalin A. (B) Splenocyte proliferation in the absence (■) or presence (▪) of fully allogeneic spleen cells. Proliferation was measured after addition of [3H]-thymidine. Values are expressed as the means ± SD of triplicate cultures, 4 to 6 months after treatment.
T-cell function after gene transfer.
(A) Splenocyte proliferation of RAG-2−/−, C57BL6, and RAG-2–transduced mice in the absence (■) or presence (▪) of concanavalin A. (B) Splenocyte proliferation in the absence (■) or presence (▪) of fully allogeneic spleen cells. Proliferation was measured after addition of [3H]-thymidine. Values are expressed as the means ± SD of triplicate cultures, 4 to 6 months after treatment.
KLH-specific T-cell proliferation after gene transfer.
After subcutaneous immunization with KLH, the lymph nodes draining the immunization site of RAG-2–transduced mice or C57BL/6 mice were cultured in the presence of increasing antigen concentrations (0, 0.125, 0.4, 2, 10, 50 ng/mL). Proliferation was measured after addition of [3H]-thymidine. Values are expressed as the means ± SD obtained in 4 RAG-2–transduced mice and 2 C57BL/6 mice.
KLH-specific T-cell proliferation after gene transfer.
After subcutaneous immunization with KLH, the lymph nodes draining the immunization site of RAG-2–transduced mice or C57BL/6 mice were cultured in the presence of increasing antigen concentrations (0, 0.125, 0.4, 2, 10, 50 ng/mL). Proliferation was measured after addition of [3H]-thymidine. Values are expressed as the means ± SD obtained in 4 RAG-2–transduced mice and 2 C57BL/6 mice.
B-cell function
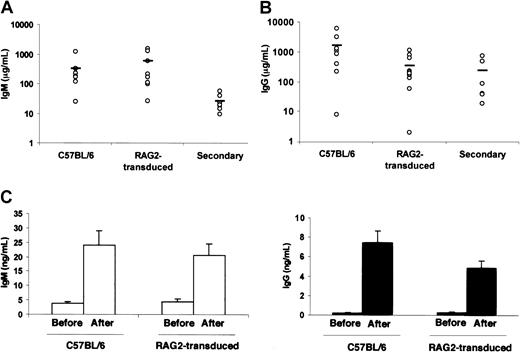
B220+sIgM+ B cells developed in all animals after gene transfer, with variable B-cell numbers in the different compartments (blood, spleen, and lymph nodes). B-cell counts remained stable throughout the study. Serum levels of IgG and IgM immunoglobulins were found to be close to those of C57BL/6 control mice from 2 to 4 months after gene transfer (Figure7A-B) and remained stable until the last time point of analysis (ie, one year after gene transfer), while RAG-2−/− mice sera did not contain any detectable immunoglobulins. Splenocytes from RAG-2–transduced mice proliferated in the presence of LPS (data not shown). RAG-2–transduced mice immunized with KLH produced antigen-specific IgM and IgG antibodies 28 days after immunization with titers similar to immunized C57BL/6 control mice (Figure 7B).
B-cell function after gene transfer.
Serum immunoglobulin IgM (A) and IgG (B) were quantified in RAG-2–transduced mice, C57BL/6 mice, and mice after secondary transplantation. Each dot represents values obtained in one mouse. (C) KLH antigen-specific IgM (■) and IgG (▪) immunoglobulins were quantified in the serum of RAG-2–transduced mice and C57BL/6 mice before and after immunization. Values are the means ± SD of data from 3 mice obtained 28 days after immunization, 4 to 6 months after gene transfer.
B-cell function after gene transfer.
Serum immunoglobulin IgM (A) and IgG (B) were quantified in RAG-2–transduced mice, C57BL/6 mice, and mice after secondary transplantation. Each dot represents values obtained in one mouse. (C) KLH antigen-specific IgM (■) and IgG (▪) immunoglobulins were quantified in the serum of RAG-2–transduced mice and C57BL/6 mice before and after immunization. Values are the means ± SD of data from 3 mice obtained 28 days after immunization, 4 to 6 months after gene transfer.
Stability of lymphoid reconstitution after RAG-2 gene transfer
RAG-2–transduced mice maintained functional levels of lymphocytes in all peripheral organs 10 to 13 months after gene transfer (n = 4). Six months after gene transfer, 4 RAG-2–transduced mice were maintained in a normal nonpathogen-free animal facility and survived well for an additional 3 months until being killed. To assess the presence of RAG-2–transduced HSCs, bone marrow cells from 7-month-old RAG-2–transduced mice were used to perform secondary transplantations into RAG-2−/− mice. Two months later, the thymuses of 2 of 7 mice that received transplants were repopulated by double-positive CD4+CD8+ cells (Figure 2). Peripheral T- and B-cell compartments were restored, as well as antibody production in all secondary treated mice (Figure 7A-B).
Toxicity
No lymphoid malignancies were observed in the 11 RAG-2–transduced mice killed between 6 months and one year after gene transfer. No pathologic changes (diarrhea, loss of weight, hair color, and aspect) associated with RAG-2 transduction was observed during this study, which included 21 mice. No macroscopic alterations, either in volume or in color, of the different organs were noticed following treatment. The histologic study of spleen, lymph nodes, and thymuses of RAG-2–transduced mice showed no structural anomalies after gene transfer (data not shown). Follicles were present in the spleen of RAG-2–transduced mice, and no tumors or abnormal lymphoid infiltrations were detected.
Discussion
We have shown that ex vivo RAG-2 retroviral gene transfer into RAG-2−/− murine hematopoietic precursor cells leads to a correction of the immune deficiency without any obvious deleterious side effects. Following transfer of the retrovirally transduced RAG-2−/− progenitors to RAG-2−/− recipients, treated animals generated peripheral T- and B-lymphoid cells, which have integrated the transgene. Both mature B- and T-cells were present in recipients, although their total counts were inferior to mock-transduced controls. Thymuses were less well reconstituted than peripheral organs. As no major cytoreductive treatment was given to recipient mice, competition between transduced cells and nontransduced lymphoid progenitors up to the TCRβ rearrangement stage could account for this observation. Secondary expansion of transduced cells, mostly in the periphery, could then lead to a close to normal size peripheral T- (and B-) lymphocyte pool.20 Preferential transduction of common lymphoid progenitors could account for a higher rate of lymphocyte transduction as compared to myeloid cells. This, however, was not confirmed by analysis of the transduction rate of CLP (ie, Sca1+ckitlo IL7R+), which was low (data not shown). In some cases, secondary transfer conversely indicated that stem cells have indeed been transduced. Improvement of HSC transduction efficiency or, alternatively, removal of the pro-B and pro-T compartments with antibodies could be an option to improve lymphocyte reconstitution. These 2 strategies are presently under investigation. RAG-2 gene transfer into Sca1+ progenitors generated a functional and diverse peripheral T-cell compartment, as shown by immunoscope analysis and proliferation assays in the presence of mitogens, KLH, and allogeneic antigens. In addition, development of intestinal intraepithelial lymphocytes was observed. In parallel, serum Ig isotype levels and antibody response of both IgM and IgG isotypes to KLH demonstrate that B cells also were functional. Immunoglobulin levels were always within normal range independently from the count of circulating B220+IgM+ cells.21 T- and B-cell numbers were stable over time, up to 12 to 14 months after gene transfer. Finally, since after secondary bone marrow transplantation, lymphocyte development was restored, it is shown that early hematopoietic progenitor cells with self-renewal capacity were successfully transduced.
Importantly, this protocol matches as closely as possible the conditions currently used in human settings since no 5′-fluorouracile was used prior to bone marrow harvesting, and Sca1+purification is a procedure partially comparable to CD34+human bone marrow purification. It should also be noticed that both primary and secondary RAG-2−/− recipients were subjected to low levels of irradiation so that competition between endogenous hematopoietic cells and transduced donor cells could take place. This result, combined with the presence of a ≥ 100-fold enrichment of provirus copies in the T-cell population as compared to the myeloid cell population of RAG-2–transduced mice supports the likelihood of a strong selective advantage of RAG-2–transduced cells over nontransduced cells, as reported in 2 other immunodeficient mouse models22,23 and in 2 clinical trials performed for children affected with SCID.4 24 Hence, even though the RAG-2 protein contributes to the lymphoid differentiation pathway at a later stage than the γc chain, the selective advantage of transduced cells is nevertheless sufficient to correct the immunodeficiency.
Another major concern raised by RAG-2 retroviral gene transfer was the potential toxicity related to the uncontrolled expression of the RAG-2 gene in several different lineages. The fact that RAG-2 protein is exclusively functional in cooperation with the RAG-1 protein argued against this possibility. Indeed, 2 models of double transgenic mice carrying both RAG-1 and RAG-2 genes under the control of the proximal lck promoter25 or the ubiquitous major histocompatibility complex (MHC) class I H2k promoter,26respectively, showed pathologic changes (ie, lymphoadenopathy, splenomegaly, and small body weight), whereas no pathologic changes were reported in single transgenic mice. It is also worth mentioning that in the former study,25 no case of lymphoma was reported in more than 300 lck-driven single transgenic RAG-1 and RAG-2 mice maintained over a period of two and a half years. In our experience, no evidence of myeloproliferation or lymphoproliferation or of autoimmune manifestations was found in the long-term reconstituted animals. Further investigations are pending to exclude the presence of illegitimate V(D)J recombination events in reconstituted animals.
Overall, it is shown that retroviral-mediated RAG-2 gene transfer leads to development of competent T- and B-cell compartments in RAG-2−/− mice without any detectable side effects. A sustained correction of the immunodeficiency was observed, as well as a selective advantage conferred by RAG-2 expression. These results set the basis for a clinical trial of RAG-2 gene transduction of CD34+ cells from RAG-2–deficient patients and opens the way to the treatment of other forms of diseases caused by V(D)J defects, such as RAG-1 or Artemis27 deficiencies.
The authors wish to thank Françoise Selz for the FACS sorting, Corinne Evra for her help at the animal facility, and Nicole Brousse, Michèle Leborgne, and Gerard Pivert for histology. We also are grateful to Frédérique Carlier, Christophe Hue, Fabian Gross, and Chantal Lagresle for their helpful assistance, to Dr Isabelle André-Schmutz for her help in the statistical analysis of the data, and to Dr Benedita Rocha for helpful discussions during the course of this work.
Prepublished online as Blood First Edition Paper, August 22, 2002; DOI 10.1182/blood-2002-03-0782.
Supported in part by INSERM, the Association Française contre les Myopathies, the Fondation Louis Jeantet, the Jeffrey Modell Foundation, and a grant from the Agence Nationale de Recherches sur le Syndrome d'Immunodéficience Acquise (SIDA) (ANRS) (F.Y.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Marina Cavazzana-Calvo, INSERM U429, Hôpital Necker-Enfants Malades, 149 rue de Sèvres, 75743 Paris Cedex 15, France; e-mail: cavazzan@necker.fr.



![Fig. 5. T-cell function after gene transfer. / (A) Splenocyte proliferation of RAG-2−/−, C57BL6, and RAG-2–transduced mice in the absence (■) or presence (▪) of concanavalin A. (B) Splenocyte proliferation in the absence (■) or presence (▪) of fully allogeneic spleen cells. Proliferation was measured after addition of [3H]-thymidine. Values are expressed as the means ± SD of triplicate cultures, 4 to 6 months after treatment.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/12/10.1182_blood-2002-03-0782/5/m_h82323451005.jpeg?Expires=1763497962&Signature=Va2~eGLp9uMf9t3tUgHc9KjHynCvB7sYVkQPGlsEJJ7PhL73Id5kb36pgoAzjlU~Zlgzv-7w8eQk70lEK674Z1aHAMdw6qHsc5Hm5zmvRUgs3SN5bjzJLWHQumvjIvMWpYWSJqT1VQrBekgkq-jPrEBrECF5EC5~nFZkVLBCRG5AFWAfP0tvQR2fOg0E42fiTYhfoAzD1eEzJwgC8VAWOG3oTr~FbS47WGZBX3OTedZcEXJKd0y2urIlz1x0xynyR-XEmJRHzELbROXeKrEp9qv~MGbxh1g9T~4RiIcofV0R7UgG7ph4JJ~jw1SmVtxyPY~~PY~-uLBSYWAlFFxgfA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. KLH-specific T-cell proliferation after gene transfer. / After subcutaneous immunization with KLH, the lymph nodes draining the immunization site of RAG-2–transduced mice or C57BL/6 mice were cultured in the presence of increasing antigen concentrations (0, 0.125, 0.4, 2, 10, 50 ng/mL). Proliferation was measured after addition of [3H]-thymidine. Values are expressed as the means ± SD obtained in 4 RAG-2–transduced mice and 2 C57BL/6 mice.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/12/10.1182_blood-2002-03-0782/5/m_h82323451006.jpeg?Expires=1763497962&Signature=Qt~TfeZ7W3o~4NrBW7ksffJymQyIxKY~Co0ZiYp8F3esHgmRkqAqiydeWb2C-0wqIIxcS0PBe-t5KZNHIuK8VqjfgJqOmwlKVM9ZRHeLc2YAOqOS~iPYTndzH4qc6CQjCvZG~Cl2vWC1azL~f-bVpOK3ZHtPK8caRcw-NgfHs7~ZnZ7tcYi9hAkFTS6-B6DoQjgQrXJIJYqP6xQy4-MtkqroIZiAjaBJ1BX8WJZYwbZnkQfm3fIwb7x7Od9qzCuwB2ilAgenzDynZ0BT85U1colqTpyT3Gh43DyjAskbUtWM3T3ooTu4T7jUlHEKgMj56nzm3uB82wNQqjUBMqFNBg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal