Epitope vaccines have shown promise for inducing cellular immune responses in animal models of infectious disease. In cases where cellular immunity was augmented, peptide vaccines composed of covalently linked minimal cytotoxic T-lymphocyte (CTL) and T-helper (TH) epitopes generally showed the most efficacy. To address a clinical vaccine strategy for cytomegalovirus (CMV) in the context of HCT (hematopoietic cell transplantation), we observed that linking the synthetically derived pan-DR epitope peptide (PADRE) or one of several tetanus TH epitopes to the immunodominant human leukocyte antigen (HLA) A*0201–restricted CTL epitope from CMV-pp65 to create a fusion peptide caused robust cytotoxic cellular immune responses in HLA A*0201/Kbtransgenic mice. Significantly, the fusion peptides are immunogenic when administered in saline solution by either subcutaneous or intranasal routes. CpG-containing single-stranded DNA (ss-oligodeoxynucleotide [ODN]) added to the fusion peptides dramatically up-regulated immune recognition by either route. Notably, target cells that either expressed full-length pp65 protein from vaccinia viruses or were sensitized with the CTL epitope encoded in the vaccine were recognized by splenic effectors from immunized animals. Visualization of murine peptide–specific CTL by flow cytometry was accomplished using an HLA A*0201 tetramer complexed with the pp65495-503 CTL epitope. TH-CTL epitope fusion peptides in combination with CpG ss-ODN represent a new strategy for parenteral or mucosal delivery of vaccines in a safe and effective manner that has applicability for control or prophylaxis of infectious disease, especially in situations such as vaccination of donors or recipients of HCT, where highly inflammatory adjuvants are not desired.
Introduction
Investigators have focused on developing transgenic (Tg) mice containing human leukocyte antigen (HLA) alleles such as A*0201 (A2.1) in the class I system or DR1 in the class II system to address the problem of selection of epitopes that bind to major histocompatibility complex (MHC) molecules in an experimental model system.1,2 Cellular immune responses to vaccines can then be studied in an easily manipulated vertebrate system with immunologic similarities to humans. We and others have characterized a repertoire of cytotoxic T-lymphocyte (CTL) epitopes specific for the immunodominant protein, cytomegalovirus (CMV)-pp65.3,4 The choice of using CTL epitopes derived from CMV was based upon the absence of a Food and Drug Administration–approved vaccine modality against this significant opportunistic infection of solid organ and hematopoietic cell transplantation (HCT) recipients or its congenital manifestations.5-7 The utility of using HLA-restricted CTL epitopes is derived from the fact that HCT recipients are HLA typed and can be selected to be potential responders to the epitope-based vaccine. Reactivation of CMV and viremia are closely monitored during the first several months after HCT, representing a unique opportunity to investigate the properties of a therapeutic vaccine.8-10 CMV-infected cells express pp65 both early and late after infection, making it an appropriate vaccine target.11,12 Vaccines incorporating them would provide a strategy to immunize against CMV infection in the clinical setting. Since CMV-pp65 contains an HLA A2.1–specific epitope that is recognized by T cells from humans and from mice of the H-2bbackground containing an HLA A2.1 or chimeric (human/mouse) A2.1/Kb transgene, it has been chosen as a model class I epitope for these studies.3,13,14 To circumvent allele specificity for the required T-helper (TH) epitope, a series of TH sequences that promiscuously bind to either human or murine class II MHC alleles have been evaluated in combination with the CMV-pp65 HLA A*0201–restricted epitope.13 15
In the last decade, investigators have studied the optimal means of delivering peptides corresponding to either CTL or THepitopes as experimental vaccines. Peptides have been emulsified in adjuvants, complexed to alum, or suspended in liposomes, to name a few of the delivery strategies.16-18 Successful epitope vaccine strategies against viruses, bacteria, and tumor antigens have been developed in mice using these delivery vehicles.19,20In addition, modification of the primary structure of peptides by incorporating lipids also has been extensively studied in both experimental animals and in man.21,22 Lipopeptides (lipidated peptides) specific for hepatitis B (HBV), HIV, and tumor antigens have been studied clinically in phase 1 and 2 trials with modest results.23-25 Alternatively, exposure of ex vivo–expanded dendritic cells to peptides as a means to stimulate cellular immunity has proven to be more effective than many parenteral vaccination regimes.26 27 However, the simplicity of injecting a stable small molecule product as a vaccine is lost when cell isolation is required to stimulate immunity.
Adjuvants, especially those that are oil-based or contain mycobacterial components, are permissible in animals, yet in many cases they are too inflammatory for human use. It would be an improvement in the vaccine field if a vaccine could be delivered without them in a safe and effective manner.28-31 An effective means has not yet been defined to deliver a vaccine with the attributes of processed T-cell epitopes, without the current efficacy problems associated with plasmid DNA vaccination or safety concerns of live viruses.32-34Alternative means to enhance the effectiveness of vaccinations with subunit protein and peptide vaccines in mice and primates using CpG ss-oligodeoxynucleotide (ODN) have been reported.35,36 Several studies have demonstrated that ss-ODN, especially CpG motifs, skew the immune response to being TH1-dominated.37 In the process of evaluating lipopeptides as vaccines, the discovery was made that covalently linking selected CTL and TH epitopes converted them into effective immunogens, without lipid modification. The highly soluble unlipidated fusion peptides can be administered parenterally or intranasally in a solution of normal saline and small amounts of dimethyl sulfoxide (DMSO). Their enhanced immunogenicity is dependent on being fused, as the component CTL and THepitopes are inactive when administered without adjuvant. We report that CpG ss-ODN further augments the activity of fusion peptides, providing a safe means to lower the amount given during an immunization. Vaccination of healthy adults and children might be accommodated with fusion peptides, because there will be limited side effects due to the formulation. This report focuses on refinements of peptide structure and delivery mechanisms to put forward a rationale approach for a therapeutic vaccine against CMV infection in the context of HCT that may have wider applicability in infectious disease and cancer.
Materials and methods
Synthetic peptides, immunogens, and ODN
pp65495-503,4 the pan-DR epitope peptide (PADRE), and tetanus (Tet) THepitopes15,38 were prepared by standard solid phase F-Moc procedures using an Applied Biosystem 432 (Foster City, CA). Peptides were purified by standard high-performance liquid chromatography methods (≥ 90%), and molecular weight of peptides was confirmed by matrix-assisted laser desorption/ionization (MALDI; Kratos, Chestnut Ridge, NY), as previously described.39 Fusion peptides were made available under the auspices of Rapid Access to Intervention Development program (Developmental Therapeutics Program, National Cancer Institute, Rockville, MD), including K25V, PAM-K25V, diPAM-K25V, and KTet830V (Table1) at purities of at least 90%. Tet639V was synthesized by Mixture Sciences (La Jolla, CA). Incomplete Freund adjuvant (IFA) was purchased from Sigma (St Louis, MO). The previously described40 41 immunostimulatory synthetic ODN 1826 (5′ TCCATGACGTTCCTGACGTT 3′) containing 2 CpG motifs was synthesized with nuclease-resistant phosphorothioate backbone by Alpha DNA (Montreal, QC, Canada). The Na+ salts of the ODN were resuspended at 5 mg/mL in 10 mM Tris (pH 7.0)/1 mM EDTA (ethylenediaminetetraacetic) acid and stored as 50 μL aliquots at −20°C before dilution in normal saline prior to injection.
Primary structure of CMV vaccine peptides
| Peptide . | Lipid molecule(s) . | Adapter sequence . | THtype . | TH epitope sequence . | Linker sequence . | CMV CTL epitope . | Carboxyl terminus . | HLA restriction . |
|---|---|---|---|---|---|---|---|---|
| K25V | None | -K-S-S- | PADRE | -A-K-X*-V-A-A-W-T-L-K-A-A-A- | None | -N-L-V-P-M-V-A-T-V- | OH | A*0201 |
| KTet830V | None | -K-S-S- | Tetanus | -Q-Y-I-K-A-N-S-K-F-I-G-I-T-E- | -A-A-A- | -N-L-V-P-M-V-A-T-V- | OH | A*0201 |
| Tet639V | None | None | Tetanus | -V-S-T-I-V-P-Y-I-G-P-A-L-N-I- | -A-A-A- | -N-L-V-P-M-V-A-T-V- | OH | A*0201 |
| Peptide . | Lipid molecule(s) . | Adapter sequence . | THtype . | TH epitope sequence . | Linker sequence . | CMV CTL epitope . | Carboxyl terminus . | HLA restriction . |
|---|---|---|---|---|---|---|---|---|
| K25V | None | -K-S-S- | PADRE | -A-K-X*-V-A-A-W-T-L-K-A-A-A- | None | -N-L-V-P-M-V-A-T-V- | OH | A*0201 |
| KTet830V | None | -K-S-S- | Tetanus | -Q-Y-I-K-A-N-S-K-F-I-G-I-T-E- | -A-A-A- | -N-L-V-P-M-V-A-T-V- | OH | A*0201 |
| Tet639V | None | None | Tetanus | -V-S-T-I-V-P-Y-I-G-P-A-L-N-I- | -A-A-A- | -N-L-V-P-M-V-A-T-V- | OH | A*0201 |
The sequences of 3 fusion peptides are shown. The derivation of the PADRE TH epitope peptide can be found in Alexander et al,38 Tet830–843 peptide is discussed in Livingston et al,21 Tet639–652 in Reece et al,71 and the A*0201 CMV CTL epitope is discussed in Longmate et al.4 X
indicates cyclohexyl-alanine.
Recombinant vaccinia virus constructs
The human ubiquitin (Ub) gene42 was amplified using the following pair of primers (5′ primer A: CAGTCAGCTAGCGTTTAAACATGCAGATCTTCGTGAAGACC, 3′ primer B: GGACAACGGCGACCGCGCGACTCCCTACCCCCCCTCAAGCGCAGGAC). Human cytomegalovirus (HCMV) (AD169) pp65 gene43 was amplified using the following pair of primers (5′ primer C: GTCCTGCGCTTGAGGGGGGGTAGGGAGTCGCGCGGTCGCCGTTGTCC and 3′ primer D: CCGGGTACCTCAACCTCGGTGCTTTTTGGGCGTC). The primers B and C were designed in a way that they not only complement each other, but also contain the Arg codon (AGG), replacing Met (ATG) at the amino terminus of pp65. The Ub gene (271 base pair [bp]) and HCMV pp65 gene polymerase chain reaction (PCR) products (1680 bp) were fused together to generate the Ub-(R)-pp65 fusion gene by PCR using primer pair A and D as described above. The PCR reaction conditions were one cycle at 94°C, 5 minutes; 5 cycles of 94°C, 1 minute; 55°C, 1 minute; 72°C, 4 minutes; followed by 20 cycles of 94°C, 1 minute; 60°C, 1 minute; and 72°C for 4 minutes. The resulting 1926-bp Ub-R-pp65 fusion gene product was gel purified and cloned into pSC11 insertion plasmid using NheI and KpnI sites to generate Ub-R-pp65-pSC11.44 The construct was verified by restriction enzyme digestion and DNA sequencing. The Ub-R-pp65 recombinant vaccinia virus (VV) (Ub-R-pp65Vac) was generated by transfecting the Ub-R-pp65-pSC11 plasmid into VV-infected Hu TK− cells. Ub-R-pp65Vac was simultaneously screened and selected for 3 rounds by color reaction of substrates (Bluogal; Sigma-Aldrich, St Louis, MO) to β-galactosidase and resistance to BrdU.13 The expression of pp65 was detected by Western blot analysis as previously described.43
Mice
HLA-A2.1/Kb transgenic (Tg)15 mice used throughout this study were bred and maintained under standard pathogen-free conditions in the American Association for Laboratory Animal Care–approved animal care facility at City of Hope. The expression of HLA-A2.1/Kb molecules was routinely confirmed by flow cytometric analyses of splenocytes from individual mice, using BB7.2 monoclonal antibody as described.15
Immunization procedures
Groups of 8- to 12-week-old Tg mice were immunized with synthetic peptides +/−ss-ODN or VVs. Either VV (107 plaque forming units) or synthetic peptides were injected using a 1-mL tuberculin syringe (Becton Dickinson, Franklin Lakes, NJ) in a volume of 100 μL of normal (N)-saline solution with concentrations of DMSO indicated in the figure legend without anesthesia at the base of the tail for the subcutaneous route. For intranasal administration, mice received anesthesia with 30 mg/kg of ketamine/xylazine cocktail (Sigma) intraperitoneally prior to treatment. A total of 30 μL (15 μL/naris) of synthetic peptides +/− ss-ODN in saline solution was administered using a pipette. For some experiments, Tg mice were boosted 2 weeks later with the same synthetic peptide/DNA combination.
Generation of CMV-specific CTL response
Spleens were aseptically removed 12 days after immunization, and splenic single-cell suspensions were produced by teasing the organs through a sterile nylon mesh as previously described.14,15 Splenocytes were stimulated in vitro (IVS) once or twice with syngeneic antigen-presenting cells (APC) and loaded with the relevant CMV-CTL epitope following a modification of a published protocol.13 Briefly, stimulator cells were syngeneic naive splenocytes pretreated for 3 days with 25 μg/mL lipopolysaccharide (LPS) and 7 μg/mL dextran sulfate (both from Sigma) at a density of 2 × 106cells/mL.45 The LPS blasts were pulsed (25 × 106 cells/100 μL) with 100 μM of the CMV-CTL epitope contained in the fusion peptide (Table 1) for 4 hours in a 37°C and 5% CO2 incubator. Spleens were pooled from each group of immunized mice, and the splenic suspensions (3 × 106) were cocultured for 7 to 8 days with 106 γ-irradiated (2400 rad, Isomedix Model 19 Gammator; Nuclear Canada, Parsippany, NJ) peptide-loaded blasts in 2 mL medium containing 10% T-Stim Culture Supplement (Collaborative Biomedical Products, Bedford, MA).
Chromium release assay
The cytotoxic activity of the cell cultures was determined by a standard 4-hour chromium release assay (CRA) following 1 or 2 IVS. To measure peptide-specific responses, T2 cells (the transporter-associated antigen processing (TAP)–deficient human cell line)46 for HLA A2.1/Kb mice were pulsed with 10 μM of the relevant peptide or an equal concentration of an unrelated synthetic sequence for 1 hour. Recognition of virally encoded CMV-pp65 was evaluated using either Jurkat HLA A2.1 transfectants13 or HLA A2.139 Epstein-Barr virus lymphoblastoid cell lines (EBV-LCL) infected overnight at multiplicity of infection (MOI) 3 with VV as described. Target cells were labeled with 200 μCi (7.4 MBq) of Na51CrO4– (ICN, Costa Mesa, CA) for 1 hour in a 37°C water bath, washed extensively, and plated in 96-well round-bottom plates at a concentration of 2000 target cells per well. The radioactivity in the supernatants was determined using a Cobra II auto γ-counter (Packard, Downers Grove, IL), and percent specific lysis was determined as described.39Experimental determinations were performed in triplicate, and assay data were taken in consideration only if spontaneous release was less than 30%. Results are reported when the average and standard deviation of experimental determinations were less than 15% of the mean. Comparisons of CTL activity using specific versus nonspecific peptides or conditions within an experiment were done using the Studentt test using SigmaPlot and SigmaStat software (SPSS, Chicago, IL).P values less than or equal to .05 are considered significant and indicated in the figure legend.
IFN-γ detection
Interferon (IFN)-γ secretion in IVS culture supernatants was measured by enzyme-linked immunosorbent assay (ELISA) following previous protocols, using paired capture (anti–IFN-γ R4-6A2) and detecting (anti–IFN-γ biotinylated XMG1.2) monoclonal antibodies (mAbs; Pharmingen, San Diego, CA).47 Collection of the supernatants was performed at 2, 24, 72, and 96 hours after IVS. Recombinant IFN-γ (Pharmingen) was used for the preparation of a standard curve. Each sample was tested in duplicate. The detection limit of the assay was 70 pg/mL IFN-γ.
Cytofluorimetric analysis
The HLA-A2 CMVpp65495-503 tetramer was refolded and purified in our laboratory using a minor modification of the procedure used by the National Institute of Allergy and Infectious Diseases Tetramer Core Facility (www.emor.edu/WHSC/TETRAMER). Briefly, HLA-A2 heavy chain and β-2-microglobulin (β2M), cloned in the vector pHN1, were expressed in Escherichia coli XA90 and refolded with the CMV pp65495-503 CTL epitope.47 The refolded HLA-A2/β2M/peptide complexes were biotinylated using the enzyme BirA (Avidity, Denver, CO) and then purified by fast performance liquid chromatography using a Sephacryl S300 gel filtration column, followed by a MonoQ ion exchange column. The purified biotinylated HLA-A2/β2M/peptide complexes were conjugated to either streptavidin-phycoerythrin (PE; Pharmingen, San Diego, CA) or to streptavidin-allophycocyanin (Molecular Probes, Eugene, OR). Labeling was typically performed using 0.5 μg of tetramer to stain 0.5 to 1 million cells in a 50 to 100 μL volume of phosphate-buffered saline/0.5% bovine serum albumin for 20 minutes. The cells were then washed and analyzed on a Becton Dickinson FACScalibur flow cytometer. A lymphocyte gate was set based on forward and side scatter and a minimum of 30 000 gated events captured. Quadrants were set based on negative controls. The numbers of tetramer-positive cells are expressed as a percentage of the total lymphocyte population.
Results
Immunization of a Tg mouse model of HLA A2.1
Results from in vitro stimulation of human peripheral blood leukocytes using the pp65495-503 peptide (Table 1) confirm that it functions to stimulate CTLp (CTL precursors) as a memory response from individuals with prior CMV exposure.13,39,47 To test whether the peptide could also stimulate de novo CTLp without prior virus exposure, a Tg HLA A2.1 mouse model was evaluated.1 A robust CTL response directed at the pp65495-503 CTL epitope was found in Tg mice that had been coimmunized with the PADRE TH epitope in IFA.13,38 These results have recently been confirmed using a mouse (C57BL/6) expressing an HLA transgene modified by substitution of the human α3 domain with the murine homolog (A2.1/Kb) used in these studies.15 In addition, we previously showed that pp65495-503–specific CTL stimulation is absolutely dependent on TH peptide coimmunization in combination with an adjuvant such as IFA, although several different THepitopes, including those from tetanus or PADRE, work equally well.15 It remained to be shown whether antigen processing in Tg HLA A2.1/Kb mice would also allow recognition of the pp65495-503 epitope in the context of a full-length protein. Mice were infected with a VV expressing recombinant CMV-pp65 (pp65Vac), previously reported to cause recognition of human APC by CMV-specific T-cell clones.13 Splenocytes from the infected mice were able to recognize human T2 target cells pulsed with the CTL epitope pp65495-5034 (data not shown). This result indicates that a pp65-specific CTL epitope is specifically recognized in Tg mice by endogenous processing of full-length pp65 protein, suggesting that it should be suitable to evaluate recognition of epitope fusion peptides.
Fusion peptides without adjuvant are highly immunogenic
Published accounts suggest that subcutaneous administration of peptides without adjuvant or lipidation induces suboptimal immunity, except in rare instances.48,49 We confirmed that adjuvant (IFA) is crucial to obtain immune responses in our model, because the combination of either PADRE or Tet830-843 THand the pp65495-503 CTL epitope as free peptides administered in normal (0.9%) saline is without activity (data not shown).15 To increase immunogenicity, both epitopes were fused to create a single peptide, an approach that had shown promise in other systems, albeit either with lipidation or strong adjuvant. The initial fusion peptide sequence to be evaluated is composed of the TH epitope PADRE and the pp65495-503 CTL epitope, referred to as K25V in Table 1. Although there are no consistent rules for success, examination of the K25V sequence using standard algorithms50,51 suggests that its significant hydrophobicity may enhance membrane association and entry into cellular protein degradation pathways and bypass the need for adjuvant.52 A dose-response experiment was carried out by giving K25V subcutaneously in 99% N-saline/1.0% DMSO (NS-D) to Tg mice at several different concentrations of peptide (Figure1). CTL activity decreased in a dose-dependent manner between 10 and 150 nanomoles (P < .001 compared to control peptide). In contrast, immunization with mixtures of TH and CTL epitopes were shown to be inactive when injected under the same conditions as the fusion peptide (data not shown), similar to what others have reported.20
K25V administered subcutaneously without adjuvant is immunogenic in Tg mice.
K25V was dissolved at 5 mM in 90% N-saline/10% DMSO and diluted in N-saline to deliver the amount of peptide shown on the x-axis. Tg HLA A2.1/Kb [n = 6 (150 nmol), 14 (100 nmol), 8 (50 nmol), and 2 (10 and 25 nmol)] mice were immunized once subcutaneously at the base of the tail with peptide and no additional adjuvant. After 2 weeks, spleens were harvested, and IVS were done as described in “Materials and methods.” Targets were T2 cells loaded with specific (pp65495-503, filled symbols) and nonspecific (p53149-157, open symbols) peptides as described in “Materials and methods.” Means and SE were calculated at each effector-target (E/T) ratio for all evaluated mice, and significantP values are indicated.
K25V administered subcutaneously without adjuvant is immunogenic in Tg mice.
K25V was dissolved at 5 mM in 90% N-saline/10% DMSO and diluted in N-saline to deliver the amount of peptide shown on the x-axis. Tg HLA A2.1/Kb [n = 6 (150 nmol), 14 (100 nmol), 8 (50 nmol), and 2 (10 and 25 nmol)] mice were immunized once subcutaneously at the base of the tail with peptide and no additional adjuvant. After 2 weeks, spleens were harvested, and IVS were done as described in “Materials and methods.” Targets were T2 cells loaded with specific (pp65495-503, filled symbols) and nonspecific (p53149-157, open symbols) peptides as described in “Materials and methods.” Means and SE were calculated at each effector-target (E/T) ratio for all evaluated mice, and significantP values are indicated.
CTL activity is accompanied by IFN-γ release after fusion peptide immunization
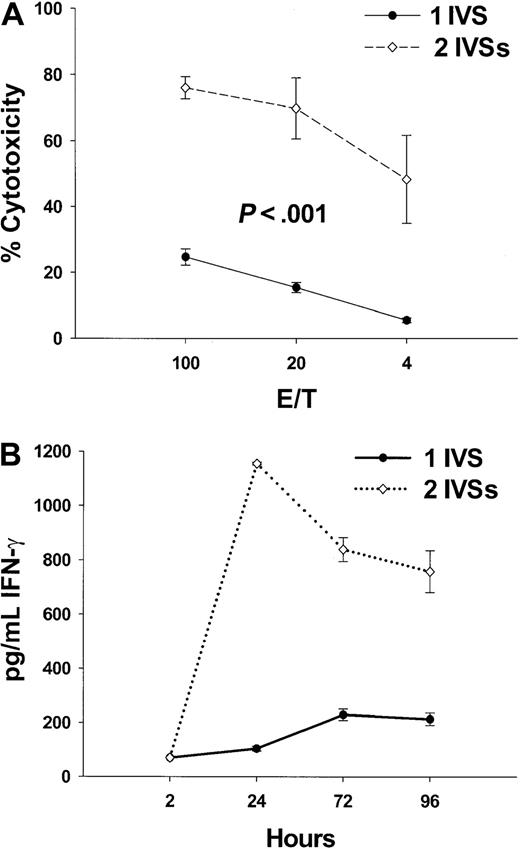
To further characterize the immune response stimulated by the fusion peptides, IFN-γ release was chosen as a reliable indicator of TH1 responses that are stimulated by vaccine candidates. Tg HLA A2.1/Kb mice were immunized with 100 nmol K25V, and IFN-γ release was quantitated by ELISA from supernatants of splenocyte cultures, and its level correlated with the peptide-specific cytotoxicity after 1 or 2 IVSs (Figure2A,B). One IVS resulted in modest IFN-γ release and corresponding cytotoxicity, while a second IVS dramatically improved the IFN-γ signal and cytotoxicity (Figure 2A,B). These data show that K25V fusion peptide is a promising immunogen, with favorable solubility and activity characteristics in physiologic saline with minimal DMSO (0.2%-3.0%).
Multiple IVS amplifies CTL response and IFN-γ release.
(A) Tg mice were vaccinated with 100 nmol of K25V fusion peptide as described in the legend to Figure 1 and boosted 2 weeks later with an additional 100 nmol of the identical peptide. Mice (n = 8) were killed after 2 weeks, spleens removed, and either one (●) or 2 (⋄) IVSs were performed followed by a CRA as described in “Materials and methods.” Values represent subtraction of nonspecific (p53149-157) from specific (pp65495-503) cytotoxicity of peptide-sensitized T2 cells as described in “Materials and methods.” (B) Aliquots of culture medium (200 μL) from IVS cultures (●, 1 IVS and ⋄, 2 IVSs) from mice immunized as described for panel A were withdrawn at the indicated times, and IFN-γ protein was measured from the undiluted fluid by ELISA as described in “Materials and methods.” The detection limit of the assay was established as 70 pg/mL using IFN-γ protein standard (Pharmingen).
Multiple IVS amplifies CTL response and IFN-γ release.
(A) Tg mice were vaccinated with 100 nmol of K25V fusion peptide as described in the legend to Figure 1 and boosted 2 weeks later with an additional 100 nmol of the identical peptide. Mice (n = 8) were killed after 2 weeks, spleens removed, and either one (●) or 2 (⋄) IVSs were performed followed by a CRA as described in “Materials and methods.” Values represent subtraction of nonspecific (p53149-157) from specific (pp65495-503) cytotoxicity of peptide-sensitized T2 cells as described in “Materials and methods.” (B) Aliquots of culture medium (200 μL) from IVS cultures (●, 1 IVS and ⋄, 2 IVSs) from mice immunized as described for panel A were withdrawn at the indicated times, and IFN-γ protein was measured from the undiluted fluid by ELISA as described in “Materials and methods.” The detection limit of the assay was established as 70 pg/mL using IFN-γ protein standard (Pharmingen).
CpG ssODN enhances immunogenicity of fusion peptides
An increase in potency of the fusion peptides is desirable, because it would reduce the amount necessary to stimulate an immune response. An effective adjuvant for a variety of vaccines in mice has been the CpG-containing ss-ODN.35,53,54 They are miscible with aqueous solvents, noninflammatory, and cost effective. Although ss-ODN have not been previously explored in conjunction with TH-CTL fusion peptides without accompanying adjuvant (IFA), their property of TH1 up-regulation suggested they might prove useful in enhancing the response to fusion peptides.55 K25V was mixed with CpG containing ss-ODN referred to as #1826 (25 μg) in NS-D and injected subcutaneously into mice. Two weeks later, spleens were removed, and one IVS (see “Materials and methods”) was performed, followed by a CRA (Figure 3A). In comparison to mice immunized without CpG ss-ODN and in which only one IVS amplification was performed (Figures 2A, 4A), there is substantial up-regulation of peptide-specific recognition in the presence of CpG ss-ODN in combination with either 50- or 100-nmol fusion peptide (Figure 3A). The dramatic effect of ss-ODN is not observed when a non–CpG ss-ODN (no. 1984)35 is used (Figure 4A).
CpG ss-ODN augments the immunogenicity of K25V fusion peptide.
(A) 25 μg of ss-ODN #1826 was mixed with fusion peptide K25V dissolved as described in the legend to Figure 1. A dose titration of peptide was set up, with a constant volume maintained by dilution with N-saline. A solution of 100 μL containing peptide and 25 μg ss-ODN was injected once subcutaneously into the following numbers of Tg mice: 100 nmol, n = 6; 50 nmol, n = 10; and 25 nmol, n = 2. After 14 days, mice were killed and spleens were removed, and one IVS was carried out as described in “Materials and methods.” CRA was performed as described in “Materials and methods” with pp65495-503 cytotoxicity represented by filled symbols and p53149-157 specificity represented by open symbols. Targets and calculation of cytotoxicity were the same as described in the legend to Figure 1. (B) 50 nmol of KTet830V (Table 1) alone (●) or with 25 μg ss-ODN #1826 (⋄) was used to immunize Tg mice (n = 6) as described in panel A. (C) The same conditions as panel B (n = 4), except the fusion peptide is Tet639V (Table 1), and symbols represent 50 nmol peptide alone (●) or with 25 μg ss-ODN #1826 (⋄).
CpG ss-ODN augments the immunogenicity of K25V fusion peptide.
(A) 25 μg of ss-ODN #1826 was mixed with fusion peptide K25V dissolved as described in the legend to Figure 1. A dose titration of peptide was set up, with a constant volume maintained by dilution with N-saline. A solution of 100 μL containing peptide and 25 μg ss-ODN was injected once subcutaneously into the following numbers of Tg mice: 100 nmol, n = 6; 50 nmol, n = 10; and 25 nmol, n = 2. After 14 days, mice were killed and spleens were removed, and one IVS was carried out as described in “Materials and methods.” CRA was performed as described in “Materials and methods” with pp65495-503 cytotoxicity represented by filled symbols and p53149-157 specificity represented by open symbols. Targets and calculation of cytotoxicity were the same as described in the legend to Figure 1. (B) 50 nmol of KTet830V (Table 1) alone (●) or with 25 μg ss-ODN #1826 (⋄) was used to immunize Tg mice (n = 6) as described in panel A. (C) The same conditions as panel B (n = 4), except the fusion peptide is Tet639V (Table 1), and symbols represent 50 nmol peptide alone (●) or with 25 μg ss-ODN #1826 (⋄).
CpG ss-ODN amplifies CTL response to K25V.
(A) Mice were immunized with K25V twice, either including CpG (♦) or non-CpG (▴), or without ss-ODN (●). Spleens were harvested after 14 days, and one IVS was performed as described in “Materials and methods.” Conditions for CRA and calculation of specific cytotoxicity were identical to those described in the legend to Figure 1. (B-G) Flow cytometry analysis of splenocytes whose CRA result is shown in panel A. Two-color flow cytometry was employed, as described in “Materials and methods,” using CD8-FITC and HLA A2.1 tetramer-PE complexed with pp65495-503 (B-D) or p53149-157 (E-G) in separate dimensions. Percentages of cells that are in the top right quadrant are shown above each profile. For each histogram, 20 000 events were collected, and electronic gates were used to exclude cells that did not fall into the small lymphocyte size range.
CpG ss-ODN amplifies CTL response to K25V.
(A) Mice were immunized with K25V twice, either including CpG (♦) or non-CpG (▴), or without ss-ODN (●). Spleens were harvested after 14 days, and one IVS was performed as described in “Materials and methods.” Conditions for CRA and calculation of specific cytotoxicity were identical to those described in the legend to Figure 1. (B-G) Flow cytometry analysis of splenocytes whose CRA result is shown in panel A. Two-color flow cytometry was employed, as described in “Materials and methods,” using CD8-FITC and HLA A2.1 tetramer-PE complexed with pp65495-503 (B-D) or p53149-157 (E-G) in separate dimensions. Percentages of cells that are in the top right quadrant are shown above each profile. For each histogram, 20 000 events were collected, and electronic gates were used to exclude cells that did not fall into the small lymphocyte size range.
Tetanus TH epitopes as part of fusion peptides mediate potent cytotoxic responses
The effect of CpG ss-ODN was also investigated in combination with 2 other fusion peptides, both containing promiscuous THepitopes from tetanus (Table 1). The KTet830V fusion peptide was given by subcutaneous injection with and without ss-ODN, and the CTL response was evaluated. KTet830V given to mice at either 50 (Figure 3B) or 100 (data not shown) nmol was not able to stimulate a vigorous CTL response without the inclusion of CpG ss-ODN in the immunization mixture. A similar effect was observed with another tetanus TH epitope called Tet639V (Table 1), although the effect of CpG ss-ODN was not as dramatic (Figure 3C). Standard measures of hydrophobicity indicate that Tet639 is similar in hydrophobicity as PADRE, but Tet830 is more hydrophilic.50 51 The data show that several TH epitopes can substitute for PADRE, but the degree of hydrophobicity may be important for CTL-stimulating activity and the ability of CpG ss-ODN to up-regulate function.
HLA A2.1 tetramer can be used to follow strength of immunization
An independent means of assessing CTL frequency apart from CRA has been the use of HLA tetramers. They provide a quantitative measure of the frequency of peptide-specific CTL that does not depend on limiting dilution or in vitro culture methods. A number of groups have explored the specificity of the pp65495-503 epitope in combination with the HLA A2.1 heavy chain modified for tetramer formation as first described by Altman and colleagues.47,56,57 We investigated whether the same tetramer preparation that worked specifically with human peripheral blood mononuclear cells would be able to distinguish pp65495-503–specific T cells from mouse spleen, as was recently shown for a human p53 HLA A2.1 CTL epitope.58 Three groups of Tg mice were immunized subcutaneously with 50 nmol K25V and a booster of the same composition with either control (non-CpG) or CpG ss-ODN, or alone. Only cytotoxicity data for the booster immunization are shown (Figure 4A), as the results of the primary immunization are consistent with Figures2A and 3A. There is DNA-independent cytotoxic recognition of the fusion peptide, although ss-ODN, especially CpG-containing DNA, up-regulated the activity, which is most apparent after a second administration of vaccine. The phenomenal specificity and activity of the K25V fusion peptide with CpG ss-ODN demonstrated by CRA prompted an examination of whether pp65495-503–specific CTL would be detectable using HLA tetramers after the second immunization. Flow cytometry using HLA tetramer–PE staining, followed by fluorescein isothiocyanate (FITC)–CD8, is shown for all 3 groups using specific (Figure 4B-D) and nonspecific HLA tetramers (Figure 4E-G). A good correlation is established between levels of stained T cells and cytotoxicity. There is insubstantial background staining with the nonspecific HLA tetramer, which provides confidence of the specificity of the interaction.
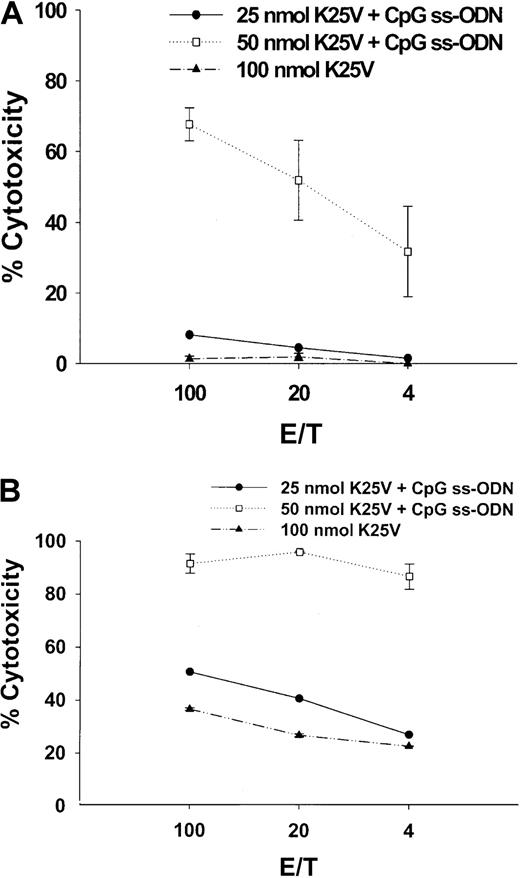
Intranasal immunization of K25V is effective in the presence of CpG ss-ODN
An investigation of mucosal immunization of fusion peptides was carried out using splenic lymphocytes to evaluate whether this route of administration resulted in systemic immunity. Past problems with introducing peptides by the intranasal route have been with the choice of adjuvant,28 since free peptides are generally not effective immunogens introduced by the mucosal route.59 It has been previously shown that CpG ss-ODN can be an effective mucosal adjuvant using protein immunogens.36 60 K25V was administered intranasally to Tg HLA A2.1/Kb mice, either alone or mixed with CpG ss-ODN as described in “Materials and methods.” A single dose of K25V at 25 or 50 nmol with CpG ss-ODN (Figure 5A) was compared to a booster of the same composition (Figure 5B) or to animals receiving either 1 (Figure 5A) or 2 (Figure 5B) doses of 100 nmol peptide without DNA. Animals were immunized for either 2 weeks (Figure 5A) or 3 weeks (Figure 5B), and subsequent IVSs and CRAs were carried out exactly as described for subcutaneous immunizations. The 25- or 50-nmol doses with DNA were effective at stimulating CTL, whereas even 2 100-nmol doses without DNA demonstrated less activity than 50-nmol treatment with DNA (Figure 5B). In contrast to the subcutaneous route (Figure 2A), there is a striking dependence on CpG ss-ODN of peptide delivered by the intranasal route.
Fusion peptides with CpG ss-ODN administered intranasally stimulate potent systemic immunity.
(A) Fusion peptide K25V, either 25 (●) or 50 nmol (■) mixed with 25 μg CpG ss-ODN or 100 nmol without ss-ODN (▴) was administered into the nares, as described in “Materials and methods.” Under anesthesia, 15 μL was introduced into each naris for a total of 30 μL total/mouse. (B) The same as panel A, except a booster of the identical amount of peptide and DNA as described for panel A was given 2 weeks after the first intranasal immunization. HLA A2.1/Kb mice were immunized for 2 (single) or 3 (booster) weeks, spleens removed, and 1 IVS was performed for 7 days. Afterward, a CRA was conducted as described in the legend to Figure 1 and in “Materials and methods.”
Fusion peptides with CpG ss-ODN administered intranasally stimulate potent systemic immunity.
(A) Fusion peptide K25V, either 25 (●) or 50 nmol (■) mixed with 25 μg CpG ss-ODN or 100 nmol without ss-ODN (▴) was administered into the nares, as described in “Materials and methods.” Under anesthesia, 15 μL was introduced into each naris for a total of 30 μL total/mouse. (B) The same as panel A, except a booster of the identical amount of peptide and DNA as described for panel A was given 2 weeks after the first intranasal immunization. HLA A2.1/Kb mice were immunized for 2 (single) or 3 (booster) weeks, spleens removed, and 1 IVS was performed for 7 days. Afterward, a CRA was conducted as described in the legend to Figure 1 and in “Materials and methods.”
Fusion peptide directs CTL recognition of endogenously processed pp65 protein
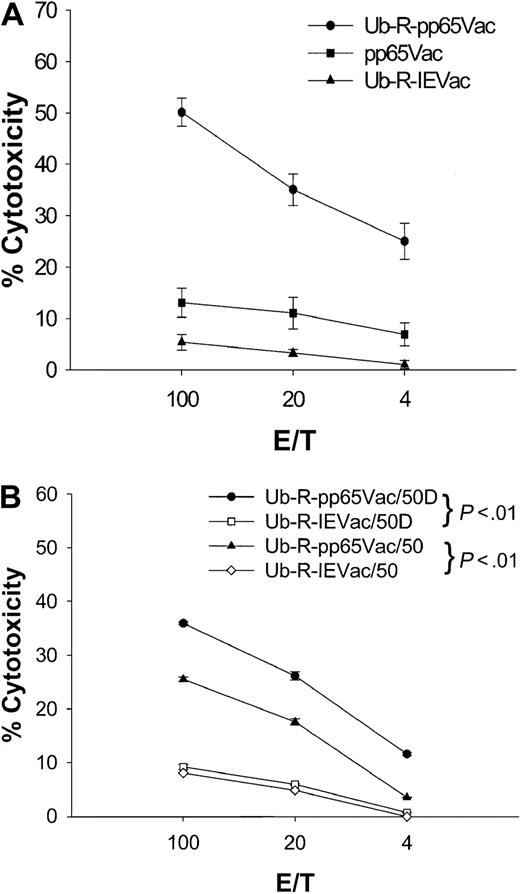
Because species specificity precludes the study of immune correlates of protection against human CMV in mice, an in vitro model to evaluate recognition of virally expressed pp65 was designed. The approach is to infect human Jurkat T cells stably expressing HLA A2.1 (JA2.1) with pp65Vac. In contrast to peptide-loaded T2 targets, virally infected targets are required to support TAP-dependent protein processing for successful recognition.46 A bulk spleen cell culture derived from a K25V immunization after repeated (5 ×) IVSs (Figure 1, 100 nmol) was used to evaluate the efficiency of recognition of pp65Vac. pp65Vac-infected JA2.1 targets were not recognized by this line (Figure 6A), whereas pp65495-503–loaded JA2.1 (data not shown) were recognized comparably to that of T2 cells (Figure 1, 100 nmol). It had been established that human pp65–specific CTL efficiently lyse pp65Vac-infected targets.13 This was further investigated, as we and others had previously observed a one-log reduction of the HLA class I transgene expression compared to the endogenous mouse class I MHC.15,61 This observation led Lemmonier and collaborators61 to construct a double knock-out Tg mouse devoid of H-2 Ia expression, which facilitated greater recognition of HLA A2.1/restricted antigens. To address this potential limitation of Tg HLA expression on the efficiency of presentation, a destabilized form of the pp65 protein was engineered according to the N-end rule model described by Varshavsky.62 This approach has been used by several groups to enhance the vaccine efficacy or recognition of viral proteins from HIV, influenza, or ovalbumin.42 63When JA2.1 infected with ubiquitinated pp65 is used as a target (Ub-R-pp65Vac), then a significant cytolytic response is detectable compared to the situation with unmodified pp65 (Figure 6A). The specificity of the response against pp65 has been confirmed by demonstrating minimal recognition of a nonspecific ubiquitinated protein from CMV (Figure 6A).
Destabilized pp65Vac enables recognition of APC by splenic effectors derived from fusion peptide immunizations.
(A) A bulk line was derived after repetitive IVSs (5 × ), as described in “Materials and methods,” from the 100-nmol immunization shown in Figure 1 that was a homogeneous CD8 T-cell population by flow cytometry (data not shown). A CRA was performed in which targets (JA2.1 T cells) were either infected with VV or pulsed with peptides (data not shown). JA2.1 cells were infected with Ub-R-pp65Vac (●) or pp65Vac (▪) for 16 hours at an MOI of 3. Nonspecific lysis is shown (Ub-R-IEVac, ▴) for VV-infected targets and was less than 5% for peptide-loaded T2 cells (data not shown). Error bars represent averages of 4 separate experiments carried out on different days. Details of the construction of Ub-R-pp65Vac are presented in “Materials and methods.” (B) HLA A2.1/Kbmice were immunized subcutaneously once with 50 nmol Tet639V alone (50) or with 25 μg CpG ss-ODN (50D) as described in the legend to Figure 3. Targets are either JA2.1 T cells infected with Ub-R-pp65Vac (● or ▵) or Ub-R-IEVac (▪ and ⋄) as described in the legend to Figure 1.
Destabilized pp65Vac enables recognition of APC by splenic effectors derived from fusion peptide immunizations.
(A) A bulk line was derived after repetitive IVSs (5 × ), as described in “Materials and methods,” from the 100-nmol immunization shown in Figure 1 that was a homogeneous CD8 T-cell population by flow cytometry (data not shown). A CRA was performed in which targets (JA2.1 T cells) were either infected with VV or pulsed with peptides (data not shown). JA2.1 cells were infected with Ub-R-pp65Vac (●) or pp65Vac (▪) for 16 hours at an MOI of 3. Nonspecific lysis is shown (Ub-R-IEVac, ▴) for VV-infected targets and was less than 5% for peptide-loaded T2 cells (data not shown). Error bars represent averages of 4 separate experiments carried out on different days. Details of the construction of Ub-R-pp65Vac are presented in “Materials and methods.” (B) HLA A2.1/Kbmice were immunized subcutaneously once with 50 nmol Tet639V alone (50) or with 25 μg CpG ss-ODN (50D) as described in the legend to Figure 3. Targets are either JA2.1 T cells infected with Ub-R-pp65Vac (● or ▵) or Ub-R-IEVac (▪ and ⋄) as described in the legend to Figure 1.
Primary immunization stimulates CTL that recognize virally expressed pp65
To evaluate whether full-length pp65 could be recognized by CTL stimulated by fusion peptide, an immunization schedule was carried out using 50 nmol of Tet639V alone or with 25 μg CpG ss-ODN administered subcutaneously. A CRA was carried out after a single primary immunization. As expected, peptide-specific responses were easily measured after one immunization for both preparations (Figure3C), but recognition of endogenously processed pp65 was also evident and more prominent with the preparation containing DNA (Figure 6B). These data confirm that fusion peptides delivered by the subcutaneous route stimulate CTL that recognize processed full-length pp65. A similar result was found when 50 nmol K25V and 25 or even 10 μg CpG ss-ODN were used to immunize by the subcutaneous or intranasal routes (data not shown). The addition of CpG ss-ODN had a major effect on recognition of full-length pp65, as shown in Figure 6B. This effect was also found after intranasal administration, since 100 nmol K25V gave a good peptide-specific response, but coadministered CpG ss-ODN was required to detect recognition of full-length pp65Vac (data not shown).
Discussion
We have shown that 3 alternative TH epitopes, together with an immunodominant CTL epitope from CMV-pp65 (HLA A2.1), can be successfully incorporated into a fusion peptide that is subject to further augmentation of its function by CpG ss-ODN. Using different Tg mice expressing HLA A*1101/Kb,64 a fusion peptide combining an HLA A*1101–restricted epitope from CMV-pp65 and PADRE were recognized equally well as K25V, and the responses were similarly enhanced with CpG ss-ODN4 (data not shown). We also reported that K25V fusion peptide and the HLA A*1101 derivative (data not shown) can be administered intranasally with CpG ss-ODN, and both compounds synergize to cause a powerful systemic immune response. The intranasal route will likely facilitate processing of the peptide by the mucosal immune system, although we have characterized only systemic immunity using splenocytes and not mucosal immune responses. What seems to be promising about the approach is that an acknowledged stimulator of TH1 responses, CpG ss-ODN synergizes with several different types of fusion peptides to augment their CTL-stimulating capacity (Table 1). The specificity of the CpG form was demonstrated by using a non-CpG form, which had minimal CTL-stimulating capacity beyond the peptide itself. Using the new technology of enumerating CD8 lymphocytes with HLA tetramers, we quantitated the frequency of pp65495-503 epitope-specific CTLs that were stimulated after immunization with fusion peptides and DNA. This experiment demonstrates that the cytotoxic activity measured by CRA can be correlated with the absolute frequency of CD8 lymphocytes enumerated by the epitope-specific HLA tetramer reagent. This method of analyzing immune response could be used with little modification to evaluate the stimulating capacity of the candidate peptide vaccine in clinical applications such as HCT or solid organ transplantation.
The function of a therapeutic CMV vaccine for HCT recipients is crucial in the time frame of immunoincompetence during the reconstitution phase, since there is an increased risk for developing CMV disease. In that context, we have examined durability of CTL memory to fusion peptide vaccines in immunocompetent Tg mice, with the finding that 50% of the original response level of a dilipidated form of K25V (Table 1) can be detected 6 months later (data not shown). Translation of these results clinically is difficult, because the Tg mice do not have a source of antigen to maintain the response, in contrast to patients who are infected with CMV. Therefore, even greater longevity of responsiveness may occur after peptide immunization of humans, especially since recent evidence suggests that CMV antigenemia drives the frequency of CMV-specific CTL monitored by HLA tetramers.65,66 Several authors have pointed out that prolongation of T-help responses is positively associated with maintenance of CMV-specific CTL.67,68 We have measured CD4 responses to fusion peptides, and their magnitude is substantial (stimulation index [SI] > 10) when using the fusion peptide as the recall antigen, in agreement with previous reports.14 The junction between the TH and CTL epitopes is a potent antigen as was pointed out by others.21 The critical question is whether noncognate CD4 TH will be an advantage in maintaining CMV-specific CTL, or is there a strict requirement for cognate TH? A definitive answer will be obtained when these fusion peptides are assessed clinically.
In the absence of a human infectious CMV protection model in immunocompetent mice, it is critical to demonstrate that naturally processed antigens from CMV will be recognized by CTLs that are stimulated by the epitope fusion vaccine.69 pp65 was modified into a form with enhanced degradation as a result of the finding that unmodified full-length pp65 was not efficiently recognized by epitope-specific murine CTL (Figure 6A). The necessity for this modification is the apparent difficulty in generating sufficient CTL epitope by the TAP-positive APC, since the T2 TAP-negative target is well-recognized when processed minimal peptide is provided (eg, pp65495-503). This might be the result of inefficient processing of the unmodified full-length protein or the 10-fold lower cell surface HLA A2.1 found on Tg mouse cells compared to endogenous MHC class I molecules (data not shown).61 Ubiquitination of pp65 coupled with destabilization through the use of an N-terminal Arg residue reduces the T½ of the protein to less than 20 minutes (Z.W. et al, in preparation). The change in T½ versus unmodified pp65 is more than 50-fold and is a possible explanation for the enhanced ability of targets that are infected with Ub-R-pp65Vac to present sufficient cognate CTL epitope to be recognized by murine CTL after fusion peptide immunization (Z.W. et al, in preparation). Enhanced recognition of target cells infected by Ub-R-pp65Vac versus unmodified pp65 was found after immunization with HLA A2.1 or A11 (data not shown) fusion peptide vaccines, making it unlikely that it is an artifact. The argument is bolstered by the finding that human CTL clones of 5 different haplotypes, which recognize pp65, lyse targets more efficiently when they are infected with Ub-R-pp65Vac versus unmodified pp65Vac (Z.W. et al, in preparation).
The results in this report provide a basis for the use of these fusion peptides in clinical immunization strategies. We and others have shown that minimal CTL epitope will expand human pp65–specific memory CTLs in vitro, and this report demonstrates the feasibility of conducting the immunization using the subcutaneous or intranasal routes in mice.39 The attractiveness of this strategy of peptide delivery without inflammatory adjuvant is that individuals such as HCT donors could easily tolerate immunization with these preparations, because both peptides and CpG DNA have limited toxicity compared to other oil- or mycobacterial-based adjuvants. One such approach is to amplify the CMV-pp65–specific memory CTL response in a donor of an HCT recipient before transplantation by administering one or more doses of peptide. As we have previously discussed, infusion of T-cell–replete bone marrow from an immunized donor with the usual “contamination” with mature T cells could provide an equivalent number of CMV-specific T cells as adoptive immunotherapy.9,13 The longevity of donor T cells transferred with either stem cells or bone marrow in the recipient to serve a protective role against CMV disease needs to be determined, especially in the context of steroid treatment of graft-versus-host disease.67,68 Substitution of ganciclovir prophylaxis and/or therapy with a vaccine approach could improve survival after HCT or organ transplant, because the adverse effects of antiviral chemotherapy would be eliminated. The increased incidence of late CMV disease might also be reversed, since delayed immune reconstitution as a result of the immunosuppressive properties of ganciclovir could be eliminated if the vaccine was found to replace antiviral chemotherapy.70 The goal of using fusion peptides in the clinic awaits cGMP production and evaluation in appropriate clinical trials.
We thank John A. Zaia for his continued support of our efforts in CMV vaccine development and for sharing reagents in support of our work. Robert F. Siliciano and Chris Buck are acknowledged for their gift of plasmids encoding ubiquitin genes. Morris Kelsey of the DTP, NCI is acknowledged for his guiding efforts toward production of clinical-grade peptide vaccine. The Bea and Edwin Wolfe Charitable Trust is gratefully acknowledged. Plasmids that encode HLA A2.1 tetramers were provided by Beckman-Coulter Immunomics (San Diego, CA). HLA-DR1 mice were a kind gift of D. Zaller (Merck, Sharpe & Dohme, Rahway, NJ). James Primus (Vanderbilt University, Nashville, TN) and EpImmune (La Jolla, CA) graciously provided HLA-A2.1/Kb and HLA-A11/Kb mice, respectively. The expert staff of the Animal Reserve Center (ARC) maintained the breeding colonies of mice that are crucial to completion of this work.
Supported by grants from the United States Public Health Service to D.J.D. (CA77544, CA30206-Project 3, AI44313, AI43267), SAIC subcontract #20XS192A, a Translational Research Award (6116-98) from the Leukemia and Lymphoma Society, and a Core grant to the City of Hope Cancer Center (CA33572). D.J.D. is the recipient of a Rapid Access to Intervention Development (RAID) award from Developmental Therapeutics Program (DTP), National Cancer Institute (NCI), which has provided all forms of the fusion peptides from various contractors.
Submitted March 27, 2002; accepted June 21, 2002. Prepublished online as Blood First Edition Paper, July 12, 2002; DOI 10.1182/blood-2002-03-0926.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Don J. Diamond, Laboratory of Vaccine Research, Fox South, Beckman Research Institute of the City of Hope, Duarte, CA 91010; e-mail: ddiamond@coh.org.

![Fig. 1. K25V administered subcutaneously without adjuvant is immunogenic in Tg mice. / K25V was dissolved at 5 mM in 90% N-saline/10% DMSO and diluted in N-saline to deliver the amount of peptide shown on the x-axis. Tg HLA A2.1/Kb [n = 6 (150 nmol), 14 (100 nmol), 8 (50 nmol), and 2 (10 and 25 nmol)] mice were immunized once subcutaneously at the base of the tail with peptide and no additional adjuvant. After 2 weeks, spleens were harvested, and IVS were done as described in “Materials and methods.” Targets were T2 cells loaded with specific (pp65495-503, filled symbols) and nonspecific (p53149-157, open symbols) peptides as described in “Materials and methods.” Means and SE were calculated at each effector-target (E/T) ratio for all evaluated mice, and significantP values are indicated.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/10/10.1182_blood-2002-03-0926/4/m_h82223412001.jpeg?Expires=1765886521&Signature=iKmttpUTPLPeKVdVhwP7BSBjFDqsPXRpdi7QoGh904nz5gc14KA4JbyeqmTCp08HyU2e11H500DfH4fIFBPfe3vu3fDXk0TSzsJDNCl0u8YNjz-dGD-IYC73O11AiIswfaDMQYcQOplhxh0ecITzES6mPT8i93LHC4kW7rzSHrRMRoLbXitQrPzd-J2a3Mqg6JZFcNbsyY5A~v9ZI-X4bWbuv9gAhjE0r6Gm2TcZOfGg~8fgEGfY64O~s7XJ9Ffl4XwLdNTuE9XnHj1G5D9~JRVl3ayxYLb3GtGY3kN7JIN5x5xANolmUCqDMNA9bH1S0odahneAkpoQ2JoIC-im4A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal