Airway dendritic cells (DCs) are held responsible for inducing sensitization to inhaled antigen, leading to eosinophilic airway inflammation, typical of asthma. However, less information is available about the role of these cells in ongoing inflammation. In a mouse model of asthma, sensitization to ovalbumin (OVA) was induced by intratracheal injection of myeloid OVA-pulsed DCs. Upon OVA aerosol challenge and induction of eosinophilic airway inflammation in sensitized mice, there was a time-dependent and almost 100-fold increase in the number of MHCII+ CD11b+CD11c+ endogenous airway DCs as well as CD11b+blood DCs. The mechanism of this increase was studied. Adoptive transfer experiments demonstrated that accumulation of airway DCs was not due to reduced migration to the mediastinal lymph nodes. Rather, the massive increase in airway and lymph node DCs was supported by an almost 3-fold expansion of myeloid CD31hiLy-6Cneg hematopoietic precursor cells in the bone marrow (BM). There was no change in any of the other 5 populations revealed by CD31/Ly-6C staining. When these CD31hiLy-6Cneg BM precursors were sorted and grown in granulocyte macrophage–colony-stimulating factor, they differentiated into MHCII+ CD11c+ DCs. The same CD31hiLy-6Cneg precursors also expressed the eotaxin receptor CCR3 and differentiated into eosinophils when grown in interleukin 5. Serum levels of eotaxin were doubled in mice with inflammation. These findings in an animal model of asthma suggest that the BM increases its output of myeloid precursors to meet the enhanced demand for DCs and eosinophils in inflamed airways.
Introduction
The role of the dendritic cell (DC) as a professional antigen-presenting cell (APC) in the primary immune response is now well established.1 Airway DCs form a network in the epithelium, capture inhaled antigen (Ag), and migrate to the mediastinal lymph nodes (MLN) where Ag is presented to recirculating naive T cells.2-5 Not surprisingly, these cells have been implicated to cause sensitization to inhaled allergens, typical of Th2-mediated allergic asthma. In a mouse model of asthma, intratracheal immunization with ovalbumin (OVA)–pulsed DCs generates Th2 effector cells that control eosinophilic airway inflammation, goblet cell hyperplasia, and bronchial hyperreactivity upon repeated challenge with OVA aerosol.6,7 These observations indicate that airway DCs are essential in the early steps of sensitization. However, less information is available on the role of DCs in stimulating memory and/or effector Th2 cells upon repeated encounter with allergens.8 In patients with stable allergic asthma, the number of airway DCs is elevated compared with healthy controls, and local allergen challenge leads to rapid accumulation of CD1a+ HLA-DR+ DCs in the airway lamina propria, suggesting that DCs also present allergens to T cells in the secondary immune response leading to airway inflammation.9
The mechanisms by which DC numbers increase in asthmatic airways include several not mutually exclusive possibilities. First, the increase could be caused by enhanced recruitment of DCs from the bloodstream into the site of airway inflammation. To support an enhanced demand for DCs in the inflamed airways, the bone marrow (BM) might enhance its output of DCs or DC progenitors. Such a mechanism would be similar to the enhanced recruitment of eosinophils into sites of allergic inflammation, supported by a release of eosinophilic progenitors from the BM.10-14 Second, enhanced differentiation of freshly recruited monocytes into DCs could also lead to increased numbers of DCs being found at sites of airway inflammation.15 In such a scenario, one would expect to find enhanced production of DC differentiation and/or growth factors within the lung. Finally, as there is continuous and high throughput migration of airway DCs from the epithelium to the draining MLNs, a small decrease of DC efflux could lead to rapid and profound accumulation of DCs within the epithelium.5,16 To study which of these mechanisms might predominate, we have used a DC-driven mouse model of asthma.6
Materials and methods
Animals
All experiments were performed with 8- to 10-week-old female Balb/c (H-2d) mice (Harlan, Zeist, The Netherlands). Mice were housed under specific pathogen-free conditions at the animal care facility at the Erasmus University Rotterdam. All of the experimental procedures used in this study were approved by the Erasmus University Committee of Animal Experiments.
Murine model of asthma
To induce sensitization to inhaled OVA, BM-derived DCs were pulsed with OVA in vitro and subsequently injected into the airways of naive mice.6 In short, BM cells were cultured for 10 days in tissue culture medium (TCM) (5% fetal calf serum [FCS] from Biocell Laboratories, Rancho Dominguez, CA; RPMI 1640, gentamycine, β-mercaptoethanol, all from Gibco BRL, Paisley, Scotland) supplemented with 20 ng/mL recombinant murine (rm) granulocyte macrophage–colony-stimulating factor (GM-CSF).17 After 9 days of culture, cells were pulsed overnight with 100 μg/mL OVA (OVA-DC) (OVA, grade V; Sigma Chemical, St Louis, MO). On day 10 of culture, cells were collected, washed, and 1 × 106 DCs were injected intratracheally in naive mice. Control mice received identical numbers of unpulsed DCs (PBS-DCs). Ten days after immunization, mice were challenged with OVA aerosol (grade III, 1% wt/vol in phosphate buffered saline [PBS], Sigma) or with PBS aerosol during a 30-minute challenge per day for 1, 3, or 7 consecutive days. In separate experiments, an additional control group consisted of naive unmanipulated mice.
Detection of airway dendritic cells in whole mounts of the trachea
At 24 hours after the last OVA challenge, animals were anesthetized and tracheal whole mounts were prepared as described earlier with a modification that the secondary antibody, used to detect rat anti–mouse major histocompatibility complex II (MHCII), was goat anti–rat F(ab′)2 fragments conjugated to horseradish peroxidase (Serotec, Oxford, United Kingdom).18 The entire trachea was mounted in Entellan (Merck, Darmstadt, Germany) and viewed under a transmission light microscope equipped with Nomarski optics (Leica, Cambridge, United Kingdom).
Collection of cells and tissues
Bronchoalveolaire lavage fluid.
At 24 hours after the last aerosol, groups of mice were killed by avertin overdose followed by exsanguination. Bronchoalveolaire lavage (BAL) was performed with 3 × 1 mL Ca2+- and Mg2+-free PBS supplemented with 0.1 mM ethylenediaminetetraacetic acid (EDTA). After red blood cells (RBCs) were lysed using ammoniumchloride lysis buffer, cytospin slides were prepared and remaining cells were used for flow cytometric analysis. Supernatants of BAL fluid (BALF) and serum were stored for enzyme-linked immunosorbent assay (ELISA) quantification of GM-CSF, interleukin 6 (IL-6) (OptEIA, PharMingen, Becton Dickinson, San Diego, CA; threshold 8 pg/mL), eotaxin, and fms-like tyrosine kinase 3 ligand (Flt-3L) (R&D Systems, Abingdon, United Kingdom; threshold 5 pg/mL).
Lymph nodes.
Lymph node (LN) cell suspensions were prepared by a 1-hour incubation at 37°C with 0.02 mg/mL DNAse I (Sigma Chemical) and 100 U/mL Collagenase IV (Life Technologies). RBCs were lysed and cells were passed through a 40-μm cell sieve (Becton Dickinson).
Blood.
Blood was collected in heparinized tubes from the iliac artery and lysed with 20 mL RBC lysis solution for 4 minutes at 4°C.
Bone marrow.
BM cells were prepared by flushing femurs and tibiae with 5 mL sterile PBS, followed by RBC lysis and passage through a 100-μm cell sieve.
Staining for major basic protein-positive eosinophils
Cytospin preparations of BALF and cultured BM were acetone fixed and blocked with 1% bovine serum albumin/PBS. Major basic protein (MBP) was detected using a rabbit anti–mouse MBP antibody (Ab) (J. J. Lee, Mayo Clinic, Scottsdale, AZ), followed by alkaline phosphatase–conjugated goat anti–rabbit Abs (Sigma) and development of signal with New Fuchsin in Tris-HCl. Slides were counterstained with Mayer haematoxilin (Merck). One investigator counted all cells.
Flow cytometric analysis on BALF, LN, and blood cells
Cells were washed in PBS containing 5% FCS and 5 mM sodium azide (FACS wash), and 1 × 106 cells were stained for 30 minutes on ice. To reduce nonspecific binding, cells were incubated with 2.4G2 blocking reagent for 15 minutes. Monoclonals used were as follows: MHCII–fluorescein isothiocyanate (MHCII-FITC) (2G9), allophycocyanin (APC)–labeled CD11c (HL3), phycoerythrin (PE)–labeled Abs against CD3 (145-2C11), B220 (RA3-6B2), and CCR3 (R&D Systems), biotin-labeled CD11b (M1/70), followed by streptavidin (SA)–PE-Cy5 (Quantum Red; Sigma). Propidium iodide (Sigma) was added for exclusion of dead cells before analysis on a FACScalibur flow cytometer using CellQuest (Becton Dickinson Immunocytometry Systems, San Jose, CA) and FlowJo software (Treestar, Costa Mesa, CA).
Flow cytometric analysis and sorting of BM cells
BM cells were stained with anti-CD31 (anti–platelet endothelial cell adhesion molecule PECAM-1; ER-MP12-bio), and anti–Ly-6C (ER-MP20-FITC; both produced in-house) followed by SA-PE or SA-PE-Cy5, allowing the discrimination of 6 distinct populations of cells.19-22 For phenotype description the following mAbs were used: CD11b-APC, CD127-PE (IL-7Rα; SB/14), CD131-PE (JORO50), CCR3-PE, CD3-PECy5 and CD4-APC (GK1.5), CD11c-APC and B220-PECy5, or Gr1-PE (RB6-8C5). Antibodies were from PharMingen or R&D Systems.
In separate sorting experiments, 80 × 106 cells were stained with CD31-bio followed by SA-PE and Ly-6C-FITC and were sorted into CD31hiLy-6Cneg and into CD31negLy-6Cmed populations under sterile conditions on a FACS Vantage flow cytometer (Becton Dickinson).
Culture of CD31hiLy-6Cneg and CD31negLy-6Cmed populations
After sorting, cells were washed twice in TCM and cultured for 7 days at 0.25 × 106/well in 24-well plates with 40 ng GM-CSF per milliliter to induce DC differentiation. In separate experiments, cells were grown at 8 × 105 cells/well in round-bottom 96-well plates in TCM supplemented with 30% FCS and 24 ng/mL murine recombinant IL-5 (rIL-5) (PharMingen) to induce eosinophil differentiation. As a control, unsorted BM was stained and cultured under identical conditions. After 7 days, cells were analyzed for expression of MHCII-FITC, CD11c-bi (N418) in combination with CD80-PE (16-10A1), CD86-PE (GL-1), or CD40-PE (3/23), followed by SA-PECy5. For eosinophil differentiation, cytospin preparations were stained with an anti–MBP Ab as described above.
Detection of labeled DCs after adoptive transfer
BM DCs were labeled using carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes, Eugene, OR), as previously described.6 CFSE+ DCs (2-5 × 106) were transferred intratracheally into mice with established eosinophilic airway inflammation (OVA-DC/3xOVA) or into control mice (PBS-DC/3xPBS). At 48 hours after the intratracheal cell transfer, mice were killed. The number of CD11c+, CFSE+ DCs was determined on BALF, MLN, and inguinal LN samples.
Statistical analysis
All experiments were performed using 3 to 10 mice per group, and per time point in kinetic experiments. Comparison of means between different groups was performed with a Kruskal-Wallis test for equality among the different groups and in the case of a significant difference, the Mann-Whitney test for unpaired data was used for comparing 2 groups (SPSS 10.0 for Windows) separately. Differences were considered significant if P < .05.
Results
OVA exposure time-dependently induces eosinophilic airway inflammation in OVA-DC–immunized mice
Sensitization was induced by intratracheal injection of 1 × 106 OVA-pulsed DCs. As a marker for inflammation in the lungs, the total number of BAL cells was measured 24 hours after 1, 3, or 7 OVA aerosol exposures in sensitized mice (Figure1A). The total recovery of BAL cells was not different from control mice (PBS-DC/PBS) after 1 OVA exposure, but sequentially increased 10-fold (P = .008) after 3 OVA exposures and 46-fold (P = .008) after 7 OVA exposures.
Effect of OVA or PBS aerosol challenge on cellular composition of BALF.
Mice were immunized on day 0 with 1 × 106 OVA-DCs or PBS-DCs. From day 10 they were challenged daily for 30 minutes on 1, 3, or 7 consecutive days with OVA or PBS aerosols. (A) Total recovery of BALF cells as a marker of airway inflammation in response to aerosol exposures (*P < .05 compared with PBS-DC/PBS). Data are expressed as mean number of cells ± SEM. (B) Differential analysis of BALF cellular content based on flow cytometric analysis. The BALF composition of naive mice is included for reference. (C) Staining of BALF cytospin preparation with an Ab specific for murine MBP, identifying eosinophils in an OVA-DC immunized animal exposed 7 times to OVA aerosol. Isotype control Ab gave negative results (not shown). Original magnification, × 400. (D) Cells expressing high granularity on SSC signal (see gate) express the CCR3 receptor (filled histogram), identifying them as eosinophils. Isotype control is indicated by the open histogram.
Effect of OVA or PBS aerosol challenge on cellular composition of BALF.
Mice were immunized on day 0 with 1 × 106 OVA-DCs or PBS-DCs. From day 10 they were challenged daily for 30 minutes on 1, 3, or 7 consecutive days with OVA or PBS aerosols. (A) Total recovery of BALF cells as a marker of airway inflammation in response to aerosol exposures (*P < .05 compared with PBS-DC/PBS). Data are expressed as mean number of cells ± SEM. (B) Differential analysis of BALF cellular content based on flow cytometric analysis. The BALF composition of naive mice is included for reference. (C) Staining of BALF cytospin preparation with an Ab specific for murine MBP, identifying eosinophils in an OVA-DC immunized animal exposed 7 times to OVA aerosol. Isotype control Ab gave negative results (not shown). Original magnification, × 400. (D) Cells expressing high granularity on SSC signal (see gate) express the CCR3 receptor (filled histogram), identifying them as eosinophils. Isotype control is indicated by the open histogram.
Differential analysis of the BALF cells using flow cytometry (Figure1B) showed a significant proportional increase in lymphocytes (CD3+ or B220+ cells) and granulocytes (based on scatter characteristics) with a concomitant decrease in alveolar macrophages/monocytes (highly autofluorescent cells) in OVA-DC/OVA mice (Figure 2A). As the discrimination of eosinophils from other polymorphonuclear granulocytes is impossible based on scatter characteristics alone, eosinophils were further characterized as nonautofluorescent highly granular (SSChi) cells expressing intermediate levels of CD11c, and lacking expression of MHCII, B220, and CD3.23 These highly granular cells also expressed the eotaxin receptor CCR3 (Figure 1D).24This method of counting eosinophils was compared with counting BALF cytospins stained with an anti–MBP Ab, yielding a highly statistically significant Pearson correlation coefficient of 0.82 (P = .0001) (Figure 1C). Performing 3 OVA aerosols induced an eosinophilia of 30.7 ± 6.0%, and 7 OVA aerosols induced an eosinophilia of 49.0% ± 5.1 of all BALF cells. Thus, OVA exposure in OVA-DC–immunized mice time-dependently induces eosinophilic airway inflammation.
Effect of OVA or PBS aerosol challenge on the number of DCs in the BALF.
Mice were immunized on day 0 with 1 × 106 OVA-DCs or PBS-DCs. From day 10 they were challenged daily for 30 minutes on 1, 3, or 7 consecutive days with OVA or PBS aerosols. (A) In OVA-DC/OVA mice (ii and iv), the FSC/SSC plot contains lymphoid cells (L), and granulocytes (G). In contrast, in PBS-DC/PBS mice (i and iii) the majority of cells are large and spontaneously autofluorescent, representing alveolar macrophages (M). A gate was set (iii and iv) on low autofluorescent cells that lacked expression of CD3 and B220. (B) Within the set gate, MHCIIhi CD11chi cells represent DCs, whereas CD11cdim MHCII− cells represent eosinophils (Eo). In our experiments, eosinophils did not express MHCII molecules. The average percentage of MHCIIhiCD11chi DCs as a percentage of total cells analyzed is indicated in the plot. (C) Kinetics of increase of DCs following OVA exposure as expressed as the absolute number of MHCIIhiCD11chi cells within the BALF (n = 5 animals per group; *P < .05 compared with PBS-DC/PBS group). Data are expressed as mean number of MHCII+/CD11c+ DCs ± SEM. (D) Top panel: gated CD11c+ MHCII+ DCs are of myeloid lineage as revealed by strong staining for CD11b (filled histogram); isotype control is indicated by the open histogram. Bottom panel: gated MHCII+CD11c+ DCs (filled histogram) do not express the eosinophil marker CCR3, whereas gated CD11cdim granular eosinophils (open histogram) clearly do.
Effect of OVA or PBS aerosol challenge on the number of DCs in the BALF.
Mice were immunized on day 0 with 1 × 106 OVA-DCs or PBS-DCs. From day 10 they were challenged daily for 30 minutes on 1, 3, or 7 consecutive days with OVA or PBS aerosols. (A) In OVA-DC/OVA mice (ii and iv), the FSC/SSC plot contains lymphoid cells (L), and granulocytes (G). In contrast, in PBS-DC/PBS mice (i and iii) the majority of cells are large and spontaneously autofluorescent, representing alveolar macrophages (M). A gate was set (iii and iv) on low autofluorescent cells that lacked expression of CD3 and B220. (B) Within the set gate, MHCIIhi CD11chi cells represent DCs, whereas CD11cdim MHCII− cells represent eosinophils (Eo). In our experiments, eosinophils did not express MHCII molecules. The average percentage of MHCIIhiCD11chi DCs as a percentage of total cells analyzed is indicated in the plot. (C) Kinetics of increase of DCs following OVA exposure as expressed as the absolute number of MHCIIhiCD11chi cells within the BALF (n = 5 animals per group; *P < .05 compared with PBS-DC/PBS group). Data are expressed as mean number of MHCII+/CD11c+ DCs ± SEM. (D) Top panel: gated CD11c+ MHCII+ DCs are of myeloid lineage as revealed by strong staining for CD11b (filled histogram); isotype control is indicated by the open histogram. Bottom panel: gated MHCII+CD11c+ DCs (filled histogram) do not express the eosinophil marker CCR3, whereas gated CD11cdim granular eosinophils (open histogram) clearly do.
OVA exposure leads to a massive increase in endogenous airway DCs in OVA-sensitized mice
To determine the number of DCs in the airways of PBS- and OVA-exposed mice, we have analyzed BALF cells 24 hours after the last OVA aerosol (Figure 2A). Dendritic cells were identified with multiparameter flow cytometry as nonautofluorescent CD11chi/MHCIIhi/B220−/CD3−cells, as described previously.3 Additional staining revealed that these cells expressed CD11b, identifying them as myeloid DCs (Figure 2D). First we verified that injected DCs could no longer be recovered from the BALF 5 days following intratracheal injection (data not shown). This eliminates the possibility that nonendogenous DCs still remaining in the BALF could confound the counting of DCs after the aerosol challenge period (day 11 to day 17 after injection). The absolute number of DCs was elevated about 10 times in OVA-DC–immunized mice challenged with 3 OVA aerosols (P = .008) and increased about 100 times after 7 OVA aerosols (P = .016) compared with control PBS-DC/PBS mice (Figure 2B). In addition to an absolute increase in cell number, the percentage of DCs found in BALF cells was similarly increased following 3 aerosols, although not significantly (P = .056), and doubled after 7 aerosols (P = .016). In control mice the number of DCs remained at low levels, comparable to the situation in unmanipulated mice.
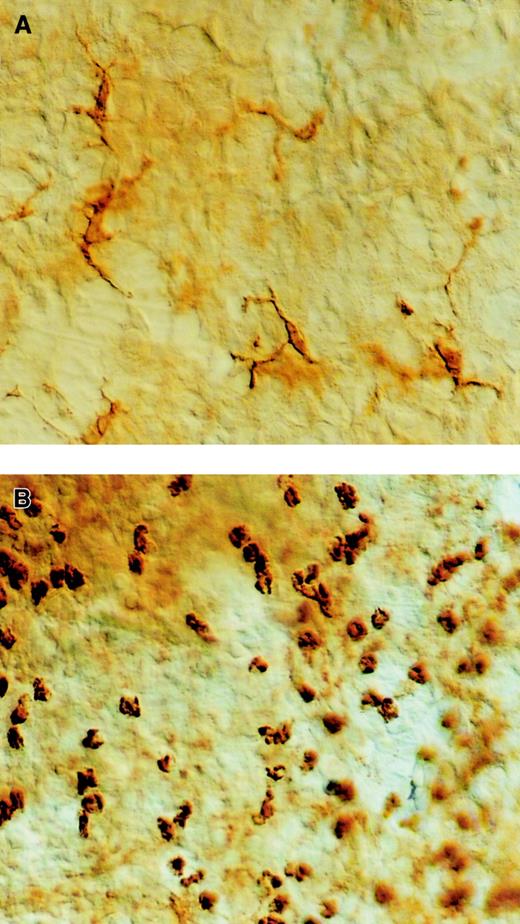
To determine if the increase in the number of BALF DCs was also supported by an increase in airway mucosal DCs, we visualized the DC network in tracheal whole mounts, as previously described.18 The pattern of the MHCII staining revealed a dendritic network in the control PBS-DC/PBS mice (Figure3A), whereas in the OVA-DC–immunized mice exposed to 3 OVA aerosols, numerous dense areas of rounded MHCII+ cells lacking the typical dendritic morphology were seen (Figure 3B). Due to the fact that MHCII+ cells were rounded and could also represent MHCII+ B cells or eosinophils, we could not directly compare DC numbers at the tissue level, although clearly, overall MHCII staining was enhanced.
Effect of OVA or PBS aerosol challenge on the number of DCs in the large conducting airways.
Mice were immunized with OVA-DCs or PBS-DCs and challenged daily with either OVA or PBS aerosol. Thereafter, whole mounts were prepared and stained with an Ab directed against I-A and I-E MHCII. (A) In PBS-DC/PBS-exposed animals nearly all MHCII+ cells demonstrate a dendritic morphology. (B) In contrast, in OVA-DC/OVA mice the MHCII+ cells are more numerous, and have a rounded appearance. They are distributed in dense clusters in the intercartilaginous area. Original magnification, × 400, Normaski optics.
Effect of OVA or PBS aerosol challenge on the number of DCs in the large conducting airways.
Mice were immunized with OVA-DCs or PBS-DCs and challenged daily with either OVA or PBS aerosol. Thereafter, whole mounts were prepared and stained with an Ab directed against I-A and I-E MHCII. (A) In PBS-DC/PBS-exposed animals nearly all MHCII+ cells demonstrate a dendritic morphology. (B) In contrast, in OVA-DC/OVA mice the MHCII+ cells are more numerous, and have a rounded appearance. They are distributed in dense clusters in the intercartilaginous area. Original magnification, × 400, Normaski optics.
OVA exposure leads to an increase in peripheral blood DCs in OVA-sensitized mice
To investigate if the accumulation of lung CD11c+CD11b+ DCs was supported by recruitment from the bloodstream, the number of DCs (CD11c+/MHCII+/B220−/CD3−cells) was determined in the blood 24 hours after the last of 3 aerosols. The percentage of MHCII+ CD11c+ blood DCs was significantly raised (OVA 0.81 ± 0.09% vs PBS 0.37 ± 0.03 vs naive 0.39 ± 0.03, P < .0001) in response to OVA challenges in OVA-DC mice (Figure4A,C).
Effect of OVA or PBS aerosol challenge on the percentage of blood DCs.
Mice were immunized with OVA-DCs or PBS-DCs and challenged daily with either OVA or PBS aerosol. (A) A gate was set on MHCII+cells within CD3−B220− cells. Numbers represent the mean percentage of cells within this gate. (B) Within the MHCII+ gate, CD11c+ cells represent DCs. Additional staining involved CD11b to discriminate myeloid (CD11b+) versus lymphoid (CD11b−) DCs. Numbers indicate the mean percentage of the population as percent of total cells within lysed blood cells (n = 9 per group). (C) Quantitative summary from unmanipulated naive mice, PBS-DC/PBS animals, and OVA-DC/OVA animals (*P < .05 compared with the naive and PBS-DC/PBS groups). Data are expressed as mean % blood DCs ± SEM of 1 experiment and are representative of multiple experiments.
Effect of OVA or PBS aerosol challenge on the percentage of blood DCs.
Mice were immunized with OVA-DCs or PBS-DCs and challenged daily with either OVA or PBS aerosol. (A) A gate was set on MHCII+cells within CD3−B220− cells. Numbers represent the mean percentage of cells within this gate. (B) Within the MHCII+ gate, CD11c+ cells represent DCs. Additional staining involved CD11b to discriminate myeloid (CD11b+) versus lymphoid (CD11b−) DCs. Numbers indicate the mean percentage of the population as percent of total cells within lysed blood cells (n = 9 per group). (C) Quantitative summary from unmanipulated naive mice, PBS-DC/PBS animals, and OVA-DC/OVA animals (*P < .05 compared with the naive and PBS-DC/PBS groups). Data are expressed as mean % blood DCs ± SEM of 1 experiment and are representative of multiple experiments.
In the blood of the control PBS-DC/PBS–immunized mice, levels were comparable with those found in untreated animals. Additional experiments were carried out to define the CD11b+ myeloid and CD11b− lymphoid population within the CD11c+ MHCII+B220−CD3− population of blood cells (Figure 4B-C). It appeared that the percentage of blood CD11b+myeloid DCs was significantly elevated in OVA-DC/OVA mice compared with PBS-DC/PBS mice (OVA 0.6 ± 0.066% vs PBS 0.231 ± 0.022%,P < .0001). In the blood of OVA-DC/OVA mice, the percentage of MHC II+ CD11b+CD11c− cells (putative monocytes) was also raised significantly over the control mice (1.00 ± 0.30% vs 0.30 ± 0.03%, P < .0001011).
OVA exposure leads to an increase in CD31hiLy-6Cneg BM cells in OVA-sensitized mice
The increased presence of CD11c+ DCs in the bloodstream during OVA challenge, despite the massive influx of DCs into the airways, suggested that DC output from the BM might be enhanced.
Staining of whole BM cells with the monoclonal Abs CD31 (ER-MP12) and Ly-6C (ER-MP20), gives rise to 6 distinct populations of BM cells, each with varying degrees of lineage commitment and progenitor potential (see Figure 5A).19 This staining was used to define the cellular composition of BM in mice with or without eosinophilic airway inflammation. Performing 1, 3, or 7 OVA aerosol exposures to OVA-DC mice sequentially induced an increase in the CD31hiLy-6Cneg population, from 4.61 ± 0.75% of total BM at baseline up to 7.84 ± 1.25% after 3 aerosols and 11.1 ± 1.48% after 7 aerosols (Figure 5A-B). There was no increase in this population after exposure to PBS aerosols. None of the 5 other distinct populations was altered significantly by OVA aerosol in OVA-DC mice or by PBS aerosol in PBS-DC mice.
Effect of OVA or PBS aerosol exposure on the cellular composition of BM.
OVA-DC– or PBS-DC–immunized mice were challenged with either 1 ×, 3 ×, or 7 × daily OVA or PBS aerosols. At 24 hours after the last challenge, BM was collected and stained for Ly-6C and CD31. (A) Using this combination of markers, 6 distinct populations can be identified. Morphologically, these populations consist of: (a) 70% blast cells and 25% lymphoid cells; (b) lymphoid cells; (c) erythroid cells; (d) myeloid progenitors and plasmacytoid cells; (e) granulocytes; (f) 75% monocytes and 20% myeloid progenitors.19 Plots represent Ly-6C/CD31 staining on BM cells taken from mice exposed 7 times to PBS or OVA aerosol. There is a clear and selective increase in the CD31hiLy-6Cneg subset in the OVA-DC/OVA group. Percentages of each population are indicated below the FACS plots. (B) Kinetics and magnitude of increase in the CD31hiLy-6Cneg BM subset following OVA or PBS challenge (n = 6-8 per group per time point, * P < .05 compared with PBS-DC/PBS). Data are expressed as mean % DC precursors ± SEM. (C) Additional staining included CD11c and B220 to delineate plasmacytoid DCs. Plots were gated on CD31hiLy-6Chi cells (population d) in PBS-DC/PBS and OVA-DC/OVA mice. Average percentage of CD11c+ B220+ within the gate is indicated.
Effect of OVA or PBS aerosol exposure on the cellular composition of BM.
OVA-DC– or PBS-DC–immunized mice were challenged with either 1 ×, 3 ×, or 7 × daily OVA or PBS aerosols. At 24 hours after the last challenge, BM was collected and stained for Ly-6C and CD31. (A) Using this combination of markers, 6 distinct populations can be identified. Morphologically, these populations consist of: (a) 70% blast cells and 25% lymphoid cells; (b) lymphoid cells; (c) erythroid cells; (d) myeloid progenitors and plasmacytoid cells; (e) granulocytes; (f) 75% monocytes and 20% myeloid progenitors.19 Plots represent Ly-6C/CD31 staining on BM cells taken from mice exposed 7 times to PBS or OVA aerosol. There is a clear and selective increase in the CD31hiLy-6Cneg subset in the OVA-DC/OVA group. Percentages of each population are indicated below the FACS plots. (B) Kinetics and magnitude of increase in the CD31hiLy-6Cneg BM subset following OVA or PBS challenge (n = 6-8 per group per time point, * P < .05 compared with PBS-DC/PBS). Data are expressed as mean % DC precursors ± SEM. (C) Additional staining included CD11c and B220 to delineate plasmacytoid DCs. Plots were gated on CD31hiLy-6Chi cells (population d) in PBS-DC/PBS and OVA-DC/OVA mice. Average percentage of CD11c+ B220+ within the gate is indicated.
To explore whether the increase of the CD31hiLy-6Cneg population was due to an increase of cells with myeloid or lymphoid commitment, additional staining was performed using the myeloid differentiation marker CD11b in combination with a CD127 Ab against the IL-7Rα chain, identifying cells with lymphoid commitment.25 26 Within the CD31hiLy-6Cneg subset, cells expressed CD11b or CD127 exclusively. After 4 aerosols, the percentage of CD11b+ cells in this subset remained constant, whereas that of CD11b−CD127+ lymphoid-committed cells was 9.5 ± 1.4% in PBS-DC/PBS mice, compared with 6.1 ± 0.4% in OVA-DC/OVA mice, indicating a small but significant (P = .004) decrease of cells with lymphoid differentiation (potential). Cells were also stained with an Ab against CD131, the common β chain of the IL-3/IL-5/GM-CSF receptor. The CD31hiLy-6Cneg subset had the highest expression compared with the other populations or whole BM. There was no difference in CD131 expression of the CD31hiLy-6Cneg subset between the PBS and the OVA exposed group.
The CD31hiLy-6Cneg BM subset gives rise to DCs and eosinophils under different culture conditions
As the CD31hiLy-6Cneg subset of cells was the only population that was increased in the BM of mice with eosinophilic airway inflammation, we examined if this subset could give rise to DCs. The CD31hiLy-6Cneg population was purified by flow cytometric sorting to 85% to 95% purity, and cultured in GM-CSF (40 ng/mL) (Figure6).
CD31hiLy-6Cneg cells give rise to DCs following culture in GM-CSF.
OVA-DC– or PBS-DC–immunized mice were challenged daily for 3 days with OVA or PBS aerosols. At 24 hours after the last challenge, BM was collected and stained with CD31 and Ly-6C antibodies. Plots shown are representative of mice within the OVA-DC/OVA group. The CD31hiLy-6Cneg and the CD31negLy-6Cmed populations were purified using flow cytometric sorting (middle panels) and subsequently cultured in the presence of GM-CSF for 7 days. The lower panels represent FACS plots of cells at the end of the culture period stained for MHCII and CD11c. Immature DCs are CD11c+ MHCII− and mature DCs are CD11c+ MHC II+.
CD31hiLy-6Cneg cells give rise to DCs following culture in GM-CSF.
OVA-DC– or PBS-DC–immunized mice were challenged daily for 3 days with OVA or PBS aerosols. At 24 hours after the last challenge, BM was collected and stained with CD31 and Ly-6C antibodies. Plots shown are representative of mice within the OVA-DC/OVA group. The CD31hiLy-6Cneg and the CD31negLy-6Cmed populations were purified using flow cytometric sorting (middle panels) and subsequently cultured in the presence of GM-CSF for 7 days. The lower panels represent FACS plots of cells at the end of the culture period stained for MHCII and CD11c. Immature DCs are CD11c+ MHCII− and mature DCs are CD11c+ MHC II+.
After 7 days culture in the presence of GM-CSF, many colonies of proliferating cells were seen. About 77% of cells were CD11c+ and more than half of these expressed MHCII, indicating maturation in culture (Figure 6C). In contrast, the CD31negLy-6Cmed population was sorted and cultured under the same conditions and yielded only 2.2% MHCII+/CD11c+cells.
Airway eosinophilia is a prominent feature of allergic airway inflammation and was also observed in our model. Therefore, we investigated whether the same CD31hiLy-6Cnegpopulation contained eosinophil precursors. First, to support the concept that cells with eosinophil potential were contained within the CD31hiLy-6Cneg population, bone marrow subsets were stained with a monoclonal antibody against the eotaxin receptor CCR3.24 Cells within this subset expressed CCR3 at intermediate levels (see Figure 7A). Mature granulocytes contained within the CD31negLy-6Cmed also contained CCR3hi mature eosinophils. Next, cells in the allergen-induced enlarged CD31hiLy-6Cnegpopulation were sorted (about 85% to 95% pure CD31hiLy-6Cneg cells) and cultured in the presence of IL-5 (24 ng/mL) for 6 days (Figure 7B-C). Eosinophils were detected after 6 days by MBP staining and morphology on cytospins. The CD31hiLy-6Cneg subset yielded a 4-fold higher number of cells (P = .029) and a higher percentage of eosinophils compared with whole BM cultured under the same conditions (CD31hiLy-6Cneg: 51.2 ± 1.1% vs whole BM: 25.6 ± 2.2%, P = .029).
CD31hiLy-6Cneg cells give rise to eosinophils following culture in IL-5.
(A) Flow cytometric staining of gated CD31hiLy-6Cneg cells reveals expression of the eotaxin receptor CCR3 (open histogram). Isotype control is indicated by the filled histogram. (B) CD31hiLy-6Cneg cells were sorted to purity using flow cytometric sorting and cultured in the presence of IL-5 for 6 days. Sorted population was obtained from bone marrow of an OVA-DC/OVA animal, exposed to 3 OVA aerosols. (C) Cultured cells have an eosinophilic cytoplasm and a donut-shaped nucleus. We next investigated whether the enhanced population of CD31hiLy-6Cneg cells responded differently to growth factor stimulation in mice with or without eosinophilic airway inflammation by comparing the growth of equal numbers of sorted CD31hiLy-6Cneg obtained from both groups. After sorting and a 7-day culture in GM-CSF there was a significant difference neither in the yield of total cells (P = .1) nor in the percentage of CD11c+ DCs derived from the CD31hiLy-6Cneg of both groups (results not shown). However, when grown in IL-5, the subset sorted from the BM of OVA-challenged animals yielded slightly more eosinophilis compared with the PBS-DC/PBS mice (51.2 ± 1.1% vs 40.5 ± 1.3%,P < .05). Cells were stained with αMBP Ab. Original magnification, × 400.
CD31hiLy-6Cneg cells give rise to eosinophils following culture in IL-5.
(A) Flow cytometric staining of gated CD31hiLy-6Cneg cells reveals expression of the eotaxin receptor CCR3 (open histogram). Isotype control is indicated by the filled histogram. (B) CD31hiLy-6Cneg cells were sorted to purity using flow cytometric sorting and cultured in the presence of IL-5 for 6 days. Sorted population was obtained from bone marrow of an OVA-DC/OVA animal, exposed to 3 OVA aerosols. (C) Cultured cells have an eosinophilic cytoplasm and a donut-shaped nucleus. We next investigated whether the enhanced population of CD31hiLy-6Cneg cells responded differently to growth factor stimulation in mice with or without eosinophilic airway inflammation by comparing the growth of equal numbers of sorted CD31hiLy-6Cneg obtained from both groups. After sorting and a 7-day culture in GM-CSF there was a significant difference neither in the yield of total cells (P = .1) nor in the percentage of CD11c+ DCs derived from the CD31hiLy-6Cneg of both groups (results not shown). However, when grown in IL-5, the subset sorted from the BM of OVA-challenged animals yielded slightly more eosinophilis compared with the PBS-DC/PBS mice (51.2 ± 1.1% vs 40.5 ± 1.3%,P < .05). Cells were stained with αMBP Ab. Original magnification, × 400.
OVA exposure does not increase the number of plasmacytoid DCs in BM
Despite the fact that none of the other populations identified by CD31 and Ly-6C staining were percentually changed, we studied these subsets in greater detail by 4-color analysis. More specifically, the percentage of CD31hiLy-6Chi cells, known to contain precursors for DCs as well as plasmacytoid DCs, was not altered by exposure to OVA.22,27 28 The percentage of plasmacytoid CD11c+ B220+ DCs within the CD31hiLy-6Chi subset was 12.2 ± 1.4% in OVA-exposed mice compared with 13.0 ± 0.8% in PBS-exposed mice after 3 aerosols (Figure 5C). However, in response to the OVA challenges, the CD31hiLy-6Chi subset, expressed more CD131 compared with the control mice (data not shown).
The percentage of CD3+CD4+ T cells (falling within the CD31medLy-6Cneg fraction) in total BM was significantly lower (0.16 ± 0.03%) in the OVA-DC/OVA group compared with the PBS-DC/PBS group (0.37 ± 0.07%;P = .001).
OVA exposure modifies the BALF and serum level of cytokines in OVA-sensitized mice
An increase in DCs could also be caused by increased local differentiation of DCs from monocytic precursors within inflamed tissues. To determine the presence of early growth and differentiation factors for DCs, we measured the content of the cytokines IL-6, GM-CSF, and Flt-3L in BALF 24 hours after 3 OVA aerosol exposures, when the number of DCs was significantly increased. IL-6 was significantly raised after 3 aerosols in OVA-DC/OVA mice compared with PBS-DC/PBS mice (OVA: 418.2 ± 74.6 pg/mL, PBS: 12.5 ± 1.9 pg/mL;P = .016). Ag challenge did not effect the GM-CSF level (OVA: 3.90 ± 1.50 pg/mL vs PBS: 9.45 ± 2.3 pg/mL;P = .111). The Flt-3L levels in BALF were just above the detection level of our assay, showing no detectable difference in the various groups.
In serum, levels of IL-6 (13.67 ± 3.39 pg/mL vs 2.55 ± 0.79 pg/mL; P = .016) and eotaxin (564 ± 62 pg/mL vs 282 ± 25 pg/mL; P = .008) were higher in the OVA-DC/OVA group compared with the PBS-DC/PBS group, whereas that of GM-CSF was below the detection limit.
OVA exposure increases DC migration toward the draining lymph nodes in OVA-sensitized mice
In addition to the mechanisms studied above, a decreased efflux to the draining MLN could contribute to an accumulation of DCs in inflamed airways. We observed that the draining MLNs of OVA-DC/OVA mice were grossly swollen compared with nondraining nodes or MLNs of PBS-DC/PBS mice. After 3 aerosol exposures, the total number (both relative and absolute) of DCs was increased in the OVA-DC/OVA group compared with the control PBS-DC/PBS group (7.00 ± 0.53% vs 2.37 ± 0.27%;P = .002). This was due primarily to an increase in the CD11bmed/hi myeloid DCs (3.5-fold increase), although the lymphoid CD11b− subset was also increased following OVA challenge (Figure 8A-B). To provide further proof that migration of airway DCs was influenced by the eosinophilic airway inflammation, we injected CFSE-labeled, BM-derived, in vitro–cultured DCs intratracheally in mice with (OVA-DC/OVA group) or without (PBS-DC/PBS) eosinophilic airway inflammation. At 48 hours after injection, mice with inflamed lungs had a small, but significantly higher number of CFSE-labeled DCs in the MLNs, compared with control mice with uninflamed lungs (9.74 ± 1.81 × 102 vs 2.41 ± 0.84 × 102 CFSE-labeled CD11c+cells; P = .029; Figure 8C), demonstrating that DC efflux to the MLNs was actually enhanced in mice with eosinophilic airway inflammation. After intratracheal injection, CFSE-labeled DCs could not be detected in peripheral LN.
Effect of OVA or PBS aerosol challenge on DC subsets within the draining mediastinal LN.
OVA-DC– or PBS-DC–immunized mice were challenged 3 times with either OVA or PBS aerosol. At 24 hours after the last challenge, mediastinal LN were collected, homogenized, and stained for the presence of MHCII+ CD11c+ DCs. (A) Within the MHCII+ population, CD11c+ cells were further characterized as CD11b+ myeloid and CD11b− lymphoid DCs. (B) In OVA-DC/OVA animals, there is an absolute and relative increase in both lymphoid and myeloid DCs compared with PBS-DC/PBS mice (*P < .05). Data are expressed as mean number of cells ± SEM of 1 experiment representative of multiple experiments. (C) To investigate whether the observed increase in DCs in the LN was caused by enhanced migration, BM DCs were labeled with CFSE, and 2 × 106 cells were injected intratracheally into mice without (PBS) or with (OVA) eosinophilic airway inflammation, and subsequently traced in the MLN and the inguinal nondraining LN. Injected DCs can be discriminated from endogenous DCs by CFSE positivity. Numbers represent the percentage of injected DCs of total cells in the LN.
Effect of OVA or PBS aerosol challenge on DC subsets within the draining mediastinal LN.
OVA-DC– or PBS-DC–immunized mice were challenged 3 times with either OVA or PBS aerosol. At 24 hours after the last challenge, mediastinal LN were collected, homogenized, and stained for the presence of MHCII+ CD11c+ DCs. (A) Within the MHCII+ population, CD11c+ cells were further characterized as CD11b+ myeloid and CD11b− lymphoid DCs. (B) In OVA-DC/OVA animals, there is an absolute and relative increase in both lymphoid and myeloid DCs compared with PBS-DC/PBS mice (*P < .05). Data are expressed as mean number of cells ± SEM of 1 experiment representative of multiple experiments. (C) To investigate whether the observed increase in DCs in the LN was caused by enhanced migration, BM DCs were labeled with CFSE, and 2 × 106 cells were injected intratracheally into mice without (PBS) or with (OVA) eosinophilic airway inflammation, and subsequently traced in the MLN and the inguinal nondraining LN. Injected DCs can be discriminated from endogenous DCs by CFSE positivity. Numbers represent the percentage of injected DCs of total cells in the LN.
Discussion
Airway DCs have been implicated in causing the sensitization to inhaled allergens, by taking up Ag in the lung mucosa, transporting it into the draining LN and finally by inducing differentiation of Th2 effector cells that can orchestrate eosinophilic airway inflammation.3,4,6 7 Despite these observations that airway DCs might be essential in the early steps of sensitization, less information is available on the role of DCs in stimulating memory and/or effector Th2 cells at times of repeated exposure to inhaled allergens. In this paper, we have demonstrated that the number of airway CD11c+ CD11b+ myeloid DCs is strongly increased within the airway epithelium and BALF following allergen challenge in sensitized animals. Within 3 days, an almost 10-fold expansion in the number of DCs was found following repeated OVA challenge, in parallel with an increase in CD4+ lymphocytes and eosinophils in the airways, reaching a 100-fold expansion at day 7. At the same time, the number of CD11c+ CD11b+myeloid DCs in the bloodstream was increased 3-fold.
The massive increase in airway DCs and the accompanying increase in circulating blood DCs led us to investigate whether the production of DCs from the BM (“dendropoiesis”) might be enhanced in mice with eosinophilic airway inflammation, to meet the enhanced demand for DCs in the inflamed lung. Numerous studies have demonstrated that the BM reacts to airway allergen challenge by increasing its output of eosinophilic precursors.10-14 Previously, repopulation experiments in irradiated and BM-reconstituted rats have shown that airway DCs stem from a rapidly dividing precursor cell in the BM.16 It is possible to discriminate distinct populations of BM cells using multiparameter flow cytometry.19,22,29,30 Here, we have used the expression of Ly-6C in combination with CD31, platelet endothelial cell adhesion molecule PECAM-1, to delineate 6 discrete populations of BM cells, each with differential lineage commitment and differentiation potential.19,22 When we stained BM cells from mice with or without eosinophilic airway inflammation, there was a striking and time-dependent increase in the population of CD31hiLy-6Cneg cells, whereas none of the other populations were affected by allergen challenge. In previous experiments, this population of BM cells was shown to contain cells with colony-forming unit (CFU) potential for granulocytes/monocytes, erythrocytes, megakaryocytes, and mast cells as well as cells with thymus repopulating capacity. More primitive precursors with long-term repopulating ability were found in the CD31medLy-6Cneg population.29 31In mice with eosinophilic airway inflammation, CD31hiLy-6Cneg cells predominantly expressed the myeloid marker CD11b, and the expression of the lymphoid differentiation marker IL-7Rα (CD127) was slightly, but significantly, decreased, suggesting an expansion of cells with myeloid potential. When CD31hiLy-6Cneg cells were sorted and subsequently grown in the DC-growth factor GM-CSF, clusters were formed in liquid culture and a majority of the outgrowth of these colonies were myeloid CD11b+CD11c+ cells with a dendritic morphology (data not shown). In contrast, when CD31negLy-6Cmed cells were grown under the same conditions, no colonies were formed and hardly any CD11c+cells grew out of the cultures. When the CD31hiLy-6Cneg DC-precursor population was sorted from both groups of mice, and grown under the same plating density and GM-CSF concentration, there was no difference in the amount or percentage of DCs that grew from these cultures. Therefore, sensitivity to GM-CSF, either by enhanced expression of the GM-CSF receptor or by modified postreceptor events, was not enhanced in mice with eosinophilic airway inflammation. In support of this, the expression of the common β chain of the IL-3/IL-5/GM-CSF on CD31hiLy-6Cneg cells was not different between mice with or without eosinophilic airway inflammation.
The fact that a time-dependent increase in CD31hiLy-6Cneg cells with DC potential was observed in mice with massive accumulation of CD11b+CD11c+ airway and blood DCs strongly suggests that the BM increased its production of DCs to meet the enhanced demand in the airways. Moreover, in mice with airway inflammation, there was no increase in the CD31hiLy-6Chi population, known to contain the plasmacytoid B220+ CD11c+ DCs and some precursors of myeloid DCs.22 The fact that we did not see an increase in any of these more mature DC populations following allergen challenge suggests that DC precursors leave the BM at an early stage of differentiation. One possibility that needs further investigation is that they were attracted to the airways through the action of particular chemokines. Allergic inflammation is accompanied by enhanced production of eotaxin in the airways.32 The subset of CD31hiLy-6C− BM precursors expressed the eotaxin receptor CCR3, suggesting that these cells might be attracted into inflamed airways.
The observed increase in CD31hiLy-6Cneg cells in BM, without any other increase in BM populations is unique for eosinophilic airway inflammation. Bacterial infection withListeria monocytogenes leads to profound changes in BM composition with a predominant time-dependent increase in CD31hiLy-6Chi monocyte precursors, CD31negLy-6Chi mature monocytes, and CD31negLy-6Cmed granulocytes. At the same time, there was a depletion of CD31medLy-6Cneglymphoid cells and more importantly, the CD31hiLy-6Cneg population described in this study.19 The changes in the BM ofListeria-infected mice reflect an increased need for granulocytes and monocytes, which are attracted to lesions consisting of mononuclear- and neutrophil-rich cell infiltrates. However, in our model of OVA-induced airway inflammation, eosinophils are strongly recruited to the airways of challenged mice together with DCs. Not surprisingly, we were able to demonstrate that the CD31hiLy-6Cneg population of BM cells could also differentiate into MBP-positive eosinophils after culture in IL-5, suggesting that enhanced production of eosinophils from the BM was induced to meet the increased demand for eosinophils. This is in line with previous experiments in which mouse BM cells were cultured in the presence of IL-5 in semisolid media to asses eosinophil–colony-forming unit (CFU-Eo) potential and showing increased CFU-Eo in mice with eosinophilic airway inflammation.11-14
The increase in CFU-Eo in challenged mice has been attributed either to the presence of a serum factor distinct from IL-5, such as eotaxin, or to migration of IL-5–producing T cells to the BM.13,14,33In support of a serum factor in our system, we measured a slightly enhanced level of the DC growth factor IL-6.34 One likely candidate that could be involved in the up-regulation of GM-CSF–responsive BM cells is eotaxin,33 of which the serum levels were indeed doubled in mice with inflammation. Further, the CD31hiLy-6Cneg cells expressed the eotaxin receptor CCR3, with identical levels in allergic and nonallergic mice (data not shown). The migration of “dendropoiesis”-promoting T cells to the BM is less likely as we measured a decrease in BM CD4+ T cells following allergen challenge, probably due to their migration to the lung.
Although the direct recruitment of immature blood DCs is the most likely explanation for the observed accumulation of lung DCs, one contributing mechanism could also be enhanced local proliferation of DCs from monocytic precursors in allergic lung. Although it was shown in irradiation experiments that there is little if any local self-renewal capacity for DCs in rat airways, this situation could be different under inflammatory conditions.16 Indeed, in our experiments, there was an increase in circulating CD11c−CD11b+MHCII+ monocytes that could be recruited to the airways and further differentiate to DCs.15 However, when we measured the concentration of the DC differentiation factors GM-CSF and Flt-3L in the airways of allergic mice, we could not detect enhanced production.34,35 One factor that enhances differentiation of mouse BM–derived DCs that was enhanced in BALF was IL-6.34 It was recently shown that enhanced expression of IL-6 in the lungs of mice receiving IL-13 and interferon-γ correlates with enhanced numbers of CD11c+DCs being found in the BALF.36 Definite proof whether locally produced IL-6, GM-CSF, or Flt-3L functionally contributes to enhanced differentiation of monocytes (precursors) to DCs in mice with airway eosinophilia awaits studies using neutralizing antibodies.
One final mechanism that could be responsible for the observed change in airway DCs in mice with airway eosinophilia would be reduced emigration of DCs. There is continuous and high throughput migration of airway DCs from the epithelium to the draining MLN and a small decrease of DC efflux could lead to rapid and profound accumulation within the epithelium.3,5,16 To our surprise, we found that the numbers of CD11c+CD11b+ myeloid and CD11c+CD11b− lymphoid DCs were increased strongly in the MLN of OVA-challenged mice. We believe that the increase in LN DCs was partly due to enhanced migration of lung-derived DCs into the MLN. In support of this, CFSE+CCR-7+ BM DCs migrated 3 times more efficiently toward the MLNs following injection into the airways of mice with established airway eosinophilia. Reduced migration of DCs to the MLN is therefore not a mechanism that contributes to accumulation of airway DCs in our model. Enhanced migration of Ag-laden DCs to the MLN during a secondary response to inhaled Ag could prove to be necessary to stimulate the recirculating pool of nonpolarized CCR7+ central memory T cells, which recirculate through the T-cell area of LN and spleen and fail to migrate into peripheral tissues.37,38 We have shown that a proportion of divided Ag-reactive T cells remain in a nonpolarized, nonrecirculating state in the MLN after immunization with DCs.4 It is possible that migratory DCs recruit these sessile partially activated cells into further cell division during renewed encounters with inhaled Ag.38
In summary, our data show that CD11b+CD11c+ DCs are massively attracted into the airways and draining LN upon OVA challenge in sensitized mice, a process supported by increased dendropoiesis in the BM. We have previously shown that systemic abolition of DCs in sensitized thymidine kinase transgenic mice immediately prior to secondary challenge completely suppresses eosinophilic airway inflammation, goblet cell hyperplasia, and IgE synthesis.18 Together, these data imply an important functional role for airway DCs not only in the induction of Th2 cells from naive precursors, but also in the maintenance of eosinophilic airway inflammation. Inhibiting the influx of DCs could prove to be a strategy for reducing airway inflammation that is typical of asthma.8
We thank C. Snoys (Cell Biology, Erasmus University) for sorting and J. Lee for antibodies.
Prepublished online as Blood First Edition Paper, July 12, 2002; DOI 10.1182/blood-2002-03-0673.
Supported by a grant from the Dutch Asthma Foundation (NAF3.2.99.37).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Leonie van Rijt, Erasmus University Rotterdam (Room Ee2263), Department of Pulmonary Medicine, Dr Molewaterplein 50, 3015 GE Rotterdam, The Netherlands; e-mail:vanrijt@longz.fgg.eur.nl.








This feature is available to Subscribers Only
Sign In or Create an Account Close Modal