Immature dendritic cells (DCs) reside in interstitial tissues (int-DC) or in the epidermis, where they capture antigen and, thereafter, mature and migrate to draining lymph nodes (LNs), where they present processed antigen to T cells. We have identified int-DCs that express both TRANCE (tumor necrosis factor–related activation-induced cytokine) and RANK (receptor activator of NF-κB) and have generated these cells from CD34+ human progenitor cells using macrophage colony-stimulating factor (M-CSF). These CD34+-derived int-DCs, which are related to macrophages, are long-lived, but addition of soluble RANK leads to significant reduction of cell viability and Bcl-2 expression. This suggests that constitutive TRANCE-RANK interaction is responsible for CD34+-derived int-DC longevity. Conversely, CD1a+ DCs express only RANK and are short-lived. However, they can be rescued from cell death either by recombinant soluble TRANCE or by CD34+-derived int-DCs. CD34+-derived int-DCs mature in response to lipopolysaccharide (LPS) plus CD40 ligand (L) and become capable of CCL21/CCL19-mediated chemotaxis and naive T-cell activation. Upon maturation, they lose TRANCE, making them, like CD1a+DCs, dependent on exogenous TRANCE for survival. These findings provide evidence that TRANCE and RANK play important roles in the homeostasis of DCs.
Introduction
The decision between cell survival and death is fundamental to the shaping of the immune system. Dendritic cells (DCs) reside in peripheral tissues in an immature state for optimal antigen uptake and, in response to inflammatory signals, mature and migrate to secondary lymphoid organs to activate antigen-specific T cells.1 Early studies using continuous [3H]thymidine labeling and skin or bone marrow transplantation found that the turnover rate of peripheral DCs was relatively slow compared with that of DCs in lymph nodes (LNs).2 In particular, it was found that Langerhans DCs (L-DCs) of the skin epithelium display a remarkably long half-life, as L-DCs could still be detected in the skin epithelium 9 weeks after skin transplantation.3 More recently, using BrdU incorporation combined with the ability to isolate specific DC subsets, the turnover kinetics of mouse DC subpopulations was measured, and it was found that the half-lives of both L-DCs and dermal interstitial DC (int-DCs) were long (about 14 days and 8 days, respectively)4 (K. Shortman, written personal communication, March 2002). On the other hand, mature DCs in secondary lymphoid organs have a much reduced lifespan,4,5 making it difficult to detect DCs in the T-cell zone only 2 days after immunization.6 A long half-life may be beneficial for immature DCs to fulfill their function as sentinels in peripheral tissues, but a fast turnover of mature DCs ensures the continuous exposure of the adaptive immune system to novel antigens.
TRANCE (tumor necrosis factor-related activation-induced cytokine) (also called RANKL/ODF/OPGL)7-10 represents a recently identified member of the tumor necrosis factor (TNF)–α superfamily and binds the signaling receptor RANK (receptor activator of NF-κB) (also called TRANCE-receptor)8 and the decoy receptor osteoprotegerin (OPG).11 Mice lacking TRANCE or RANK display severely reduced osteoclastogenesis, show defects in early differentiation of T and B cells, lack LNs, and fail to develop mammary glands.12-14 TRANCE is expressed by osteoblasts and fibroblasts,9,10 activated T cells,7subcapsular sinus macrophages,10 metallophilic macrophages (C.G.F.M., unpublished data, June 2000), and certain myeloma.15 RANK is widely expressed in the myelomonocytic lineage, ranging from osteoclast precursors to mature DCs.8,16 Some cell types, such as mammary epithelial cells14 and LN founder cells,17 coexpress TRANCE and RANK. In addition to playing a key role in osteoclastogenesis, TRANCE-RANK interaction has been shown to prolong the survival of epithelial cells14 and mature DCs.18 Recently, it has been shown in a mouse diabetes model that TRANCE is implicated in the establishment of regulatory T cells.19
In view of the finding that TRANCE is involved in the survival of mature DCs, we investigated the role of TRANCE and RANK in the survival of immature DCs. We show that CD14+ int-DCs in human dermis and liver coexpress TRANCE and RANK. We have generated macrophage-related cells from CD34+ human progenitors using macrophage colony-stimulating factor M-CSF and found that these cells carry markers characteristic of dermal int-DCs20-22and express TRANCE and RANK. These cells, which we refer to as CD34+-derived int-DCs, are long-lived in culture, but addition of soluble RANK leads to significant reduction of cell viability and Bcl-2 expression, suggesting that constitutive TRANCE-RANK interaction is associated with CD34+-derived int-DC longevity. Conversely, CD1a+ DCs express RANK but lack TRANCE and are short-lived. However, they can be rescued from cell death either by recombinant soluble TRANCE or by CD34+-derived int-DCs. CD34+-derived int-DCs mature in response to lipopolysaccharide (LPS)/CD40L and, once mature, migrate in a CCL21/CCL19-chemotactic gradient and induce proliferation of naive CD4+ T cells. Upon maturation they lose TRANCE but retain RANK expression. These results underpin the importance of TRANCE and RANK for immature DC homeostasis and provide further evidence for the important function of these TNF family members in the immune system.
Materials and methods
Immunohistochemistry
Acetone-fixed cryostat sections of normal healthy human skin were incubated with the following antibodies (Ab): mouse anti-TRANCE (R&D Systems, Abingdon, United Kingdom), goat anti-RANK (R&D Systems), mouse anti-CD14 (Immunotech/Coulter, Villepinte, France), mouse anti-CD1a (Pharmingen, Le-Pont-de-Claix, France), rabbit anti–coagulation factor XIIIa (FXIIIa) (Calbiochem, San Diego, CA), and rat anti–M-CSF receptor (Zymed, San Francisco, CA). TRANCE was revealed by biotinylated horse anti–mouse Ab (Vector, Burlingame, CA) followed by horseradish peroxidase (HRP)–conjugated (brown) or alkaline phosphatase (AP)–conjugated (blue) avidin-biotin complex (ABC; Vector). RANK was revealed by biotinylated rabbit anti–goat Ab (DAKO, Trappes, France) and AP-ABC (Vector). Other antigens were revealed by HRP-conjugated goat anti–rabbit Ab (DAKO) or HRP-conjugated goat anti–mouse/rat Ab (The Binding site, Birmingham, United Kingdom). Development of AP was with Fast Blue (Vector) and HRP with 3,3′ diaminobenzidine (DAB; DAKO).
Cell culture
Human skin DCs were isolated as described.23Briefly, human skin obtained from plastic surgery was cleaned of fat, rehydrated, and floated onto a double layer of culture medium containing 1% fetal calf serum (FCS) (upper) and 20% FCS (lower). After 16 hours' incubation at 37°C, nonadherent cells were harvested from the lower layer and analyzed for CD14/RANK and CD14/TRANCE expression by fluorescence-activated cell sorter (FACS) (Beckton-Dickinson, Le-Pont-de-Claix, France). Liver pieces were obtained from partial hepatectomy for colorectal metastases situated al least 3 cm from the tumor. Liver pieces were cut into 0.5 cm2 squares, washed in phosphate-buffered saline (PBS), and cultured overnight in complete medium (RPMI 1640, 10% FCS, 2 mM glutamine, 100 μg/mL streptomycin, and 100 U/mL penicillin). Nonadherent cells that migrated out of the tissue into the media were collected and DC enriched by centrifugation over a 12% optiprep gradient (Nycomed, Sigma, St Louis, MO). The yield was typically 0.5 × 105 cells/g of liver tissue. Cells were analyzed for CD14, RANK, and TRANCE expression by flow cytometry (Beckman-Coulter, Fullerton, CA). Umbilical cord blood was collected after consent and processed according to institutional guidelines. CD34+ cells were purified from cord blood using anti-CD34–coated magnetic beads (Miltenyi, Paris, France) and were cultured for 5-6 days in RPMI medium containing 10% endotoxin-free FCS (Life Technologies, Cergy-Pontoise, France), 25 ng/mL stem cell factor (SCF) (R&D Systems), 3 ng/mL TNF-α (R&D Systems), and 200 U/mL granulocyte-macrophage colony-stimulating factor (GM-CSF) (Schering-Plough, Kenilworth, NJ), yielding CD1a+CD14− and CD1a−CD14+ precursors.24,25 To generate CD34+-derived int-DCs, the CD14+CD1a− precursors were sorted on a FACSVantage and grown in 25 ng/mL M-CSF (R&D Systems) for 4-6 days.25 To generate immature CD1a+ L-DCs, the CD1a+CD14− precursors were sorted on a FACSVantage and grown in 200 U/mL of GM-CSF (Schering-Plough) for 4 days.25 As internal control for the mixed lymphocyte reaction, mature CD1a+ DCs were generated by culturing the sorted CD14+CD1a− precursors for 4-6 days in 200 U/mL GM-CSF and 3 ng/mL TNF-α.25
Phenotypic analysis
Expression of specific markers was determined by flow cytometry on a FACSCalibur (Becton Dickinson) and the following Ab: CD14-FITC, CD4-FITC, HLA DR–FITC (Becton Dickinson), CD1a-PE (Immunotech/Coulter), M-CSF receptor (Zymed), FXIIIa (Calbiochem), TRANCE (R&D Systems), RANK (R&D Systems and Santa Cruz Biotechnology, Santa Cruz, CA), CD40-PE, CD40L-PE, CD80-PE, CD83-PE, CD11b-FITC, CD11c-PE, CD21-FITC, CD35-FITC, CD16-FITC, CD64-FITC (Immunotech/Coulter), CD86-FITC, and CD32-FITC (Pharmingen). Secondary Ab were all FITC-F(ab′)2 goat Ab from Jackson ImmunoResearch (West Grove, PA). The analyses were done using the CellQuest software (Beckton-Dickinson). 15 × 106 COBS cells were transfected by electoporation with a plasmid coding for the flag-tagged full-length human TRANCE cDNA (kindly provided by Y. Choi) under the control of a viral promotor. Cells were detached 24 hours after transfection, fixed in 4% PBS-buffered paraformaldehyde, and incubated with isotype control Ab, anti-Flag Ab (Stratagene, La Jolla, CA), and anti-TRANCE Ab (R&D Systems).
Reverse transcriptase–polymerase chain reaction and Western blot analyses
Total RNA was extracted from CD34+-derived int-DCs, unsorted CD34+-derived DCs (with or without maturation by CD40L), and total peripheral blood T cells, activated by anti–CD3/CD28 mAb. RNA was reverse-transcribed using standard procedures and subject to polymerase chain reaction (PCR) with the following primer pairs: TRANCE: 5′-AAATCCCAAGTTCTCATACCC and 5′-TCTCATAAGGTCAACCCGTAA, RANK: 5′-GTACACACACGGCAAAC and 5′-TGCTCTGTGTCCCCGTGAAGC, OPG 5′-CGCAAAAGTGTGGAATAGATGT and 5′-TGAGTGACAGTTTTGGGAAAGTG. β-actin primers were from Clontech (Palo Alto, CA). Cells were lysed in radioimmunoprecipitation assay (RIPA) buffer (10 mM Tris-Cl, pH 7.4, 150 mM NaCl, 1 mM EDTA [ethylenediaminetetraacetic acid], 1% Triton X-100, 0.5% Na-deoxyycholate, 0.1% sodium dodecyl sulfate [SDS], aprotinin, phenylmethylsulfonyl fluoride [PMSF], leupeptin). Cleared cell lysates were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and blotted onto nylon membrane. As control, 10 ng RANK-Fc (R&D Systems) and 50 ng soluble TRANCE (Peprotech, Rocky Hill, NJ) were loaded. Nylon membranes were saturated in Tris-buffered 5% milk and incubated with anti-RANK (R&D Systems) and anti-TRANCE (C-20, Santa Cruz Biotechnologies) Ab. Western blots were developed using the ECL-kit (Amersham-Pharmacia, Little Chalfont, United Kingdom) or NBC/BCIP coloration (Pierce, Rockford, IL).
Cell survival studies
CD34+-derived int-DCs were cultured at 105 cells/mL in the presence of either 100 ng/mL human IgG1 (Sigma) or human RANK-Fc (R&D Systems). IgG1 and RANK-Fc were renewed every 3 days. After 12 days, CD34+-derived int-DC viability was determined by trypan blue exclusion of dead cells, and cells were stained for intracellular Bcl-2 using fluorescein isothiocyanate (FITC)–conjugated Ab (DAKO). 2 × 105 L-DCs were washed and placed into a 12-well plate in cytokine-free medium under the following conditions: L-DCs alone, L-DCs in the presence of 50 ng/mL recombinant human TRANCE (Peprotech), in the presence of 105CD34+-derived int-DCs with 500 ng/mL IgG1, or 500 ng/mL RANK-Fc. After 2 days, cell viability was determined by trypan blue exclusion of dead cells, and cells were double-stained with anti–Bcl-2-FITC (DAKO) and anti–CD14-PE (Becton Dickinson) or anti–CD1a-PE (Coulter). Student unpaired t test was used to analyze statistical significance.
Phagocytosis assay
A recombinant strain of Mycobacterium bovisbacillus Calmette-Guérin (BCG) (Myc409) was used expressing a mutated form of the green fluorescent protein (GFP) fromAequoria victoria. The gfp gene was cloned under the control of the strong mycobacterial promoter pBlaF* and the expression cassette inserted into the BCG chromosome by means of an integrative vector derived from the mycobacteriophage Ms6. On day 1 of infection, CD34+-derived int-DCs were infected in complete medium lacking antibiotics with Myc409 at a multiplicity of infection (MOI) of either 1 or 10 bacteria for 1 int-DC. Cells were washed 4 hours later and complete medium without antibiotics added. Cells were harvested 20 hours later, and phagocytosed Myc409 bacilli were detected by flow cytometry.
Chemotactic cell migration
Chemotactic cell migration was assessed using a chemotaxis Boyden microchamber with a standard 5-μm pore polycarbonate filter (Neuroprobe, Gaithersburg, MD).26 Migration of 105 unstimulated, LPS-, and LPS/CD40L-treated CD34+-derived int-DCs was analyzed in response to 100 ng/mL CCL20, 500 ng/mL CCL19, and 100 ng/mL CCL21 (R&D Systems) after incubation at 37°C for 1 hour and 30 minutes.
Allogeneic mixed lymphocyte reaction assay
Unstimulated, LPS-, and LPS/CD40L-stimulated CD34+-derived int-DCs, as well as CD14+precursor cell–derived CD1a+ DCs, were treated with mitomycin C (Sigma). Graded doses of stimulator cells were seeded with 2 × 104 T cells in microtest culture plates in RPMI medium containing 10% FCS. T-cell proliferation was measured after 5 days of culture following an overnight incubation with 1 μCi (0.037 MBq) of [3H]thymidine.
Results
Identification of TRANCE+RANK+ cells in human skin
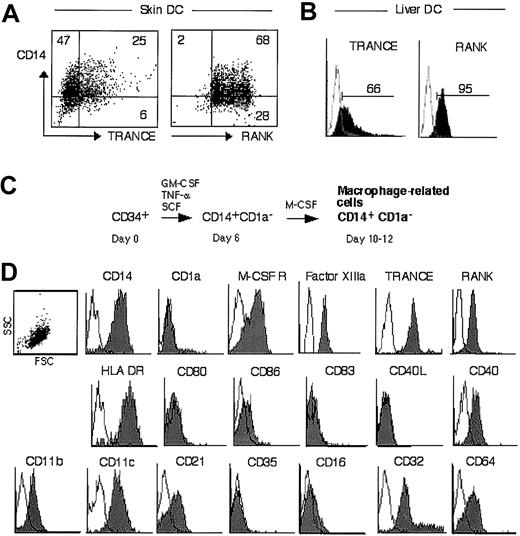
We analyzed the distribution of DC survival factors in peripheral tissues and observed cells in the dermis of human skin that expressed both TRANCE and RANK (Figure1A, indicated by arrows). They were in the vicinity of cells expressing only RANK, which could be vascular endothelial cells or CD1a+ L-DCs.8 Further double-labeling analyses revealed that TRANCE+ cells also expressed Factor (F) XIIIa (Figure 1B), CD14 (Figure 1C), and M-CSF receptor (Figure 1D), but lacked CD1a (Figure 1E). Close to blood vessels (arrows, Figure 1B), TRANCE+ cells lacked FXIIIa, suggesting that they acquire in the stromal environment this marker that is characteristic of the dermal monocyte/macrophage subset.20 27 CD1a+ cells in the dermis (Figure1E, between arrowheads) were often found in the vicinity of TRANCE+ cells but lacked TRANCE expression. Taken together, these findings suggest that TRANCE and RANK are expressed by a dermal monocyte/macrophage subset in healthy human skin.
Identification of dermal TRANCE+RANK+ cells.
Immunolabeling of healthy human skin for TRANCE, RANK, and other antigens as indicated (B-E). (A) Arrows highlight double-positive cells. (B) Arrows point to dermal blood vessels. (E) Arrowheads highlight CD1a+ cells in the dermis. (F) Incubation with TRANCE isotype control and rabbit preimmune serum. In all panels, ep indicates epidermis; d, dermis. Original magnification: A, C, D × 160; B, E, F × 125. Development was done using Fast Blue and 3,3′ diaminobenzidine (DAB).
Identification of dermal TRANCE+RANK+ cells.
Immunolabeling of healthy human skin for TRANCE, RANK, and other antigens as indicated (B-E). (A) Arrows highlight double-positive cells. (B) Arrows point to dermal blood vessels. (E) Arrowheads highlight CD1a+ cells in the dermis. (F) Incubation with TRANCE isotype control and rabbit preimmune serum. In all panels, ep indicates epidermis; d, dermis. Original magnification: A, C, D × 160; B, E, F × 125. Development was done using Fast Blue and 3,3′ diaminobenzidine (DAB).
Int-DCs express TRANCE and RANK
To verify the immunohistochemical findings, CD14+cells were purified from human skin and analyzed for TRANCE and RANK expression by flow cytometry. As shown in Figure2A, all CD14+ cells expressed RANK, and a subset also expressed TRANCE. Next, we purified int-DCs from liver, an organ that, compared with heart or kidney, is relatively rich in int-DCs,28 and tested the cells for TRANCE and RANK expression. As shown in Figure 2B, liver DCs expressed RANK and also TRANCE, albeit more weakly than RANK. These data suggest that CD14+ int-DCs express both TRANCE and RANK. The low TRANCE expression could be related to the spontaneous maturation of ex vivo–purified DCs29 30 combined with the fact that TRANCE is down-regulated on mature DCs (see below).
TRANCE and RANK are novel int-DC antigens.
CD14+ DCs purified from human skin (A) and liver (B) were analyzed for TRANCE and RANK expression by FACS. Percentage of positive cells is indicated. (C) Generation of macrophage-related cells from CD34+ progenitors (CD34+-derived int-DCs). Cord blood CD34+ cells were cultured for 6 days in SCF, GM-CSF, and TNFα, yielding a CD14+CD1a− population, which was FACS-sorted and grown in M-CSF for 4-6 days.25(D) Antigen expression of CD34+-derived int-DCs. The SSC/FSC scattergram profile is shown, and no electronic gates were set. The expression of surface markers was determined by flow cytometry using specific antibodies. White histograms represent isotype controls, and specific labeling is shown in gray.
TRANCE and RANK are novel int-DC antigens.
CD14+ DCs purified from human skin (A) and liver (B) were analyzed for TRANCE and RANK expression by FACS. Percentage of positive cells is indicated. (C) Generation of macrophage-related cells from CD34+ progenitors (CD34+-derived int-DCs). Cord blood CD34+ cells were cultured for 6 days in SCF, GM-CSF, and TNFα, yielding a CD14+CD1a− population, which was FACS-sorted and grown in M-CSF for 4-6 days.25(D) Antigen expression of CD34+-derived int-DCs. The SSC/FSC scattergram profile is shown, and no electronic gates were set. The expression of surface markers was determined by flow cytometry using specific antibodies. White histograms represent isotype controls, and specific labeling is shown in gray.
To study the role of TRANCE and RANK on int-DCs we sought to generate these cells in vitro. However, monocyte-derived DCs grown in the presence of GM-CSF and IL-4 carry the CD1a antigen but not CD14 or TRANCE (Sallusto and Lanzavecchia31 and data not shown). We therefore generated macrophage-related cells from CD34+progenitors using a previously described method25 (Figure2C). Human CD34+ progenitors were grown in SCF, GM-CSF, and TNF-α and, after 6 days, the CD14+CD1a−intermediate population was FACS-sorted and cultured in M-CSF for 4-6 days. This procedure yielded a homogenous cell population that expressed CD14, M-CSF receptor, Factor XIIIa, TRANCE, and RANK but lacked CD1a (Figure 2D). In addition, the cells carried the following cell surface antigens: HLA DR, CD40, and complement (CD11b, CD11c, CD21) and Fc-receptors (CD32, CD64). They weakly expressed CD86 and lacked CD80, CD83, CD40L, CD35, and CD16. Although these cells were generated in M-CSF, we refer to these cells as CD34+-derived int-DCs to emphasize the fact that they can mature into professional antigen-presenting cells (APCs).
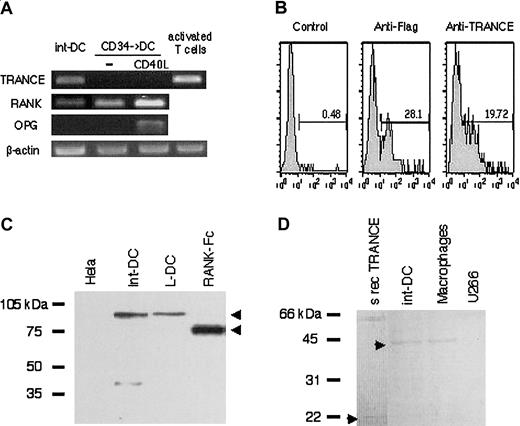
To confirm TRANCE and RANK expression, we performed reverse transcriptase (RT)–PCR analyses on cDNA prepared from CD34+-derived int-DCs as well as from CD40L-matured DCs and activated T cells known to express RANK and TRANCE, respectively.7,8 As shown in Figure3A, CD34+-derived int-DCs transcribe TRANCE and RANK but not OPG, which acts as a decoy receptor for TRANCE.11 We also verified the specificity of the Ab directed against TRANCE, used in the immunohistochemical and flow cytometry studies, by transfecting COBS cells with Flag-tagged human TRANCE cDNA.7 As shown in Figure 3B, 28% of transfected cells are recognized by the anti-Flag Ab and 20% are recognized by the anti-TRANCE Ab. Finally, we detected TRANCE and RANK expression by Western blot analysis (Figure 3C-D). Full-length RANK and recombinant soluble RANK-Fc migrated with an apparent molecular weight of about 90 kDa and about 70 kDa, respectively, and full-length TRANCE and recombinant soluble TRANCE with an apparent molecular weight of about 42 kDa and about 20 kDa, respectively (arrowheads). Taken together, CD34-derived int-DCs resemble DCs of interstitial tissues on the basis of expression of common markers including TRANCE and RANK.
TRANCE and RANK are expressed by CD34+-derived int-DCs.
(A) CD34+-derived int-DCs, unstimulated or CD40L-stimulated DCs, generated in the presence of GM-CSF and TNFα25 and anti–CD3/CD28-activated T cells were tested for the transcription of TRANCE, RANK, OPG, and β-actin by RT-PCR. (B) COBS cells were transfected with Flag-tagged human TRANCE cDNA, and cDNA expression was analyzed by anti-Flag and anti-TRANCE Ab. Percentage of positive cells is indicated. Detection of RANK (C) and TRANCE (D) by Western blot in the indicated cells. Full-length RANK and recombinant soluble RANK-Fc migrated with an apparent molecular weight of about 90 kDa and about 70 kDa, respectively, and full-length TRANCE and recombinant soluble TRANCE with an apparent molecular weight of about 42 kDa and about 20 kDa, respectively. CD1a+ L-DCs and macrophages served as positive controls; Hela cells and the myeloma U266 served as negative controls.
TRANCE and RANK are expressed by CD34+-derived int-DCs.
(A) CD34+-derived int-DCs, unstimulated or CD40L-stimulated DCs, generated in the presence of GM-CSF and TNFα25 and anti–CD3/CD28-activated T cells were tested for the transcription of TRANCE, RANK, OPG, and β-actin by RT-PCR. (B) COBS cells were transfected with Flag-tagged human TRANCE cDNA, and cDNA expression was analyzed by anti-Flag and anti-TRANCE Ab. Percentage of positive cells is indicated. Detection of RANK (C) and TRANCE (D) by Western blot in the indicated cells. Full-length RANK and recombinant soluble RANK-Fc migrated with an apparent molecular weight of about 90 kDa and about 70 kDa, respectively, and full-length TRANCE and recombinant soluble TRANCE with an apparent molecular weight of about 42 kDa and about 20 kDa, respectively. CD1a+ L-DCs and macrophages served as positive controls; Hela cells and the myeloma U266 served as negative controls.
CD34+-derived int-DCs survival is mediated by TRANCE-RANK interaction
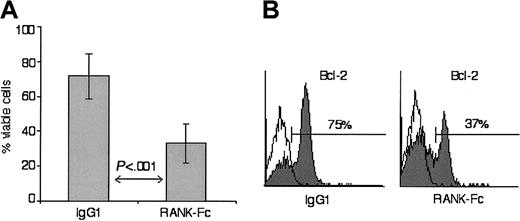
We observed that CD34+-derived int-DCs remained immature and viable in culture for more than 2 weeks without addition of cytokines. In light of the role of TRANCE in mature DC survival,18 we wondered whether this longevity was the consequence of constitutive RANK signaling caused by TRANCE and RANK coexpression. To address this question, CD34+-derived int-DCs were cultured for 12 days in the presence of control IgG1 or soluble RANK-Fc fusion protein in order to interrupt TRANCE interaction with membrane RANK. After this prolonged culture, 72% of IgG1-treated CD34+-derived int-DCs were still alive, whereas in the RANK-Fc–treated samples, only 33% remained viable (Figure4A). Reduction of cell viability by addition of RANK-Fc is reflected in Bcl-2 levels, as in the presence of soluble RANK-Fc, 2-fold fewer CD34+-derived int-DCs expressed Bcl-2 (Figure 4B). Identical results were obtained with soluble RANK lacking the cytoplasmic domain (data not shown). These results suggest that CD34+-derived int-DCs are long-lived due to Bcl-2 expression sustained by constitutive TRANCE-RANK ligation.
CD34+-derived int-DC longevity is mediated by constitutive TRANCE-RANK ligation.
(A) CD34+-derived int-DCs were cultured for 12 days in the presence of either human IgG1 or RANK-Fc. Cell viability was determined by trypan blue exclusion of dead cells. The results are expressed as the means of 5 independent experiments with SD of the data. (B) IgG1- or RANK-Fc–treated CD34+-derived int-DCs were stained for intracellular Bcl-2, and the percentage of Bcl-2–expressing cells is indicated. Results are representative of 5 independent experiments.
CD34+-derived int-DC longevity is mediated by constitutive TRANCE-RANK ligation.
(A) CD34+-derived int-DCs were cultured for 12 days in the presence of either human IgG1 or RANK-Fc. Cell viability was determined by trypan blue exclusion of dead cells. The results are expressed as the means of 5 independent experiments with SD of the data. (B) IgG1- or RANK-Fc–treated CD34+-derived int-DCs were stained for intracellular Bcl-2, and the percentage of Bcl-2–expressing cells is indicated. Results are representative of 5 independent experiments.
CD34+-derived int-DCs can rescue L-DCs from cell death
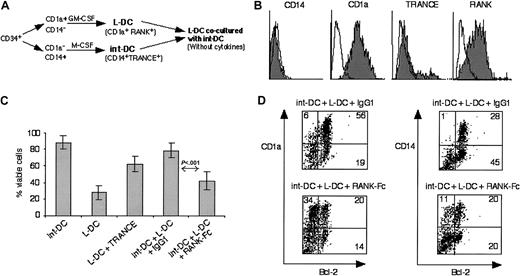
Besides TRANCE+RANK+ int-DCs, interstitial dermal tissue also contained cells that expressed RANK but lacked TRANCE (Figure 1A). These might be L-DCs migrating from the dermal vasculature to the epidermis or leaving the epidermis to home to draining LN. Immature CD1a+ DCs (L-DCs) derived from CD34+ progenitors in the presence of GM-CSF (Figure5A) expressed RANK but lacked TRANCE (Figure 5B). Placed into fresh culture medium in the absence of cytokines, a great number of L-DCs underwent cell death, in contrast to CD34+-derived int-DCs (Figure 5C). Addition of soluble recombinant TRANCE partially rescued L-DCs from cell death. To test whether CD34+-derived int-DCs can likewise rescue L-DCs from cell death, L-DCs were cocultured with CD34+-derived int-DCs (ratio: 2 L-DCs to 1 CD34+-derived int-DC) in the presence of control IgG1 and RANK-Fc. Total cell viability of these cocultures was 79% in the presence of control IgG1, compared with only 41% in the presence of RANK-Fc. This suggests that CD34+-derived int-DCs can support L-DC cell viability by intercellular TRANCE-RANK engagement. To ascertain that L-DCs were rescued from cell death, we analyzed Bcl-2 expression in the cocultures. In the presence of IgG1, 56% of all cells express CD1a and Bcl-2, however, in the presence of RANK-Fc, only 20% of all cells express CD1a and Bcl-2. Identical results were obtained with soluble RANK lacking the cytoplasmic domain (data not shown). Separation of L-DCs and int-DCs by a transwell membrane did not rescue L-DCs from cell death, indicating that cell-cell contact is required and that TRANCE is not shed by int-DCs (data not shown). These results suggest that immature DCs that express RANK but lack TRANCE are short-lived, but their viability can be prolonged by soluble TRANCE or by coculture with TRANCE+ CD34+-derived int-DCs.
CD34+-derived int-DCs sustain viability of L-DCs.
(A) Schematic description of the experiment. CD34+progenitor cells were cultured in SCF, GM-CSF, and TNFα, and at day 6 the CD1a+CD14− and the CD1a−CD14+ precursors were cell sorted. L-DCs were generated from the CD1a+CD14− precursors in the presence of GM-CSF,25 and CD34+-derived int-DCs were generated from the CD1a−CD14+precursors by culture in M-CSF. L-DCs and CD34+-derived int-DCs were washed and cocultured for 2 days in medium lacking cytokines. (B) L-DCs expressed CD1a and RANK but lacked CD14 and TRANCE. White histograms represent isotype controls, and specific labeling is shown in gray. (C) Percentage cell viability of int-DCs and L-DCs after 2 days in cytokine-free medium: int-DCs and L-DCs alone, L-DCs with soluble TRANCE, coculture of in-DCs and L-DCs in the presence of IgG1 or RANK-Fc. Cell viability was determined by trypan blue exclusion of dead cells. The results are expressed as the means of 5 independent experiments with SD of the data. (D) Double-labeling of cells in the cocultures using anti–Bcl-2-FITC and anti–CD14-PE or anti–CD1a-PE. Percentages of labeled cells are indicated in the quadrants. The data are representative of 3 independent experiments.
CD34+-derived int-DCs sustain viability of L-DCs.
(A) Schematic description of the experiment. CD34+progenitor cells were cultured in SCF, GM-CSF, and TNFα, and at day 6 the CD1a+CD14− and the CD1a−CD14+ precursors were cell sorted. L-DCs were generated from the CD1a+CD14− precursors in the presence of GM-CSF,25 and CD34+-derived int-DCs were generated from the CD1a−CD14+precursors by culture in M-CSF. L-DCs and CD34+-derived int-DCs were washed and cocultured for 2 days in medium lacking cytokines. (B) L-DCs expressed CD1a and RANK but lacked CD14 and TRANCE. White histograms represent isotype controls, and specific labeling is shown in gray. (C) Percentage cell viability of int-DCs and L-DCs after 2 days in cytokine-free medium: int-DCs and L-DCs alone, L-DCs with soluble TRANCE, coculture of in-DCs and L-DCs in the presence of IgG1 or RANK-Fc. Cell viability was determined by trypan blue exclusion of dead cells. The results are expressed as the means of 5 independent experiments with SD of the data. (D) Double-labeling of cells in the cocultures using anti–Bcl-2-FITC and anti–CD14-PE or anti–CD1a-PE. Percentages of labeled cells are indicated in the quadrants. The data are representative of 3 independent experiments.
CD34+-derived int-DCs capture antigen
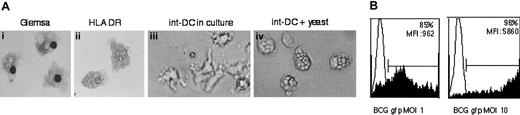
CD34+-derived int-DCs are generated in the presence of M-CSF and express CD14 but are distinct from typical macrophages by the absence of CD16 (Figure 2D) and complete lack of adherence to plastic cell surfaces (Figure 6A). Giemsa coloration or staining for HLA DR revealed a veiled cell morphology, an extensive cytoplasm with numerous vacuoles, suggesting active endocytosis. Indeed, after the addition of heat-killed yeast S cerevisiae, CD34+-derived int-DCs were filled with yeast bodies (Figure 6A). To better assess antigen capture, Myc409, a live strain of recombinant Mycobacterium bovis BCG expressing GFP was added to CD34+-derived int-DCs for 4 hours and uptake of Myc409 was measured by flow cytometry. As shown in Figure 6B, at a multiplicity of infection (MOI) of only onemycobacterium per cell, 85% of CD34+-derived int-DCs captured Myc409 with a mean fluorescence intensity (MFI) of 962 units. Increasing the dose to an MOI of 10 resulted in virtually all cells capturing Myc409 with an MFI that has increased 6-fold. This shows that CD34+-derived int-DCs are capable of rapidly capturing a great number of microorganisms.
CD34+-derived int-DCs efficiently capture antigen.
(A) May-Grünwald-Giemsa staining and anti–HLA DR labeling (revealed in DAB) of cytospun CD34+-derived int-DCs (panels Ai and Aii, original magnification, × 400). CD34+-derived int-DCs were incubated for 2 hours with heat-killed yeast and then thoroughly washed. Compare with CD34+-derived int-DCs in culture without yeast (panels Aiii and Aiv, original magnification, × 350). (B) Myc409, a recombinant Mycobacterium bovis BCG strain expressing GFP, was added for 4 hours to CD34+-derived int-DCs at an MOI of 1 or 10. Capture of Myc409 was measured by flow cytometry. The data are representative of 2 independent experiments.
CD34+-derived int-DCs efficiently capture antigen.
(A) May-Grünwald-Giemsa staining and anti–HLA DR labeling (revealed in DAB) of cytospun CD34+-derived int-DCs (panels Ai and Aii, original magnification, × 400). CD34+-derived int-DCs were incubated for 2 hours with heat-killed yeast and then thoroughly washed. Compare with CD34+-derived int-DCs in culture without yeast (panels Aiii and Aiv, original magnification, × 350). (B) Myc409, a recombinant Mycobacterium bovis BCG strain expressing GFP, was added for 4 hours to CD34+-derived int-DCs at an MOI of 1 or 10. Capture of Myc409 was measured by flow cytometry. The data are representative of 2 independent experiments.
Maturation into APC in response to LPS/CD40L
Next, we wondered whether CD34+-derived int-DCs would mature into professional APCs upon encounter with DC maturation signals. Stimulation with endotoxin resulted in cell aggregation (Figure 7A), increased expression of CD80 and CD40, but diminished CD11b and CD14 expression (Figure 7B). A small proportion of cells expressed CD83. The up-regulation of CD40 prompted us to subsequently provide an anti-CD40 (Figure 7A) or a CD40L signal (Figure 7B). This led to (1) formation of large free-floating cell aggregates with distinct dendritic cell extensions (Figure 7A), (2) a rise in the levels of HLA DR with acquisition of CD83, and (3) increased expression of CD80 and CD86. LPS/CD40L-stimulated cells lost TRANCE expression but continued to express RANK (Figure 7B).
CD34+-derived int-DCs mature in response to LPS/CD40L.
CD34+-derived int-DCs were stimulated for 24 hours with 1 μg/mL LPS (int-DC + LPS) and then for 48 hours with either 10 μg/mL anti-CD40 (G-28.5, American Type Culture Collection [ATCC]) (int-DC + LPS + αCD40) (A) or CD40L on transfected L-cells (int-DC + LPS + CD40L) (B). (A) Cell morphology as seen by microscopy. (B) FACS-measured antigenic expression, using the same Ab as in Figure 2. Percentages of labeled cells and the MFI for CD40 expression are indicated. White histograms represent isotype controls, and specific labeling is shown in gray. The data are representative of 5 independent experiments.
CD34+-derived int-DCs mature in response to LPS/CD40L.
CD34+-derived int-DCs were stimulated for 24 hours with 1 μg/mL LPS (int-DC + LPS) and then for 48 hours with either 10 μg/mL anti-CD40 (G-28.5, American Type Culture Collection [ATCC]) (int-DC + LPS + αCD40) (A) or CD40L on transfected L-cells (int-DC + LPS + CD40L) (B). (A) Cell morphology as seen by microscopy. (B) FACS-measured antigenic expression, using the same Ab as in Figure 2. Percentages of labeled cells and the MFI for CD40 expression are indicated. White histograms represent isotype controls, and specific labeling is shown in gray. The data are representative of 5 independent experiments.
We next addressed the question of whether untreated or stimulated CD34+-derived int-DCs would migrate in response to the chemokines CCL20 (MIP3α) CCL19 (MIP3β) or CCL21 (6Ckine) (Figure8A). CCL20 was not an efficient chemoattractant for either untreated or stimulated cells. LPS-stimulated cells responded weakly to CCL19, but LPS/CD40L-stimulated cells migrated in great numbers in response to CCL19 and CCL21. Then we compared the ability of untreated or stimulated CD34+-derived int-DCs to induce proliferation of alloreactive naive CD4+ T cells. As shown in Figure 8B, LPS/CD40L-stimulated cells induced vigorous proliferation of T cells, but untreated or LPS-stimulated cells were unable to induce T-cell proliferation. The data suggest that, in response to pathogen and CD40L, CD34+-derived int-DCs mature into professional APCs.
Mature CD34+-derived int-DCs migrate in response to chemokines and activated naive T cells.
(A) Unstimulated and stimulated CD34+-derived int-DCs were tested for chemotactic migration in response to CCL20, CCL19, and CCL21. Each assay was performed in duplicate, and the results are expressed as the means ± SD of migrating cells per 2 fields. (B) Naive T cell stimulatory ability of unstimulated or stimulated CD34+-derived int-DCs and CD14+ precursor cell–derived mature CD1a+ DCs, generated in the presence of GMCSF and TNFα. Graded doses of DCs were seeded with 2 × 104 cord blood–purified T cells in microtest culture plates in RPMI medium containing 10% FCS. T-cell proliferation was measured after 5 days of culture following an overnight incubation with 1 μCi (0.037 MBq) of [3H]thymidine. Results are expressed as mean cpm ± SD of triplicate cultures.
Mature CD34+-derived int-DCs migrate in response to chemokines and activated naive T cells.
(A) Unstimulated and stimulated CD34+-derived int-DCs were tested for chemotactic migration in response to CCL20, CCL19, and CCL21. Each assay was performed in duplicate, and the results are expressed as the means ± SD of migrating cells per 2 fields. (B) Naive T cell stimulatory ability of unstimulated or stimulated CD34+-derived int-DCs and CD14+ precursor cell–derived mature CD1a+ DCs, generated in the presence of GMCSF and TNFα. Graded doses of DCs were seeded with 2 × 104 cord blood–purified T cells in microtest culture plates in RPMI medium containing 10% FCS. T-cell proliferation was measured after 5 days of culture following an overnight incubation with 1 μCi (0.037 MBq) of [3H]thymidine. Results are expressed as mean cpm ± SD of triplicate cultures.
Discussion
TRANCE and RANK are markers for interstitial DC
In this report, we have shown that an interstitial cell population in the human skin coexpresses TRANCE and RANK. We have characterized these cells further and observed that they carry markers characteristic of dermal int-DCs, that is, CD14, Factor XIIIa, and MCSF-R.20-22 In addition, CD14+ DCs purified from human skin and liver also expressed TRANCE and RANK. Cells that express these markers could be generated from CD34+progenitors by growth in GM-CSF, TNF-α, and SCF, followed by culturing the CD14+ subset in M-CSF. These cells capture antigen efficiently and mature into professional APCs in response to LPS/CD40L.
Given the plasticity of the myelomonocytic cell lineage, the phenotypic distinction between macrophages and immature DCs in the healthy interstitial environment is difficult. Human macrophages and immature DCs share a great number of antigenic markers, such as Factor XIIIa and CD11c. However, in contrast to macrophages, which are terminally differentiated, immature DCs can further differentiate into professional APCs capable of activating naive T cells. The cells we have generated in vitro share antigenic markers with macrophages, yet because they are (1) nonadherent, (2) display a veiled cell morphology, and (3) can further differentiate into mature DCs capable of activating naive T cells, it is appropriate to consider them to be immature DCs rather than macrophages. Our observations concur with those of Lu and colleagues, who showed that cells can been grown from liver stem cells that likewise resemble macrophages and immature DCs and that can mature into professional APCs.32 Also, the M-CSF–dependent subcapsular sinus macrophages that express weakly CD11c but strongly sialoadhesin, a marker shared with macrophages, can differentiate into professional APCs.33 Finally, it has been found that a subpopulation of monocytes cultured in M-CSF has the capacity to undergo differentiation into mature DCs.34,35 These observations suggest the existence of an uncommitted myeloid cell within interstitial tissues, which has been termed by some investigators “precursor DC”28 or “dendrophage.”36
It is important to underline the diversity of interstitial tissues, which stems from heterogeneous cell composition and from the influence of neighboring environments. For instance, dermal int-DCs are exposed on one hand to GM-CSF secreted by neighboring keratinocytes, and on the other hand to M-CSF and IL-6 produced by endothelial cells and collagen-associated fibroblasts. This changing cytokine milieu is likely to create a spaciotemporal heterogeneity in the int-DC population. Such heterogeneity was observed by Meunier and colleagues, who noted among major histocompatibility complex (MHC)–II+ dermal cells a low number of CD1a+Factor XIIIa+ DCs.37 Further support for the dynamic nature of the int-DC population is provided by the observation that human dermal CD14+ cells can differentiate into bona fide L-DCs in the presence of GM-CSF and TGF-β1.38 Interstitial tissues also differ with respect to antigenic load. When associated with the gastrointestinal tract or with respiratory surfaces, interstitial tissues are constantly encountering microbial stimuli, which will induce DC maturation and hasten their turnover rate.2 39 These observations suggest heterogeneity and functional diversity among the interstitial APCs.
DC viability
Measurements of steady-state turnover rates for mouse dermal int-DCs and L-DCs isolated from cutaneous-draining LNs and skin showed that both peripheral DC subsets display slow renewal in the skin.4 Dermal int-DCs obtained from skin require 14 days for BrdU incorporation by 77% of the cells, and 27% of L-DCs are labeled during the same time period. In contrast, once migrated to the draining LNs, DCs showed rapid turnover, which is even faster after microbial product stimulation (K. Shortman, written personal communication, March 2002). We found that CD34+-derived int-DCs were long-lived in culture and insensitive to cytokine-free medium for 2 days. In view of the role of TRANCE and RANK in cell survival,14,18 we hypothesized that the longevity of CD34+-derived int-DCs could be due to constitutive engagement of RANK by TRANCE. Indeed, addition of soluble RANK led to significant loss of Bcl-2 expression and cell death. By coexpressing TRANCE and RANK, CD34+-derived int-DCs appear to maintain cell viability in an autocrine survival loop. As our data suggest that TRANCE is not shed from the membrane, the simplest scenario is that TRANCE and RANK are constitutively ligated on the same cell. Yet we cannot formally exclude the possibility that cell-to-cell contact between int-DCs is required to maintain cell viability, but this is likely to occur given that int-DCs are frequently clustered around vessels and are considered highly mobile. On the other hand, mature CD34+-derived int-DCs down-regulated TRANCE while retaining RANK, suggesting that as CD34+-derived int-DCs mature they become short-lived and increasingly dependent upon exogenous factors for survival. This finding would be in accord with the faster turnover kinetics of DCs in draining LNs. CD1a+L-DCs expressed RANK but lacked TRANCE and died rapidly in cytokine-free medium. L-DCs could be rescued from cell death by addition of either soluble recombinant TRANCE or TRANCE expressed by CD34+-derived int-DCs. As our data suggest that TRANCE is not shed, cell contact between int-DCs and L-DCs would be necessary to support cell viability. This would confine int-DC to L-DC interaction to the interstitial space when L-DCs migrate from the dermis toward the epithelium or from the epidermis toward draining LNs. On the other hand, in view of the finding that DCs can send dendrites across an intact epithelial barrier,40 it may not be too surprising if int-DCs and L-DCs could interact by an analogous mechanism. One can also anticipate that epithelial cells may contribute to L-DC longevity, and it may be important to address these issues in further experiments. In support of the idea that TRANCE+ int-DC influences L-DC viability and, thus, cell number, is the observation that osteopetroticop/op mice, which lack dermal F4/80+macrophages,41 show only 60% of normal L-DC numbers.42 We have observed in mice that dermal F4/80+ cells express TRANCE and RANK and that L-DCs express only RANK (C.G.F.M. and Y. Choi, data not shown, May 2001).
Functional int-DC subsets
CD34+-derived int-DCs were efficient antigen-capturing cells as they rapidly internalized a great number of yeast bodies andMycobacterium bovis bacilli. In response to LPS/CD40L, CD34+-derived int-DCs matured into professional APCs displaying high levels of MHC-II and costimulatory molecules and acquiring expression of CD83. LPS/CD40L-stimulated CD34+-derived int-DCs responded to the chemoattractants CCL19 and CCL20, which would facilitate migration to draining LNs and could efficiently stimulate proliferation of naive CD4+ T cells. This suggests that int-DCs play an important role in the defense against invading pathogens and in the link between the innate arm and the adaptive immune system. In healthy human dermis, CD14+cells outnumber CD1a+ cells, but it is likely that in response to infection or inflammation conditions (when cytokines such as GM-CSF and IL4 or interferon [IFN]α are induced), DC precursors will preferentially differentiate into CD1a+ DCs. We hypothesize that, although both DC subsets—when mature—are equally potent T-cell stimulators, they may direct differently the adaptive immune response. For instance, it has been shown that while CD1a+ DCs direct T-cell polarization toward the Th1 type, CD1a− DCs generate Th0 effector T cells.43 We are currently investigating effector T-cell polarization by CD34+-derived int-DCs. Because CD14+ int-DCs outnumber CD1a+ int-DCs in healthy tissue, it is plausible that these cells are implicated in peripheral tolerance.44,45 In this context, their longevity could help detect and phagocytose apoptotic cells,46,47 which in healthy tissue with low antigenic load is usually a rare event. In support of a role of long-lived CD14+ int-DCs in tolerance is the finding that liver DCs have been associated with long-term graft tolerance and have been found in the T-cell zone of draining LNs up to 2 months after injection into the host.48 Also, it has been observed in a model of cytotoxic T lymphocytes (CTLs) induction to tumor antigens that continuous antigen presentation can be associated with T-cell tolerization.49 The role of TRANCE and RANK in immature DC survival could provide a molecular framework to further address the role of DCs in tolerance and tumor or microbial immunity.
We are grateful to C. Gardner, R. Blanque, and S. Roman-Roman (Aventis, Romainville, France) for kindly providing recombinant RANK-Fc and for helpful discussions. The plasmid-encoding human TRANCE cDNA was kindly provided by Y. Choi (University of Pennsylvania School of Medicine, Philadelphia, PA). We thank the staff of the Bourg-La-Reine maternity ward for providing umbilical cord blood. Special thanks to Z. Maciorowski (Institut Curie, Paris, France) for cell sorting. We also thank J. Davoust (Institut Gustave Roussy, Villejuif, France) and Y. Choi for helpful discussions and reading of the manuscript.
Prepublished online as Blood First Edition Paper, July 5, 2002; DOI 10.1182/blood-2002-01-0312.
Supported by INSERM, Université Pierre and Marie Curie, Institut Curie, Hoechst-Marion-Roussel, a grant from the Medical Research Council, and the Roche Organ Transplantation and Research Foundation. I.C. is a recipient of a postdoctoral fellowship from ARC (Association pour la Recherche contre le Cancer), and M.-C.D.-N. is a recipient of a postdoctoral fellowship from the Hoechst-Marion-Roussel Research Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Chris G. F. Mueller, INSERM U255, Centre de Recherches Biomedicales des Cordeliers, 15 rue de l'Ecole de Medecine, Paris 75270, cedex 6; e-mail:chmuller@infobiogen.fr.


![Fig. 7. CD34+-derived int-DCs mature in response to LPS/CD40L. / CD34+-derived int-DCs were stimulated for 24 hours with 1 μg/mL LPS (int-DC + LPS) and then for 48 hours with either 10 μg/mL anti-CD40 (G-28.5, American Type Culture Collection [ATCC]) (int-DC + LPS + αCD40) (A) or CD40L on transfected L-cells (int-DC + LPS + CD40L) (B). (A) Cell morphology as seen by microscopy. (B) FACS-measured antigenic expression, using the same Ab as in Figure 2. Percentages of labeled cells and the MFI for CD40 expression are indicated. White histograms represent isotype controls, and specific labeling is shown in gray. The data are representative of 5 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/10/10.1182_blood-2002-01-0312/4/m_h82223386007.jpeg?Expires=1769167519&Signature=19r7rql~kHRz64AsQoGKjvqN~8sDv2x9b9haXX6UB~hL4P7ISFLz2rpZCKxI9x~OM4MZxaUK2ADWqYquuz09OwpcNZEDfi1YRqVAx~5B6aKjQ4rWNDm6b20i3~9YFkn9kBM9xLL-9bxiyNJZy56Yub1mPQZhXj5fGvJ-5-EDLLcGPnzVzf8GuFGzWlE4k4wkOY6-6M7zgWGMy0ehQPTzGt6UNdk3--rMXgGBIALTEvXIWK5x0ajOB-UTRgDhlqPupb2ZENB-Rz8EJmNcmgQDYvW-CcK5Pl4Hmhm8SrT3nKLPCdIjfsLA8X0CCpU4EoRdVyn6vshw~iSdZwZ0sh34EQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 8. Mature CD34+-derived int-DCs migrate in response to chemokines and activated naive T cells. / (A) Unstimulated and stimulated CD34+-derived int-DCs were tested for chemotactic migration in response to CCL20, CCL19, and CCL21. Each assay was performed in duplicate, and the results are expressed as the means ± SD of migrating cells per 2 fields. (B) Naive T cell stimulatory ability of unstimulated or stimulated CD34+-derived int-DCs and CD14+ precursor cell–derived mature CD1a+ DCs, generated in the presence of GMCSF and TNFα. Graded doses of DCs were seeded with 2 × 104 cord blood–purified T cells in microtest culture plates in RPMI medium containing 10% FCS. T-cell proliferation was measured after 5 days of culture following an overnight incubation with 1 μCi (0.037 MBq) of [3H]thymidine. Results are expressed as mean cpm ± SD of triplicate cultures.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/10/10.1182_blood-2002-01-0312/4/m_h82223386008.jpeg?Expires=1769167519&Signature=0YxvQex-KyRlGHmHVMsmlzSe25xiNo2T8AjKHbfiNKati-rc5q~k~n-UphHKmz~58gjSMrf0Gr99iRbCJPQsQiNwZsXDQhyS6Vm0rC1HgILO3QkVlWr~Zf-uTJaaxPjn2xgsOH8MAp2i5jqbsrtOoAyBPk0N5mWeFCZICFNoCmWiI30l14s-vucjNzA9TQbh1KN2GOav~fQObS1RELbi234QFG~YWqefwSRQjJLbd0K4N6hbnwWRBDHFRi3sHa44p4NY7MLRfqkmBuFFv48OOGr1qzdt3zqXpyyXP2GJLsk9rfPqYyBZmDPhfE3-KYdguzzu9I04EZJ7gvzqNA9Q0g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal