Malarial anemia is associated with a shift in iron distribution from functional to storage compartments. This suggests a relative deficit in erythropoietin production or action similar to that observed in other infections. Our study in Kenyan children with asymptomatic malaria aimed at investigating whether malaria causes increased erythropoiesis, and whether the erythropoietic response appeared appropriate for the degree of resulting anemia. Longitudinal and baseline data were used from a trial with a 2 × 2 factorial design, in which 328 anemic Kenyan children were randomly assigned to receive either iron or placebo, and sulfadoxine-pyrimethamine or placebo. Erythropoiesis was evaluated by serum concentrations of erythropoietin and soluble transferrin receptor. Prospectively collected data showed that malarial infection resulted in decreased hemoglobin concentrations, and increased serum concentrations of erythropoietin and transferrin receptor. Conversely, disappearance of malarial antigenemia resulted in increased hemoglobin concentrations, and decreased concentrations of these serum indicators. Additionally, our baseline data showed that current or recent malarial infection is associated with increased serum concentrations of erythropoietin and transferrin receptor, and that these were as high as or perhaps even higher than values of children without malarial infection and without inflammation. Our findings indicate that in asymptomatic malaria, the erythropoietic response is adequate for the degree of anemia, and that inflammation probably plays no or only a minor role in the pathogenesis of the resulting anemia. Further research is needed to demonstrate the role of deficient erythropoietin production or action in the pathogenesis of the anemia of symptomatic malaria.
Introduction
Impaired erythropoiesis is believed to play an important role in the pathogenesis of malarial anemia, and may exacerbate anemia due to malaria-induced hemolysis.1Several mechanisms might explain this erythropoietic suppression. Malarial anemia is associated with a shift of iron distribution from functional compartments—comprising metabolically active iron that is required for normal functions—toward storage compartments that constitute an iron reserve.2 This suggests a relative deficit in erythropoietin production or decreased marrow responsiveness to erythropoietin in malaria, similar to that observed in other infections.3,4 These effects are probably mediated by proinflammatory cytokines such as tumor necrosis factor (TNF) and interleukin 1 (IL-1).3 4
Observational evidence supports a possible role of proinflammatory cytokines in the pathogenesis of malarial anemia. Serum erythropoietin concentrations are increased in acute, symptomatic malaria5-7 but appear lower than expected for the degree of anemia.8 Proinflammatory cytokines such as TNF, IL-1, and IL-6 are released by monocytes and suppress erythropoietin synthesis in adults with symptomatic malaria.9,10Similar observations have been made in children with persistent, asymptomatic Plasmodium falciparum malaria.11
Reduced erythropoietin production or action might possibly explain reports12 of reduced numbers of red cell precursors in acute malaria. Evidence for erythroid hypoplasia is supported by ferrokinetic studies showing reduced incorporation of iron into red cells during acute malaria,13 and showing decreased serum soluble transferrin receptor (sTfR) concentrations during episodes of febrile malaria.14,15 Concentrations of sTfR measure both erythropoietic activity and the deficit in the erythron of iron; they are not influenced by the inflammatory response to infections.16-18
Studies from Zaire, however, failed to show an effect on sTfR concentrations of malaria,19,20 whereas reports from cross-sectional studies among nonhospitalized Africans give support to increased sTfR concentration in malaria.21-23 Erythroid hyperplasia with dyserythropoiesis may also occur in malaria, and appears more common in patients with severe anemia and low-grade parasitemia than in those with acute malaria.12
We used longitudinal data to investigate whether malaria and iron deficiency in asymptomatic Kenyan children independently cause increased erythropoiesis. Study of pathogenic mechanisms in asymptomatic malaria is important because there are strong indications that mild anemia may progress to severe anemia in children with malaria but remaining without or with few symptoms. Erythropoiesis was evaluated by serum concentrations of erythropoietin and sTfR.24,25 The data used were collected as part of a randomized controlled trial to determine the efficacy in improving iron status of intermittent administration of iron and sulfadoxine-pyrimethamine (SP). In addition, we used the baseline data from this trial to evaluate whether observational data indicated that the erythropoietic response to asymptomatic malaria was appropriate for the degree of resulting anemia. Detailed descriptions of study design and other results are published elsewhere.26
Patients, materials, and methods
Area and population
The study was conducted in Mtito Andei Division, Eastern Province, Kenya, which is located halfway on the road and rail link between Nairobi and Mombasa. The area has been described in more detail elsewhere.23 26 Clinical records of outpatient and inpatient attendance and fever incidence in Kibwezi Rural Health Centre, located at a distance of 40 km, show that malaria transmission is highly seasonal, and follows 2 annual peaks in rainfall distribution in October through January and March through May. Children were recruited at the start of the rainy seasons in the period 1998 to 2000. An earlier survey among children aged 2 to 36 months in this area had shown prevalences of malaria, anemia (hemoglobin concentration < 110 g/L), Ascaris lumbricoides, and Trichuris trichiura of 31%, 72%, 3%, and 5%, respectively (H.V., unpublished results, July 1997). Malaria infections reported were exclusively due to Plasmodium falciparum. No hookworm infections were found. There is no active malaria control program in the area, and no other epidemiologic or entomologic studies of malaria have been conducted previously.
A research clinic was established in the area and children were recruited from neighboring communities at the start of rainy seasons in the period 1998 to 2000. The study received ethical approval from the African Medical and Research Foundation and the Kenya Medical Research Institute. Informed consent was obtained from community leaders and local government officials, and from parents of participating children, in accordance with the Helsinki protocol. Children included in the study and their siblings received free medical care for common childhood illnesses.
Design and procedures
The study consisted of a double-blind trial with a 2 × 2 factorial design with an intervention period of 12 weeks. Children randomly selected from communities in the study area (n = 328) were randomly allocated to receive either iron supplement or placebo, and either sulfadoxine-pyrimethamine or placebo. Iron was administered twice weekly as ferrous fumarate in a 6.25 g/L suspension at a target dose of 6 mg elemental iron kg−1 body weight, wk−1. Sulfadoxine-pyrimethamine was administered every 4 weeks at therapeutic doses (25 mg and 1.25 mg kg−1body weight, respectively).
Eligibility criteria at randomization were as follows: hemoglobin concentrations 60 g/L to 110 g/L; age 2 to 36 months; axillary temperature less than 37.50°C; absence of symptoms suggestive of malaria or anemia, or of any systemic illness occurring in combination with a dipstick test result indicating current or recent malarial infection; parents reported their intention to stay in the study area during the intervention period; parental consent given; absence reported of allergy to sulfa drugs; and no sulfa drugs used in the previous 3 weeks.
Children were withdrawn and treated as appropriate if hemoglobin concentrations were less than 50 g/L, if they met one or more criteria of severe and complicated malaria,27 or if they had manifestations of other severe disease.
Children were medically examined at baseline and at 12 weeks. At both visits, samples of capillary blood from each child were assayed by dipstick test to detect current or recent malarial infection, and hemoglobin concentration was measured. Children were always promptly treated for common illnesses, or referred if needed; details are described elsewhere.26
Laboratory measurements
Hemoglobin concentrations were determined by photometer (HemoCue, Ängelholm, Sweden), and dipstick tests (Model ML02; AMRAD/ICT, Sydney, Australia) were used for rapid detection in blood of antigens (histidine-rich protein-2) specific to P falciparum.28 In patients with manifestations of malaria, this test has sensitivity and specificity estimates more than 95% in detecting parasitemia as determined by microscopy.29-32 A negative test result is highly predictive for the absence of malarial infection, whereas a positive test result probably has less predictive value for the presence of infection.33 Resources were lacking for routine microscopic examination of blood smears.
Procedures for collection and handling of blood samples have been described elsewhere.23 Ferritin concentrations were measured by immunoassay (Roche, Mannheim, Germany) on an Elecsys electrochemiluminescense analyzer. C-reactive protein concentration was determined as an indicator of inflammation in an assay (Orion Diagnostics, Espoo, Finland) adapted to allow measurement in low volumes of ranges from zero to highly elevated values. This method correlated excellently with standard methods (Beckman Coulter, Brea, CA; Roche). Serum transferrin receptor concentrations were estimated by enzyme-linked immunosorbent assay (Ramco, DPC, Los Angeles, CA) according to instructions from the manufacturer. Erythropoietin was measured by chemiluminescent immunoassay (Diagnostic Products, Los Angeles, CA). Insufficient blood was available for determination of all hematologic indicators in all blood samples: as a result, the total number of children included in the subgroups was sometimes less than the total number of children investigated (n = 328).
Statistical analysis
Data cleaning and standard analysis was undertaken using SPSS (version 7.5.2; SPSS, Chicago, IL). Cross-sectional data from the baseline were used to compare blood samples testing positive and negative when assayed by malaria dipstick test with regard to hemoglobin concentration, and serum concentrations of ferritin, erythropoietin, and sTfR. Values of serum indicators were normalized by log transformation before analysis.
The cross-sectional data were subsequently used to assess serum concentrations of ferritin, erythropoietin, and sTfR in children with a positive malaria dipstick test in comparison with what might be expected for the resulting degrees of anemia and inflammation.24 25 For this purpose, children were divided into 3 groups. Group 1 comprised children whose blood tests indicated the absence of both malarial infection and inflammation (serum C–reactive protein concentration ≤ 10 mg/L). Group 2 comprised children without malarial infection as indicated by a negative dipstick test result, and with inflammation (serum C–reactive protein concentration > 10 mg/L). Group 3 comprised children with current or recent malarial infection as indicated by a positive dipstick test result. Hemoglobin classes were defined using cutoff values that were selected to balance the number of children in categories formed by cross-tabulation with the groups into which children had been divided. Serum concentrations of ferritin, erythropoietin, and sTfR from group 3 were subsequently compared with those from groups 1 and 2, assuming independent t-distributions of log-transformed values.
In a subsequent analysis, change in hematologic indicators was calculated as the difference in values after and before intervention. For serum indicators, these changes were either positively or negatively skewed within intervention groups and could not be normalized. Thus, we could not use linear regression models to evaluate the response of these indicators to malaria or iron supplementation. The effects on hematologic indicators of iron supplementation were assessed in children without malarial infection at both baseline and at 12 weeks, thus excluding children with a positive dipstick test result at either of these points in time. In addition, we excluded children receiving SP in this analysis, regardless of whether it was given alone or in combination with iron. Significance of differences in the distributions was determined by t test in the case of change in hemoglobin concentrations, and by the Mann-Whitney test in the remaining indicators.
Finally, we examined the effects of asymptomatic malaria in children receiving either SP or placebo, thus excluding children receiving iron. These children were divided into 3 groups. Group A comprised children without infection at either baseline or at 12 weeks as assessed by dipstick test. Group B contained children who became infected during the trial (dipstick test result negative at baseline, and positive at 12 weeks), whereas group C comprised children with a positive dipstick test at baseline, and a negative result at 12 weeks. The results from groups B and C were both compared with those from group A, and significance of differences in the distributions were determined by the Mann-Whitney test. Data from children with a positive dipstick test both at baseline and at 12 weeks were not considered because parasite density might not have been the same at the 2 time points. Thus, it would be difficult to interpret the meaning of any differences in the parameters between these time points.
Results
Findings from the cross-sectional data of the effect of malaria on hematologic indicators are shown in Table1. The prevalence of current or recent malarial infection in these children as indicated by a positive dipstick test result was 0%, 20%, 29%, and 50% for children aged 2 to 6 months, 6 to 12 months, 12 to 24 months, and 24 to 36 months, respectively. Malarial infection was associated with a mild reduction of hemoglobin concentrations, mild inflammation as indicated by serum C–reactive protein concentration, and increased serum concentrations of erythropoietin, sTfR, and ferritin.
Effect of malarial infection on various hematologic indicators; cross-sectional data from the baseline
| Indicator . | No malaria infection (negative dipstick test result) . | Current or recent malarial infection (positive dipstick test result) . | Difference (95% CI*) or factor† . | ||
|---|---|---|---|---|---|
| n . | Mean . | n . | Mean . | ||
| Hemoglobin concentration‡, g/L | 228 | 97.3 | 100 | 89.9 | 7.4 (5.0–9.9) |
| Serum erythropoietin concentration, IU/L | 216 | 21.4 | 92 | 49.6 | 2.3† (1.9–2.9) |
| Serum soluble transferrin receptor concentration, mg/L | 219 | 3.1 | 94 | 4.7 | 1.5† (1.4–1.7) |
| Serum ferritin concentration, μg/L | 213 | 11.2 | 91 | 28.1 | 2.5† (1.8–3.4) |
| Serum C-reactive protein concentration, mg/L | 219 | 14.7 | 96 | 23.9 | 1.6† (1.4–1.8) |
| Indicator . | No malaria infection (negative dipstick test result) . | Current or recent malarial infection (positive dipstick test result) . | Difference (95% CI*) or factor† . | ||
|---|---|---|---|---|---|
| n . | Mean . | n . | Mean . | ||
| Hemoglobin concentration‡, g/L | 228 | 97.3 | 100 | 89.9 | 7.4 (5.0–9.9) |
| Serum erythropoietin concentration, IU/L | 216 | 21.4 | 92 | 49.6 | 2.3† (1.9–2.9) |
| Serum soluble transferrin receptor concentration, mg/L | 219 | 3.1 | 94 | 4.7 | 1.5† (1.4–1.7) |
| Serum ferritin concentration, μg/L | 213 | 11.2 | 91 | 28.1 | 2.5† (1.8–3.4) |
| Serum C-reactive protein concentration, mg/L | 219 | 14.7 | 96 | 23.9 | 1.6† (1.4–1.8) |
Group estimates are geometric.
CI indicates confidence interval; estimated effects were all highly significant upon testing (P < .001).
Factors indicate the proportional change in values measured in children with current or recent infection relative to those in their counterparts without infection.
Group estimates are arithmetric means.
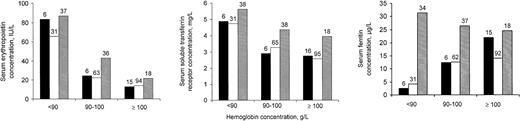
Serum concentrations of erythropoietin and sTfR from children with current or recent malarial infection as indicated by dipstick test were as high as or perhaps even beyond the values of children without malaria, regardless of whether serum C–reactive protein concentration values from children in groups 1 or 2 indicated the presence or absence of inflammation (Figure 1). Blood samples from children in group 3 had somewhat higher serum C–reactive protein concentrations than their counterparts in group 2 (not shown).
Serum indicators (geometric means) in relation to hemoglobin concentration, for 3 groups of children.
Group 1 (black bars): children whose blood tests indicated the absence of both malarial infection and inflammation; group 2 (open bars): children without malarial infection, and with inflammation; group 3 (shaded bars): children with current or recent malarial infection (positive dipstick test result). Numbers above bars indicate group sample sizes. See text for further explanation.
Serum indicators (geometric means) in relation to hemoglobin concentration, for 3 groups of children.
Group 1 (black bars): children whose blood tests indicated the absence of both malarial infection and inflammation; group 2 (open bars): children without malarial infection, and with inflammation; group 3 (shaded bars): children with current or recent malarial infection (positive dipstick test result). Numbers above bars indicate group sample sizes. See text for further explanation.
Hemoglobin concentrations increased more in children who received iron than in their counterparts who received iron placebo (16.0 g/L versus 4.1 g/L; difference: 11.9 g/L; 95% confidence interval: 7.9-15.9 g/L). The corresponding changes in concentrations of serum indicators are shown in Figure 2. Iron supplementation led to decreased serum concentrations of erythropoietin and sTfR, and increased serum ferritin concentrations. There was no evidence of an effect of iron on serum C–reactive protein concentrations (not shown), or of differences between the groups compared regarding baseline characteristics (not shown).
Effect of iron supplementation on change in serum indicators.
Box plots indicate 25th and 75th percentiles (box) and the median (thick line across each box). Vertical lines indicate highest and lowest values, excluding outliers (5 largest and 5 smallest values). Group sample sizes are indicated in parentheses; Pvalues indicate the level of significance when testing for differences in distributions relative to children receiving placebo (Mann-Whitney test). See “Statistical analysis” for further explanation.
Effect of iron supplementation on change in serum indicators.
Box plots indicate 25th and 75th percentiles (box) and the median (thick line across each box). Vertical lines indicate highest and lowest values, excluding outliers (5 largest and 5 smallest values). Group sample sizes are indicated in parentheses; Pvalues indicate the level of significance when testing for differences in distributions relative to children receiving placebo (Mann-Whitney test). See “Statistical analysis” for further explanation.
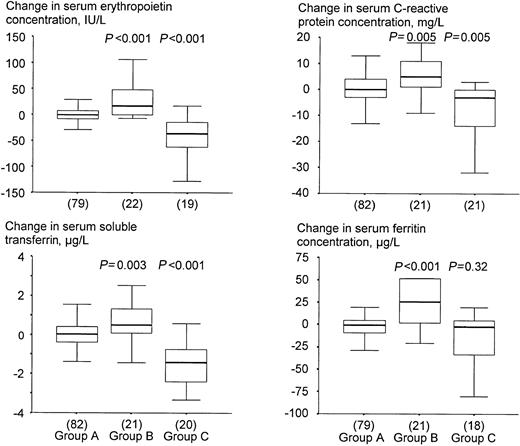
Children who became infected by malaria in the course of the study had decreased hemoglobin concentrations (Table2), and increased serum concentrations of erythropoietin, sTfR, C–reactive protein, and ferritin at 12 weeks when compared with baseline (Figure 3). Conversely, those with both a positive dipstick test result at baseline and a negative dipstick test result at 12 weeks, had increased hemoglobin concentrations (Table 2), and decreased serum concentrations of erythropoietin, sTfR, C–reactive protein and ferritin at 12 weeks when compared with baseline (Figure 3). Exclusion of children who received SP did not lead to different conclusions (not shown).
Effect of asymptomatic malaria on hemoglobin concentrations (longitudinal data)
| Group . | Dipstick test result . | n . | Change in hemoglobin concentration, g/L . | ||
|---|---|---|---|---|---|
| At baseline . | At 12 wk . | Difference in values after and before intervention . | Compared with group A* . | ||
| A | Negative | Negative | 83 | 5.4 ± 8.8 | — |
| B | Negative | Positive | 22 | −7.8 ± 19.2 | −13.3 (−4.6 to 22.0) |
| C | Positive | Negative | 21 | 20.4 ± 11.5 | 15.0 (10.4 to 19.5) |
| Group . | Dipstick test result . | n . | Change in hemoglobin concentration, g/L . | ||
|---|---|---|---|---|---|
| At baseline . | At 12 wk . | Difference in values after and before intervention . | Compared with group A* . | ||
| A | Negative | Negative | 83 | 5.4 ± 8.8 | — |
| B | Negative | Positive | 22 | −7.8 ± 19.2 | −13.3 (−4.6 to 22.0) |
| C | Positive | Negative | 21 | 20.4 ± 11.5 | 15.0 (10.4 to 19.5) |
Mean ± standard deviation (95% confidence interval).
Calculated as the change in hemoglobin concentration in either group B or C minus the corresponding change in group A.
Effect of asymptomatic malaria on change in serum indicators.
Group A: children without infection either at baseline or at 12 weeks; group B: children who became infected during the trial; group C: children with a positive dipstick test at baseline and a negative result at 12 weeks. P values indicate the level of significance when testing for differences in distributions of sampled populations of groups B and C relative to group A (Mann-Whitney test). See the legend to Figure 2 and “Statistical analysis” for further explanation.
Effect of asymptomatic malaria on change in serum indicators.
Group A: children without infection either at baseline or at 12 weeks; group B: children who became infected during the trial; group C: children with a positive dipstick test at baseline and a negative result at 12 weeks. P values indicate the level of significance when testing for differences in distributions of sampled populations of groups B and C relative to group A (Mann-Whitney test). See the legend to Figure 2 and “Statistical analysis” for further explanation.
Discussion
Our findings showed prospectively that the development of asymptomatic malaria resulted in decreased hemoglobin concentrations, and increased serum concentrations of erythropoietin and transferrin receptor. Conversely, the disappearance of malarial antigenemia resulted in increased hemoglobin concentrations, and decreased concentrations of these serum indicators. In addition, our baseline data showed that current or recent malarial infection is associated with increased serum concentrations of erythropoietin and transferrin receptor, and that these were as high as or perhaps even higher than the values of children without malarial infection and without inflammation.
Many African children presenting to hospitals with malarial infection and severe, acutely life-threatening anemia have no symptoms or recent history of symptoms of malaria attacks.34 It appears that at least in a large proportion of these children, severe anemia has developed following prolonged exposure to chronic or repeated infection with low levels of parasitemia. Likewise, reports from many community-based surveys in malaria-endemic areas indicate that a substantial proportion of children may have severe anemia. The vast majority of these children are asymptomatic, and they often have low-level parasitemia. Thus, we believe that our present report on the erythropoietic response in children with asymptomatic malaria may also contribute to understanding the pathogenesis of severe, life-threatening malarial anemia.
To assess the effects of iron supplementation, we excluded from the analysis children with malarial infection at baseline or after 12 weeks to eliminate the possibility of confounding due to the effects of malaria. We observed that iron supplementation leads to both increased hemoglobin concentrations and decreased serum erythropoietin concentrations. This is in agreement with reports that iron-deficient erythropoiesis leads to appropriately increased serum concentrations of erythropoietin and hypoproliferative erythropoiesis but an impaired responsiveness to erythropoietin.35
In addition, we found that iron supplementation led to decreased sTfR concentrations. This corroborates our understanding that the expression of transferrin receptors on the cell surface of the erythroblast can be modified to reflect cellular iron requirements, resulting in an increased density of surface transferrin receptors in iron-deficient cells.18 Serum soluble transferrin receptors presumably result from cellular degradation of membrane-bound receptors. In the absence of hemolysis or other causes of increased erythroid iron turnover, increased sTfR concentrations reliably indicate iron deficiency.36
In an earlier report,37 it was shown that the dipstick test employed might give positive results for up to 2 weeks after clearance of parasitaemia. In most cases (70%), however, results became negative within 7 days after initiation of curative chemotherapy.37 In our study, such false positives may have led to misclassification of infection status at baseline, and thus to underestimates in the effect of malarial infection. Despite this possible bias, we found that a positive dipstick test result was associated with decreased hemoglobin concentrations, and with increased serum concentrations of erythropoietin, transferrin receptor and C–reactive protein (Table 1). Thus, we believe that our conclusions drawn from these and other observations (Table 2, Figures 1 and 3) are correct. In children with a negative dipstick test result at baseline, a subsequent positive dipstick test validly indicates a newly acquired infection.
Malaria causes a shift of iron distribution from functional toward storage compartments,2 which has also been observed in a range of other infections.3,4,38-40 Thus, in malaria, stainable macrophage iron stores are often present in bone marrow41,42 while serum ferritin concentrations are increased.2,42 43
Ferritin is an iron storage protein, but its serum concentration also increases in infection, probably as part of a host immune response.44 This was confirmed by our results. We also found that asymptomatic malaria results in increased serum concentrations of C–reactive protein, which is a highly responsive acute phase reactant. Serum ferritin concentrations less than 12 μg/L are highly predictive of depleted iron stores,45 whereas values above this range, when coupled with anemia, indicate inflammation but may mask coexisting iron deficiency.23
Anemia, decreased or inappropriately low erythropoiesis, and the shift in iron distribution from functional to storage compartments are observed in malaria and a range of other infections. As also indicated in several other reports,44,46 this suggests that the pathogenic mechanisms underlying the anemia of chronic disease also play a role in the development of malarial anemia. Several workers raised42 and reviewed46,47 the possibility that acute, symptomatic malaria leads to iron sequestration in the mononuclear phagocyte system, thus resulting in iron-limited erythropoiesis. There are several reasons to believe that this is not the case. First, the observed presence of iron in bone marrow corroborates experimental evidence4 that iron therapy in patients with inflammatory diseases does not result in correction of anemia. Second, contrary to a state of iron deficiency, iron absorption is normal or somewhat decreased in the anemia of chronic disease,40 and absorbed iron is effectively incorporated in the erythron.48 Third, instead of being incorporated into hemoglobin, it appears that iron taken up in developing red cells is stored in ferritin and hemosiderin.48 Fourth, expression of transferrin receptors on individual erythroblasts is increased in iron deficiency but decreased in anemia of chronic disease.18,49 50 If our assumption were true that also asymptomatic malaria does not lead to iron sequestration, then this would suggest that the increased serum transferrin receptor concentrations observed in children with malarial infection in our study are primarily due to increased erythropoiesis.
The anemia of chronic disease is primarily due to reduced erythropoietin production and reduced responsiveness of erythroid progenitor cells to erythropoietin3,4 under influence of proinflammatory cytokines such as tumor necrosis factor and interleukin-1. Therapy using recombinant erythropoietin can correct the anemia of chronic disease but not of iron deficiency.3Thus, the distinctive abnormalities in body iron distribution appear to be a by-product of these mechanisms.51 It cannot be ruled out, however, that some iron sequestration occurs in malaria in hemazoin, a product from hemoglobin degradation by malaria parasites that is found in circulating or phagocytosed red cells.
Contrary to what is observed in the anemia of chronic disease,3,4 we found no evidence that erythropoietin production was impaired in asymptomatic malaria (Figure 1). In addition, serum transferrin receptor concentrations in children with current or recent malarial infection appeared appropriate for the degree of anemia (Figure 1). In a study among asymptomatic children, Mockenhaupt et al21 also reported increased serum transferrin receptor concentrations when adjusting for hemoglobin concentration. These findings provide evidence that the erythropoietic response of asymptomatic malaria is adequate for the degree of anemia, and that inflammation probably plays no or only a minor role in the pathogenesis. This indicates that the malaria-associated anemia observed in the present study is primarily due to hemolysis.
We found that a positive dipstick test result was associated with a minor increase in serum C–reactive protein concentration, indicating that malarial infection was associated with a minor degree of inflammation. Acute malaria is probably associated with higher parasite densities and thus more intense inflammation. Thus, it seems likely that the decrease in sTfR concentrations observed in acute malaria14 15 might be due, in addition to hemolysis, to deficient erythropoietin production or suppressed marrow response to erythropoietin.
In summary, our findings indicate that in asymptomatic malaria, the erythropoietic response is adequate for the degree of resulting anemia. Inflammation and the pathogenic mechanisms involved in the anemia of chronic disease probably play no or a minor role. Further research is needed to demonstrate the role of deficient erythropoietin production or action in the pathogenesis of the anemia of acute, symptomatic malaria.
H.V., C.E.W., and F.J.K. were responsible for study design and interpretation of results. R.K. was responsible for biochemical analyses and assisted in the interpretation of their results. H.V. carried out the data analysis. H.V., S.M.N., R.K., M.M.M., S.van L., R.H., and C.S. collected the data.
We are grateful for outstanding assistance by Pascal Kyulle and Jones Mwau, chiefs of Kathekani and Nthunguni locations, respectively, as well as village elders, community health workers, traditional birth attendants, and the parents of study children. In addition, we thank Mike Aballa Wanga, Jacobien Veenemans, Rikkert van der Valk, Anneleen Kuijsten, Stefan de Vogel, Mariëlle Maas, and Shanomae Rose for excellent assistance in data collection, and Riekie te Stroet, medical technican, Eemland Hospital, Amersfoort, The Netherlands, for biochemical analyses of serum samples.
Prepublished online as Blood First Edition Paper, July 25, 2002; DOI 10.1182/blood-2001-12-0228.
Supported by the Netherlands Foundation for the Advancement of Tropical Research (NWO/WOTRO; grant WV93-273).
Correspondence:Clive E. West, Division of Human Nutrition and Epidemiology, Wageningen University, PO Box 8129, 6700 EV Wageningen, The Netherlands; e-mail:clive.west@staff.nutepi.wau.nl.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal