In 1943, the first description of familial idiopathic methemoglobinemia in the United Kingdom was reported in 2 members of one family. Five years later, Quentin Gibson (then of Queen's University, Belfast, Ireland) correctly identified the pathway involved in the reduction of methemoglobin in the family, thereby describing the first hereditary trait involving a specific enzyme deficiency. Recessive congenital methemoglobinemia (RCM) is caused by a deficiency of reduced nicotinamide adenine dinucleotide (NADH)–cytochrome b5 reductase. One of the original propositi with the type 1 disorder has now been traced. He was found to be a compound heterozygote harboring 2 previously undescribed mutations in exon 9, a point mutation Gly873Ala predicting a Gly291Asp substitution, and a 3-bp in-frame deletion of codon 255 (GAG), predicting loss of glutamic acid. A brother and a surviving sister are heterozygous; each bears one of the mutations. Thirty-three different mutations have now been recorded for RCM. The original authors' optimism that RCM would provide material for future genetic studies has been amply justified.
Introduction
In an era dominated by functional genomics, it is salutary to reflect that the first hereditary disorder involving an enzyme deficiency was discovered just over half a century ago by Quentin Gibson.1 In 1943, Dr James Deeny, a general practitioner, described 2 brothers, Russell and Fred Martin from Banbridge in Northern Ireland, who had a blue appearance.2When Russell was treated with vitamin C, he turned pink, and Dr Deeny assumed that he had corrected an underlying heart condition. However, the cardiologists in Belfast were more skeptical and were unable to find any abnormality in either brother. The conundrum attracted the attention of the physiologist Henry Barcroft, who carried out a detailed study of Dr Deeny's cases during treatment and found raised levels of methemoglobin in 2 members of the Martin family.3 Gibson correctly defined the pathway involved in the reduction of methemoglobin in the family, and, in so doing, he described the first hereditary trait involving a specific enzyme deficiency.1
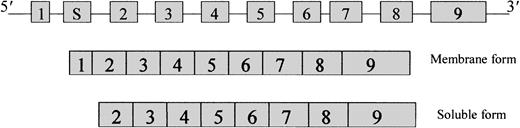
The disorder recessive congenital methemoglobinemia (RCM; McKusick no. 250 800) is caused by a deficiency of reduced nicotinamide adenine dinucleotide (NADH)–cytochrome b5 reductase (cytb5r: E.C.1.6.2.2). Two forms of cytb5r are known, a soluble form and a membrane-bound form, and are localized in different cellular compartments. The soluble form is present mainly in red cells4 and is involved in the reduction of methemoglobin.5 The membrane-bound form is found mainly in the endoplasmic reticulum and outer mitochondrial membrane,6 where it participates in the desaturation and elongation of fatty acids and in the biosynthesis of cholesterol and P-450–mediated drug metabolism. The cytb5r gene is 31-kb long, contains 9 exons, and has been localized to chromosome 22q 13-qter. Both forms of the enzyme are generated from tissue-specific alternative transcripts (Figure 1), which give rise to the 275-amino acid soluble form7 and the 300-amino acid membrane-bound form. They have an identical hydrophilic catalytic domain but differ at the N-termini, where the membrane-bound form has 25 additional hydrophobic amino acids. There are 2 distinct clinical forms of cytb5r deficiency. Type 1 is characterized clinically by a single symptom, cyanosis, and biochemically by a deficiency of the red cell–soluble form of the enzyme.8 In type 2, cyanosis is accompanied by severe mental retardation and neurologic impairment involving the soluble and the membrane-bound forms of the enzyme.9
Organization of the
cytb5r gene showing the arrangement of the 9 exons and the 2 mRNA transcripts that code for the membrane-bound and soluble forms.
Organization of the
cytb5r gene showing the arrangement of the 9 exons and the 2 mRNA transcripts that code for the membrane-bound and soluble forms.
We were curious to know the actual mutations involved in Gibson's landmark discovery, and eventually we had the opportunity to analyze blood from one of the propositi, Fred, who had emigrated to Australia in 1968, and from 2 surviving siblings.
Patients, materials, and methods
Case history
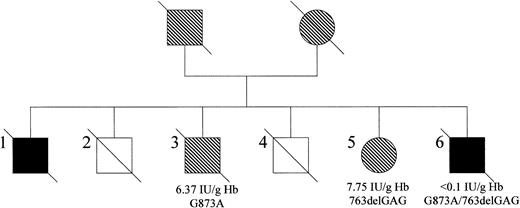
The pedigree for the family originally reported by Deeny et al and Barcroft et al2 3 is shown in Figure2. Three surviving family members were available for study—one of the original propositi (#6, FM) who has RCM type 1, a brother (#3, BM), and a sister (#5, EH). Unfortunately, during the course of this study, both Fred Martin and his brother BM died.
The family with methemoglobinemia as reported by Barcroft et al.3
Affected males are represented by black squares, male carriers by shaded squares, and female carriers by shaded circles. Three members of the family were available for the present study (#3, #5, and #6). Cytb5r activity and phenotype are indicated for each subject. The status of the remaining family members could not be established because they were deceased at the time of study.
The family with methemoglobinemia as reported by Barcroft et al.3
Affected males are represented by black squares, male carriers by shaded squares, and female carriers by shaded circles. Three members of the family were available for the present study (#3, #5, and #6). Cytb5r activity and phenotype are indicated for each subject. The status of the remaining family members could not be established because they were deceased at the time of study.
Polymerase chain reaction amplification of genomic DNA
Genomic DNA was isolated from buffy coats by the Nucleon BACC 1 DNA extraction kit (Nucleon Biosciences, Manchester, United Kingdom). Nucleotide sequences of the 9 exons were obtained from the GenBank database (accession numbers M28705 to M28713), and the following primers were designed: EX1F, 5′-gcgacagagcgagcgcggcg-3′; EX1R, 5′-gtcacctcccgcaggccaac-3′; EX1SF, 5′-cattctgagccaggcctcctgg-3′; EX1SR, 5′-ctgttctaaccgggaggaagtg-3′; EX2F, 5′-ctttctggatgagggtggtg-3′; EX2R, 5′-gaggcagtgagtgtggttca-3′; EX3F, 5′-gttctggccaccttgtttgt-3′; EX3R, 5′-ccttcccactctcattccaa-3′; EX4F, 5′-caggccaggctttgaatg-3′; EX4R, 5′-cccttctttggcttttgttg-3′; EX5F, 5′-gtacacgaggctggtggttt-3′; EX5R, 5′-agctggcctgacgagagtc-3′; EX67F, 5′-cctctacctcggacctcaca-3′; EX67R, 5′-gtcatccccagaatctcagc-3′; EX8F, 5′-gagtcccctcctgaaagctc-3′; EX8R, 5′-gaaaggactctgcctctgga-3′; EX9F, 5′-gggatcagcctctccattct-3′; and EX9R, 5′-ggcaggacgtactctgaagg-3′.
RNA isolation, cDNA synthesis, and cloning
Mononuclear cells were separated from venous blood using Ficoll-Hypaque (Life Technologies, Paisley, United Kingdom). RNA was prepared using Trizol reagent (Life Technologies). A nested polymerase chain reaction (PCR) protocol involving 2 rounds of PCR was used to amplify the full-length cytb5r cDNA. First-round PCR reactions were set up using forward (5′-gcgacagagcgagcgcggcg-3′) and reverse (5′-ggtggccgtgtgaccggtgc-3′) primers. The second round of PCR used forward (5′-gcatggatccatgggggcccagctcagcacg-3′) and reverse (5′-gcatgaattccctcagaagacgaagcagcgc-3′) primers located inside the first-round primers. The BamHI andEcoRI sites, located in the nested forward and reverse primers, respectively (shown underlined), permitted directional cloning of the full-length cytb5r cDNA into the pGEM-T Easy vector (Promega, Southampton, United Kingdom). Plasmid DNA containing the appropriately sized insert on restriction with BamHI and EcoRI sites was subjected to DNA sequencing.
Sequencing
PCR products were purified using Concert Rapid PCR Purification System (Life Technologies) and were sequenced using ABI Prism BigDye Terminator Cycle Sequencing Ready Reaction Kit Version on ABI 3100 DNA Genetic Analyzer (Applied Biosytems, Warrington, United Kingdom).
Results and discussion
The cytb5r activity, measured using the NADH-ferricyanide method,10 was less than 0.1 IU/g hemoglobin in FM, 6.37 IU/g hemoglobin in BM, and 7.75 IU/g hemoglobin in EH (normal range, 11.51-29.9 IU/g hemoglobin). These results suggested that BM and EH were heterozygote carriers of a cyt5r mutation. DNA sequencing of PCR products of the 9 exons revealed 2 mutations in exon 9 of FM, one in each allele. The first was a point mutation of G to A at codon 291, which would cause a change of glycine to aspartic acid. The second was a 3-bp in-frame deletion resulting in the loss of codon 255 and the deletion of glutamic acid. Each sibling carries one mutation—G to A at codon 291 in BM and the 3-bp deletion causing loss of codon 255 in EH. This was confirmed by cloning and sequencing cDNA from all 3 subjects. Thus, enzymic activity analysis and DNA sequencing indicate that FM is a compound heterozygote and that his siblings BM and EH are heterozygotes for cyt5r mutations.
In the late 1940s, when Gibson was investigating methemoglobinemia, Warburg and his colleagues11 had just shown that sugar is oxidized to pyruvate as methemoglobin is reduced to hemoglobin in intact red cells. It was also known that methylene blue accelerates the rate of reduction of methemoglobin. Gibson1 showed that methylene blue could reduce methemoglobin in the patients' red cells, but incubation of the cells with sugar alone had little effect. He postulated that these patients had an enzymatic defect. Later he showed that iodoacetic acid, an inhibitor of glyceraldehyde-3-phosphate-dehydrogenase, inhibited the reduction of methemoglobin when sugar, but not lactate, was added as substrate, and he deduced that the proposed enzyme defect affected the reaction between Coenzyme I (ie, NAD) and methemoglobin. This deduction was confirmed in many subsequent investigations. An intriguing account of this early work has been recorded by Gibson.12
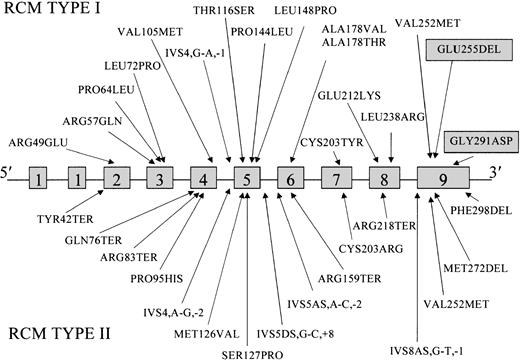
These 2 hitherto unidentified mutations in the family originally studied by Gibson bring to 33 the number of different mutations described in patients with RCM (Figure3). Previously, 15 mutations had been associated with the type 1 disorder13-20 and 16 with type 2 RCM.17,20-27 Mutations have been found in all exons but 1 and 1S. Mutations that cause exon skipping and those predicted to give truncated proteins with severe impairment of function have been described only in type 2, except in one child in whom neurologic abnormalities were not observed at the age of 1 year.19
Locations of the mutations reported to date in the
cytb5r gene of patients with types 2 and 2 RCM.Mutations are shown in relation to the 9 exons of the 31-kb gene. Novel mutations found in the present study are indicated in the shaded boxes.
Locations of the mutations reported to date in the
cytb5r gene of patients with types 2 and 2 RCM.Mutations are shown in relation to the 9 exons of the 31-kb gene. Novel mutations found in the present study are indicated in the shaded boxes.
Because the structure of cytb5r has recently been resolved by x-ray crystallography,28 it is possible to interpret the effects of mutations on enzyme function. Although the 2 novel exon 9 mutations are located outside the FAD and NADH binding sites, each would be expected to have a deleterious effect on enzyme activity (Figure4). Loss of E255 would affect the packed hydrophobic environment formed by I177, L206, A208, T237, M256, M278, and C283, and the presence of aspartic acid at codon 291 instead of the nonpolar glycine would also lead to perturbation of the secondary structure.
Predicted translation, demarcation of soluble and membrane forms, and location of secondary structure elements of cytb5r.
Predicted translation, demarcation of soluble and membrane forms, and location of secondary structure elements of cytb5r.
In 1943, Deeny et al2 reported the first case of RCM in the United Kingdom, and it was hoped that the mode of inheritance would be defined and the genetic basis of the disorder established. More than 40 years later the cytb5r gene was cloned, thereby allowing the genetic basis of the disorder to be defined. It took another 20 years for the mutations in the original family to be identified.
We thank Dr Quentin Gibson for making available his original notebooks and the propositi's sister, EH, for providing family histories.
Prepublished online as Blood First Edition Paper, July 5, 2002; DOI 10.1182/blood-2002-05-1405.
Supported by the Northern Ireland Leukaemia Research Fund.
References
Author notes
Melanie J. Percy, Department of Hematology, Floor C, Belfast City Hospital, Lisburn Road, Belfast, BT9 7AB, Northern Ireland; e-mail:melanie.percy@bll.n-i.nhs.uk.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal