Abstract
Factor V Leiden (FVL) is associated with venous thrombosis; however, an association between FVL and arterial thrombosis remains controversial. We investigated FVL as a risk factor for myocardial infarction (MI), ischemic stroke (IS), or non-MI ischemic heart disease (non-MI–IHD). The design was 3 case-control studies and 3 prospective studies with 21 years' follow-up. The setting was the general population in Copenhagen, Denmark. The participants for The Copenhagen City Heart Study were 20- to 95-year-old participants without cardiovascular disease (control population, n = 7907) or participants diagnosed with MI (n = 469), IS (n = 231), or non-MI–IHD (n = 365). In addition, 3 independent patient populations from Copenhagen University Hospital with MI (n = 493), IS (n = 231), or non-MI–IHD (n = 448) were included. We measured FVL genotype; major cardiovascular risk factors; and MI, IS, and non-MI–IHD incidence and prevalence. Prevalences of FVL heterozygotes and homozygotes in control subjects from the general population were 7.7% and 0.2%. Odds ratios and relative risks of MI in FVL carriers (heterozygotes + homozygotes) versus noncarriers were 1.24 (95% confidence interval [CI], 0.91-1.69) and 0.83 (0.58-1.20) in case-control and prospective studies, respectively. Corresponding risks for IS were 0.92 (95% CI, 0.56-1.53) and 0.68 (0.45-1.04), and for non-MI–IHD 1.01 (95% CI, 0.71-1.44) and 0.97 (0.66-1.42). Findings from The Copenhagen City Heart Study suggest that FVL is not associated with MI, IS, or non-MI–IHD.
Introduction
Since the first report of activated protein C resistance (APC-R) in 1993,1 and later the demonstration of the Arg506Gln mutation in coagulation factor V (factor V Leiden, FVL) as the genetic defect responsible for most APC-R cases,2-4 numerous studies have established FVL as the most frequent hereditary thrombophilic disorder predisposing carriers to venous thrombosis.
Despite considerable effort in assessing FVL as a risk factor for myocardial infarction (MI) and ischemic stroke (IS), the role of FVL in arterial thrombosis remains controversial. Most studies on FVL and arterial thrombosis have demonstrated insignificant or borderline significant associations, some studies favoring a risk reduction in FVL carriers,5-16 others an increase.17-44
The present study (1) estimated risk of thrombosis in atherosclerotic arteries (MI or IS) as well as risk of atherosclerosis without thrombosis (non-MI–ischemic heart disease [non-MI–IHD]) in FVL carriers, using a population-based study; (2) examined if FVL interacts with established cardiovascular risk factors in predicting MI, IS, and non-MI–IHD; and finally (3) systematically combined our data with previously published studies on FVL and risk of arterial thrombosis using meta-analyses.
For these purposes, we conducted 3 case-control studies each including more than 8000 individuals aged 20 to 98 years. In addition, we carried out 3 prospective studies, including more than 9000 individuals with a follow-up time of 21 years. Finally, we present 2 meta-analyses on FVL and risk of MI (all and premature) and IS (adult and childhood) covering data on approximately 31 000 individuals from 35 studies.
Patients, materials, and methods
Study design
Case-control studies.
Three case populations comprising 493 MI patients, 231 IS patients, and 448 non-MI–IHD patients collected at Copenhagen University Hospital were compared with 7907 control subjects from The Copenhagen City Heart Study (free of MI, IS, and non-MI–IHD) (Figure1, study 1-3).
Prospective studies.
Three prospective studies were conducted within The Copenhagen City Heart Study (Figure 1, study 4-6). The cohort was defined as participants in The Copenhagen City Heart Study who attended the 1991-1994 examination and gave blood for DNA analysis. All outcomes (either MI, IS, or non-MI–IHD) were recorded in the follow-up period from 1976 through 1997. Individuals diagnosed with either endpoint before entry into The Copenhagen City Heart Study were excluded from the relevant study.
Subjects
The Copenhagen City Heart Study.
The Copenhagen City Heart Study is a prospective cardiovascular study of individuals selected based on the Central-Population-Register Code to reflect the adult Danish general population. Those invited were stratified into 5-year age groups from 20 to 95 years, with main emphasis placed on the 35- to 70-year-olds. In 1976-1978, a total of 19 329 individuals were invited of whom 74% (14 223) participated. In 1981-1983, the original cohort, supplemented with 500 in the 20- to 25-year-old group, was invited and 70% (12 698) participated. Finally, in 1991-1994, the cohort, further supplemented with 3000 in the 20- to 49-year-old group, was invited and 61% (10 135) participated. Less than 1% was non-Caucasian.
All 3 examinations included a self-administered questionnaire, a physical examination, and blood samples.45 46 In the 1991-1994 examination, additional blood samples for DNA extraction were drawn. DNA was available on 9259 participants of whom 9253 were genotyped for FVL.
Information on diagnoses of IHD (World Health Organization [WHO] International Classification of Diseases, 8th ed, codes 410-414) and ischemic cerebrovascular disease (codes 432-435) were gathered until the end of 1997 from the Danish National Hospital Discharge Register, from the Danish National Register of Cause of Death, and from medical records from general practitioners and hospitals. Of the 834 individuals with IHD, 365 had non-MI–IHD and 469 had suffered an MI. Experienced cardiologists verified the diagnosis of MI requiring the presence of at least 2 of the following criteria: (1) characteristic chest pain, (2) elevated cardiac enzymes, and (3) electrocardiographic changes indicative of MI. Experienced neurologists verified the 410 cases with IS on the basis of sudden onset of focal neurologic symptoms lasting more than 24 hours and a computerized tomography (CT) scan excluding hemorrhage. Among IS cases, 70 and 49 individuals were also diagnosed with MI and non-MI–IHD, respectively.
The study was approved by the Danish ethics committee for the City of Copenhagen and Frederiksberg (No. 100.2039/91). Approval was obtained from the Herlev University Hospital institutional review board for these studies. Informed consent was provided according to the Declaration of Helsinki.
Copenhagen University Hospital.
Patients with IHD were identified among 992 consecutive patients from the Greater Copenhagen area referred for coronary angiography because of angina pectoris from 1991 through 1993. Of these patients, 948 had definite IHD; 941 patients, aged 20-81, were genotyped for FVL. Less than 1% was non-Caucasian.
The diagnosis of MI (n = 493) was established by using the same criteria as in The Copenhagen City Heart Study; the remaining 448 patients had non-MI–IHD. The diagnosis of MI was first given by a referring doctor and was later confirmed by a cardiologist at Copenhagen University Hospital.
Experienced neurologists and vascular surgeons identified 231 patients with IS among 528 patients with more than 50% carotid artery stenosis examined at Copenhagen University Hospital. Ischemic stroke was diagnosed using the same criteria as those applied in The Copenhagen City Heart Study.
Through the use of the Central-Population-Register Code (which unambiguously identifies a person), a search was conducted to clarify potential overlap of cases between populations. Five patients from Copenhagen University Hospital with MI, 1 with non-MI–IHD, and 3 with IS were also registered in The Copenhagen City Heart Study with MI, non-MI–IHD, or IS. These individuals were not excluded from analyses.
Biochemical analyses
The Arg506Gln mutation was identified by using polymerase chain reaction followed by restriction enzyme digestion and agarose gel electrophoresis.47 To avoid misclassification of FVL genotypes, all participants had their genotypes determined from the agarose gel by 2 independent investigators. Furthermore, data entry was compared with a hard copy by 2 independent investigators.
Blood samples from participants in The Copenhagen City Heart Study were drawn in the nonfasting state, whereas samples from patients with MI, non-MI–IHD, and IS were drawn after an overnight fast. Colorimetric and turbidimetric assays were used to measure plasma levels of total cholesterol, high-density lipoprotein (HDL)–cholesterol, triglycerides, and fibrinogen (all Boehringer Mannheim, Mannheim, Germany), and lipoprotein(a) total mass (DAKO A/S, Glostrup, Denmark).
Statistical analyses
Case-control and prospective studies were analyzed by using SPSS.48 Except for analysis of genotype distribution, heterozygous and homozygous individuals were combined (carriers) and compared with noncarriers. Two-sided P < .05 were considered significant. Categorical variables were compared by using Pearson chi-square test. Student t test compared means in 2 group comparisons; plasma triglycerides, lipoprotein(a), and HDL-cholesterol levels were logarithmically transformed to approach normal distribution. As age was not normally distributed, Mann-WhitneyU test compared age in 2 group comparisons.
With the use of an unmatched case-control design, unconditional logistic regression analyses assessed associations between FVL and MI, IS, and non-MI–IHD. Results are given as age-adjusted odds ratios with 95% confidence intervals (CIs). Log-rank tests and Kaplan-Meier curves are given for prospective data. Cox regression analysis was used to estimate relative risk with 95% CIs adjusted for differences in age at entry into The Copenhagen City Heart Study.
Logistic and Cox regression analysis were also performed after stratifying on medians of age, body mass index, cholesterol, HDL cholesterol, lipoprotein(a), triglycerides, and fibrinogen as well as on sex, smoking habits, hypertension, and diabetes mellitus. Formal testing of bivariate multiplicative interaction was done by introducing the 2-factor interaction term in the logistic or Cox regression models. Statistical power was calculated by using GraphPad Statmate.49
Meta-analyses
Only case-control, case-referent, and prospective studies presented in English that correlated FVL genotype (determined on all participants irrespective of APC-R measurements) to either MI or IS were included in meta-analyses. Only a few studies besides our own reported data on non-MI–IHD; therefore, this meta-analysis was not performed. Included studies had to use WHO criteria in the diagnosis of MI (as done in the present study) and in the case of IS only include cases with sudden onset of focal neurologic symptoms lasting more than 24 hours with at least one CT or magnetic resonance scan excluding hemorrhage. Meta-analyses on FVL and IS were performed separately for children and adults, as heterogeneity tests suggested dissimilar association between FVL and IS in these groups. For MI, a separate meta-analysis was likewise performed for premature MI; however, evidence for heterogeneity was not present in MI studies.
A PubMed search was done in October 2001 by using the search terms “Factor V, fv:q506, or APC resistance” combined with the terms “atherosclerosis, ischemic heart disease, myocardial infarction, acute coronary syndrome, ischemic cerebrovascular disease, or stroke.” This search yielded 303 references. Eighteen, 6, 8, and 7 studies met the criteria for inclusion into the meta-analysis on FVL and MI, premature MI, adult IS, and childhood IS. In one instance, 2 studies appeared to cover an overlapping patient population,17,50 one was therefore excluded.17
Only a small number of studies present data for each sex separately. Sex-stratified meta-analyses were therefore only possible with MI as an endpoint and included 2 and 5 studies in women and men, respectively.
Statistical analysis
Data as presented in the original studies were entered into Review Manager.51 In a few cases, only FVL genotype frequencies were reported. In these instances the actual number of FVL individuals was therefore calculated. Heterozygous and homozygous FVL carriers were combined, and comparisons were done for FVL carriers versus noncarriers. Data from our own study were included, combining cases from Copenhagen University Hospitals with those from The Copenhagen City Heart Study, while using the same control group as that used in the case-control studies. Weighted odds ratios with 95% CIs were calculated by using random-effects models. A chi-square test was performed to test for heterogeneity. Visual inspection of funnel-plots was done to check for publication bias.
Results
Except for total cholesterol in MI patients, body mass index in IS patients, prevalence of smokers in non-MI–IHD patients from Copenhagen University Hospital, and lipoprotein(a) levels in IS and non-MI–IHD participants from The Copenhagen City Heart Study, all major cardiovascular risk factors were more prevalent among those with MI, non-MI–IHD, or IS than among control subjects (Table1). In control subjects from the general population, 612 (7.7%) and 17 (0.2%) individuals were heterozygous and homozygous FVL carriers. These frequencies did not differ from those observed in individuals with MI, IS, or non-MI–IHD (Table2) or from those predicted by the Hardy-Weinberg equilibrium (chi-square test, P = .28).
Characteristics of participants
| . | Control . | Myocardial infarction . | Ischemic stroke . | Non-MI ischemic heart disease . | |||
|---|---|---|---|---|---|---|---|
| CCHS . | Patients . | CCHS . | Patients . | CCHS . | Patients . | CCHS . | |
| No. of individuals | 7907 | 493 | 469 | 231 | 410 | 448 | 365 |
| Age, y | 56 ± 0.2 | 58 ± 0.4* | 68 ± 0.4‡ | 63 ± 0.5‡ | 69 ± 0.4‡ | 58 ± 0.5* | 69 ± 0.5‡ |
| Body mass index, kg/m2 | 25.5 ± 0.05 | 26.4 ± 0.2‡ | 26.7 ± 0.2‡ | 25.5 ± 0.3 | 26.1 ± 0.2‡ | 26.4 ± 0.2‡ | 26.5 ± 0.2‡ |
| Cholesterol, mM | 6.1 ± 0.01 | 6.2 ± 0.06 | 6.6 ± 0.05‡ | 6.5 ± 0.08‡ | 6.5 ± 0.06‡ | 6.3 ± 0.06† | 6.4 ± 0.07‡ |
| HDL cholesterol, mM | 1.6 ± 0.01 | 1.1 ± 0.02‡ | 1.4 ± 0.02‡ | 1.4 ± 0.03‡ | 1.5 ± 0.02‡ | 1.2 ± 0.02‡ | 1.5 ± 0.03† |
| Lipoprotein(a), mg/L | 300 ± 4 | 621 ± 29‡ | 397 ± 20* | 442 ± 41† | 361 ± 22 | 573 ± 30‡ | 334 ± 20 |
| Triglycerides, mM | 1.8 ± 0.02 | 2.4 ± 0.1‡ | 2.4 ± 0.1‡ | 2.3 ± 0.1‡ | 2.2 ± 0.1‡ | 2.2 ± 0.1‡ | 2.2 ± 0.1‡ |
| Fibrinogen, mg/dL | 3.0 ± 0.01 | — | 3.6 ± 0.04‡ | — | 3.5 ± 0.04‡ | — | 3.5 ± 0.05‡ |
| Women, % | 57 | 20‡ | 36‡ | 39‡ | 48‡ | 33‡ | 46‡ |
| Smoking, % | 73 | 88‡ | 84‡ | 80* | 80† | 76 | 82‡ |
| Hypertension, % | 17 | 31‡ | 35‡ | 46‡ | 40‡ | 29‡ | 35‡ |
| Diabetes mellitus, % | 3 | 11‡ | 9‡ | 14‡ | 8‡ | 9‡ | 6‡ |
| . | Control . | Myocardial infarction . | Ischemic stroke . | Non-MI ischemic heart disease . | |||
|---|---|---|---|---|---|---|---|
| CCHS . | Patients . | CCHS . | Patients . | CCHS . | Patients . | CCHS . | |
| No. of individuals | 7907 | 493 | 469 | 231 | 410 | 448 | 365 |
| Age, y | 56 ± 0.2 | 58 ± 0.4* | 68 ± 0.4‡ | 63 ± 0.5‡ | 69 ± 0.4‡ | 58 ± 0.5* | 69 ± 0.5‡ |
| Body mass index, kg/m2 | 25.5 ± 0.05 | 26.4 ± 0.2‡ | 26.7 ± 0.2‡ | 25.5 ± 0.3 | 26.1 ± 0.2‡ | 26.4 ± 0.2‡ | 26.5 ± 0.2‡ |
| Cholesterol, mM | 6.1 ± 0.01 | 6.2 ± 0.06 | 6.6 ± 0.05‡ | 6.5 ± 0.08‡ | 6.5 ± 0.06‡ | 6.3 ± 0.06† | 6.4 ± 0.07‡ |
| HDL cholesterol, mM | 1.6 ± 0.01 | 1.1 ± 0.02‡ | 1.4 ± 0.02‡ | 1.4 ± 0.03‡ | 1.5 ± 0.02‡ | 1.2 ± 0.02‡ | 1.5 ± 0.03† |
| Lipoprotein(a), mg/L | 300 ± 4 | 621 ± 29‡ | 397 ± 20* | 442 ± 41† | 361 ± 22 | 573 ± 30‡ | 334 ± 20 |
| Triglycerides, mM | 1.8 ± 0.02 | 2.4 ± 0.1‡ | 2.4 ± 0.1‡ | 2.3 ± 0.1‡ | 2.2 ± 0.1‡ | 2.2 ± 0.1‡ | 2.2 ± 0.1‡ |
| Fibrinogen, mg/dL | 3.0 ± 0.01 | — | 3.6 ± 0.04‡ | — | 3.5 ± 0.04‡ | — | 3.5 ± 0.05‡ |
| Women, % | 57 | 20‡ | 36‡ | 39‡ | 48‡ | 33‡ | 46‡ |
| Smoking, % | 73 | 88‡ | 84‡ | 80* | 80† | 76 | 82‡ |
| Hypertension, % | 17 | 31‡ | 35‡ | 46‡ | 40‡ | 29‡ | 35‡ |
| Diabetes mellitus, % | 3 | 11‡ | 9‡ | 14‡ | 8‡ | 9‡ | 6‡ |
Values are means ± SE or frequencies.
MI indicates myocardial infarction; CCHS, The Copenhagen City Heart Study; HDL, high-density lipoprotein.
P < .05;
P < .01;
P < .001 compared with controls using Mann-Whitney U test, Pearson chi-square test, or Studentt test.
Factor V Leiden genotype frequencies
| . | Control . | Myocardial infarction . | Ischemic stroke . | Non-MI ischemic heart disease . | |||
|---|---|---|---|---|---|---|---|
| CCHS . | Patients . | CCHS . | Patients . | CCHS . | Patients . | CCHS . | |
| Wild-type | 7278 (92.0) | 445 (90.3) | 438 (93.4) | 214 (92.6) | 387 (94.4) | 412 (92.0) | 337 (92.3) |
| Heterozygote | 612 (7.7) | 47 (9.5) | 30 (6.4) | 16 (6.9) | 22 (5.4) | 34 (7.6) | 26 (7.1) |
| Homozygote | 17 (0.2) | 1 (0.2) | 1 (0.2) | 1 (0.4) | 1 (0.2) | 2 (0.4) | 2 (0.5) |
| χ-square test | — | P = .36 | P = .57 | P = .71 | P = .21 | P = .60 | P = .39 |
| . | Control . | Myocardial infarction . | Ischemic stroke . | Non-MI ischemic heart disease . | |||
|---|---|---|---|---|---|---|---|
| CCHS . | Patients . | CCHS . | Patients . | CCHS . | Patients . | CCHS . | |
| Wild-type | 7278 (92.0) | 445 (90.3) | 438 (93.4) | 214 (92.6) | 387 (94.4) | 412 (92.0) | 337 (92.3) |
| Heterozygote | 612 (7.7) | 47 (9.5) | 30 (6.4) | 16 (6.9) | 22 (5.4) | 34 (7.6) | 26 (7.1) |
| Homozygote | 17 (0.2) | 1 (0.2) | 1 (0.2) | 1 (0.4) | 1 (0.2) | 2 (0.4) | 2 (0.5) |
| χ-square test | — | P = .36 | P = .57 | P = .71 | P = .21 | P = .60 | P = .39 |
Values represent number of individuals and percentages in parenthesis. Chi-square test versus controls using 2 × 3 tables.
CCHS indicates The Copenhagen City Heart Study; MI, myocardial infarction.
Risk of MI (study 1 and 4)
Age-adjusted odds ratios for MI in FVL carriers was 1.24 (95% CI, 0.91-1.69) (Table 3). FVL was associated with increased MI risk (in study 1) in individuals without hypertension and in those with low triglycerides (Table 3); however, tests of multiplicative interaction did not support interaction between FVL and hypertension or triglycerides firmly.
Risk of myocardial infarction, ischemic stroke, and nonmyocardial infarction–ischemic heart disease in carriers (heterozygotes + homozygotes) of factor V Leiden
| Interaction test stratification . | Myocardial infarction . | Ischemic stroke . | Non-MI ischemic heart disease . | |||
|---|---|---|---|---|---|---|
| Study 1 . | Study 4 . | Study 2 . | Study 5 . | Study 3 . | Study 6 . | |
| Case-control OR . | Prospective RR . | Case-control OR . | Prospective RR . | Case-control OR . | Prospective RR . | |
| Case/control subjects | 493/7907 | 469/8707 | 231/7907 | 410/8835 | 448/7907 | 365/8818 |
| Overall | 1.24 (0.91-1.69) | 0.83 (0.58-1.20) | 0.92 (0.56-1.53) | 0.68 (0.45-1.04) | 1.01 (0.71-1.44) | 0.97 (0.66-1.42) |
| Sex | P = .21 | P = .72 | P = .81 | P = .20 | P = .06 | P = .74 |
| Female | 0.78 (0.34-1.80) | 0.91 (0.51-1.65) | 0.99 (0.45-2.18) | 0.88 (0.51-1.52) | 1.55 (0.92-2.60) | 1.04 (0.60-1.79) |
| Male | 1.34 (0.94-1.90) | 0.80 (0.50-1.27) | 0.88 (0.46-1.72) | 0.51 (0.26-0.99) | 0.77 (0.47-1.24) | 0.91 (0.53-1.54) |
| Age | P = .42 | P = .63 | P = .53 | P = .45 | P = .43 | P = .87 |
| Median or younger | 1.27 (0.83-1.92) | 0.81 (0.47-1.40) | 1.05 (0.54-2.04) | 0.42 (0.20-0.89) | 0.88 (0.52-1.49) | 0.93 (0.53-1.63) |
| Greater than median | 1.21 (0.76-1.93) | 0.85 (0.52-1.39) | 0.79 (0.36-1.71) | 0.96 (0.57-1.59) | 1.04 (0.62-1.73) | 1.01 (0.60-1.72) |
| Smoking | P = .31 | P = .61 | P = .48 | P = .17 | P = .95 | P = .99 |
| No | 0.84 (0.88-3.83) | 0.68 (0.25-1.87) | 1.30 (0.50-3.41) | 1.12 (0.51-2.43) | 1.03 (0.51-2.10) | 0.99 (0.40-2.48) |
| Yes | 1.19 (0.84-1.69) | 0.88 (0.60-1.30) | 0.86 (0.47-1.56) | 0.55 (0.33-0.93) | 1.03 (0.67-1.57) | 0.99 (0.64-1.51) |
| Hypertension | P = .07 | P = .80 | P = .07 | P = .53 | P = .68 | P = .56 |
| No | 1.46 (1.02-2.08) | 0.85 (0.54-1.34) | 0.51 (0.21-1.26) | 0.75 (0.44-1.26) | 1.04 (0.68-1.59) | 1.03 (0.65-1.65) |
| Yes | 0.73 (0.38-1.40) | 0.77 (0.42-1.42) | 1.37 (0.73-2.60) | 0.57 (0.28-1.16) | 0.87 (0.45-1.67) | 0.82 (0.42-1.62) |
| Diabetes mellitus | P = .76 | P = .96 | P = .35 | P = .74 | P = .74 | P = .12 |
| No | 1.25 (0.90-1.74) | 0.85 (0.58-1.24) | 1.03 (0.61-1.73) | 0.68 (0.44-1.06) | 0.99 (0.68-1.43) | 1.03 (0.70-1.52) |
| Yes | 1.01 (0.34-3.01) | 0.78 (0.19-3.25) | 0.36 (0.05-2.87) | 0.48 (0.07-3.48) | 1.12 (0.34-0.39) | — |
| Body mass index | P = .40 | P = .21 | P = .67 | P = .56 | P = .13 | P = .31 |
| Median or less | 1.38 (0.84-2.28) | 1.08 (0.64-1.84) | 0.77 (0.33-1.78) | 0.58 (0.30-1.13) | 0.52 (0.24-1.13) | 1.17 (0.67-2.03) |
| Greater than median | 1.06 (0.68-1.64) | 0.69 (0.41-1.14) | 0.97 (0.48-1.93) | 0.76 (0.44-1.31) | 1.03 (0.63-1.67) | 0.81 (0.47-1.39) |
| Cholesterol | P = .64 | P = .60 | P = .58 | P = .18 | P = .89 | P = .62 |
| Median or less | 1.17 (0.72-1.89) | 0.73 (0.40-1.34) | 1.06 (0.48-2.33) | 0.45 (0.20-1.01) | 0.92 (0.53-1.62) | 1.08 (0.61-1.90) |
| Greater than median | 1.38 (0.89-2.14) | 0.90 (0.57-1.42) | 0.71 (0.34-1.48) | 0.84 (0.51-1.38) | 0.88 (0.51-1.51) | 0.90 (0.53-1.52) |
| HDL cholesterol | P = .35 | P = .50 | P = .37 | P = .51 | P = .53 | P = .17 |
| Median or less | 1.15 (0.81-1.64) | 0.89 (0.59-1.35) | 0.54 (0.25-1.18) | 0.61 (0.35-1.06) | 0.80 (0.51-1.26) | 1.19 (0.75-1.88) |
| Greater than median | 1.82 (0.76-4.34) | 0.67 (0.31-1.43) | 1.49 (0.70-3.14) | 0.78 (0.41-1.49) | 1.10 (0.47-2.57) | 0.66 (0.32-1.34) |
| Triglycerides | P = .05 | P = .25 | P = .75 | P = .11 | P = .27 | P = .50 |
| Median or less | 1.86 (1.14-3.03) | 1.10 (0.61-1.99) | 0.98 (0.42-2.27) | 0.38 (0.16-0.93) | 1.29 (0.73-2.27) | 1.09 (0.62-1.93) |
| Greater than median | 0.97 (0.64-1.45) | 0.71 (0.45-1.13) | 0.73 (0.36-1.45) | 0.86 (0.53-1.39) | 0.86 (0.54-1.35) | 0.86 (0.51-1.46) |
| Lipoprotein(a) | P = .83 | P = .36 | P = .31 | P = .41 | P = .22 | P = .32 |
| Median or less | 1.19 (0.65-2.18) | 0.68 (0.37-1.24) | 0.68 (0.21-2.20) | 0.55 (0.27-1.11) | 1.39 (0.78-2.45) | 0.77 (0.41-1.45) |
| Greater than median | 1.27 (0.88-1.85) | 0.96 (0.61-1.52) | 1.03 (0.47-2.27) | 0.79 (0.47-1.34) | 0.88 (0.56-1.38) | 1.15 (0.71-1.88) |
| Fibrinogen | P = .80 | P = .92 | P= .81 | |||
| Median or less | — | 0.86 (0.45-1.63) | — | 0.68 (0.32-1.46) | — | 0.89 (0.47-1.70) |
| Greater than median | — | 0.79 (0.50-1.25) | — | 0.74 (0.44-1.22) | — | 1.00 (0.61-1.64) |
| Interaction test stratification . | Myocardial infarction . | Ischemic stroke . | Non-MI ischemic heart disease . | |||
|---|---|---|---|---|---|---|
| Study 1 . | Study 4 . | Study 2 . | Study 5 . | Study 3 . | Study 6 . | |
| Case-control OR . | Prospective RR . | Case-control OR . | Prospective RR . | Case-control OR . | Prospective RR . | |
| Case/control subjects | 493/7907 | 469/8707 | 231/7907 | 410/8835 | 448/7907 | 365/8818 |
| Overall | 1.24 (0.91-1.69) | 0.83 (0.58-1.20) | 0.92 (0.56-1.53) | 0.68 (0.45-1.04) | 1.01 (0.71-1.44) | 0.97 (0.66-1.42) |
| Sex | P = .21 | P = .72 | P = .81 | P = .20 | P = .06 | P = .74 |
| Female | 0.78 (0.34-1.80) | 0.91 (0.51-1.65) | 0.99 (0.45-2.18) | 0.88 (0.51-1.52) | 1.55 (0.92-2.60) | 1.04 (0.60-1.79) |
| Male | 1.34 (0.94-1.90) | 0.80 (0.50-1.27) | 0.88 (0.46-1.72) | 0.51 (0.26-0.99) | 0.77 (0.47-1.24) | 0.91 (0.53-1.54) |
| Age | P = .42 | P = .63 | P = .53 | P = .45 | P = .43 | P = .87 |
| Median or younger | 1.27 (0.83-1.92) | 0.81 (0.47-1.40) | 1.05 (0.54-2.04) | 0.42 (0.20-0.89) | 0.88 (0.52-1.49) | 0.93 (0.53-1.63) |
| Greater than median | 1.21 (0.76-1.93) | 0.85 (0.52-1.39) | 0.79 (0.36-1.71) | 0.96 (0.57-1.59) | 1.04 (0.62-1.73) | 1.01 (0.60-1.72) |
| Smoking | P = .31 | P = .61 | P = .48 | P = .17 | P = .95 | P = .99 |
| No | 0.84 (0.88-3.83) | 0.68 (0.25-1.87) | 1.30 (0.50-3.41) | 1.12 (0.51-2.43) | 1.03 (0.51-2.10) | 0.99 (0.40-2.48) |
| Yes | 1.19 (0.84-1.69) | 0.88 (0.60-1.30) | 0.86 (0.47-1.56) | 0.55 (0.33-0.93) | 1.03 (0.67-1.57) | 0.99 (0.64-1.51) |
| Hypertension | P = .07 | P = .80 | P = .07 | P = .53 | P = .68 | P = .56 |
| No | 1.46 (1.02-2.08) | 0.85 (0.54-1.34) | 0.51 (0.21-1.26) | 0.75 (0.44-1.26) | 1.04 (0.68-1.59) | 1.03 (0.65-1.65) |
| Yes | 0.73 (0.38-1.40) | 0.77 (0.42-1.42) | 1.37 (0.73-2.60) | 0.57 (0.28-1.16) | 0.87 (0.45-1.67) | 0.82 (0.42-1.62) |
| Diabetes mellitus | P = .76 | P = .96 | P = .35 | P = .74 | P = .74 | P = .12 |
| No | 1.25 (0.90-1.74) | 0.85 (0.58-1.24) | 1.03 (0.61-1.73) | 0.68 (0.44-1.06) | 0.99 (0.68-1.43) | 1.03 (0.70-1.52) |
| Yes | 1.01 (0.34-3.01) | 0.78 (0.19-3.25) | 0.36 (0.05-2.87) | 0.48 (0.07-3.48) | 1.12 (0.34-0.39) | — |
| Body mass index | P = .40 | P = .21 | P = .67 | P = .56 | P = .13 | P = .31 |
| Median or less | 1.38 (0.84-2.28) | 1.08 (0.64-1.84) | 0.77 (0.33-1.78) | 0.58 (0.30-1.13) | 0.52 (0.24-1.13) | 1.17 (0.67-2.03) |
| Greater than median | 1.06 (0.68-1.64) | 0.69 (0.41-1.14) | 0.97 (0.48-1.93) | 0.76 (0.44-1.31) | 1.03 (0.63-1.67) | 0.81 (0.47-1.39) |
| Cholesterol | P = .64 | P = .60 | P = .58 | P = .18 | P = .89 | P = .62 |
| Median or less | 1.17 (0.72-1.89) | 0.73 (0.40-1.34) | 1.06 (0.48-2.33) | 0.45 (0.20-1.01) | 0.92 (0.53-1.62) | 1.08 (0.61-1.90) |
| Greater than median | 1.38 (0.89-2.14) | 0.90 (0.57-1.42) | 0.71 (0.34-1.48) | 0.84 (0.51-1.38) | 0.88 (0.51-1.51) | 0.90 (0.53-1.52) |
| HDL cholesterol | P = .35 | P = .50 | P = .37 | P = .51 | P = .53 | P = .17 |
| Median or less | 1.15 (0.81-1.64) | 0.89 (0.59-1.35) | 0.54 (0.25-1.18) | 0.61 (0.35-1.06) | 0.80 (0.51-1.26) | 1.19 (0.75-1.88) |
| Greater than median | 1.82 (0.76-4.34) | 0.67 (0.31-1.43) | 1.49 (0.70-3.14) | 0.78 (0.41-1.49) | 1.10 (0.47-2.57) | 0.66 (0.32-1.34) |
| Triglycerides | P = .05 | P = .25 | P = .75 | P = .11 | P = .27 | P = .50 |
| Median or less | 1.86 (1.14-3.03) | 1.10 (0.61-1.99) | 0.98 (0.42-2.27) | 0.38 (0.16-0.93) | 1.29 (0.73-2.27) | 1.09 (0.62-1.93) |
| Greater than median | 0.97 (0.64-1.45) | 0.71 (0.45-1.13) | 0.73 (0.36-1.45) | 0.86 (0.53-1.39) | 0.86 (0.54-1.35) | 0.86 (0.51-1.46) |
| Lipoprotein(a) | P = .83 | P = .36 | P = .31 | P = .41 | P = .22 | P = .32 |
| Median or less | 1.19 (0.65-2.18) | 0.68 (0.37-1.24) | 0.68 (0.21-2.20) | 0.55 (0.27-1.11) | 1.39 (0.78-2.45) | 0.77 (0.41-1.45) |
| Greater than median | 1.27 (0.88-1.85) | 0.96 (0.61-1.52) | 1.03 (0.47-2.27) | 0.79 (0.47-1.34) | 0.88 (0.56-1.38) | 1.15 (0.71-1.88) |
| Fibrinogen | P = .80 | P = .92 | P= .81 | |||
| Median or less | — | 0.86 (0.45-1.63) | — | 0.68 (0.32-1.46) | — | 0.89 (0.47-1.70) |
| Greater than median | — | 0.79 (0.50-1.25) | — | 0.74 (0.44-1.22) | — | 1.00 (0.61-1.64) |
Values represent odds ratios (OR) and relative risks (RR) with 95% confidence intervals. Calculations are based on slightly varying numbers according to availability of data for each covariate.
All continuous variables, except for age, were stratified according to the median determined in the control group; these medians were body mass index 25 kg/m2, cholesterol 6.1 mM, HDL cholesterol 1.5 mM, lipoprotein(a) 173 mg/dL, triglycerides 1.5 mM, fibrinogen 3.0 mg/dL. For age, the median was determined among case subjects in the relevant study. Age medians were 59, 52, 64, 55, 59, and 55 years in studies 1 through 6, respectively.
MI indicates myocardial infarction; HDL, high-density lipoprotein.
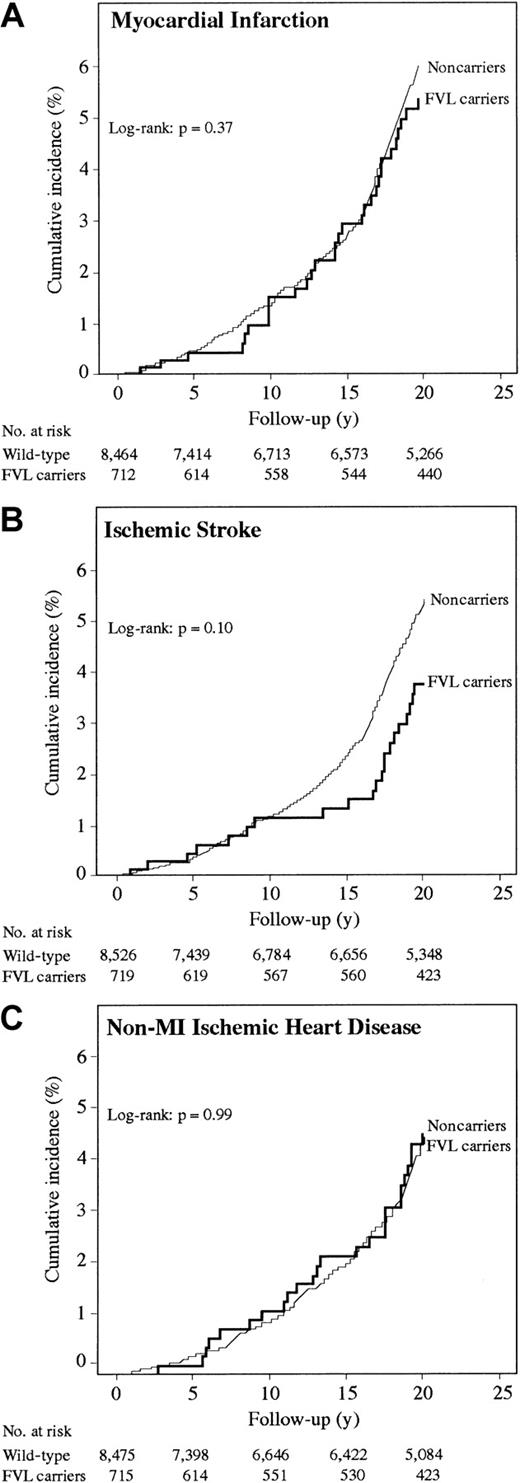
During 156.420 person-years, The Copenhagen City Heart Study cohort experienced 469 MI events, producing an incidence rate of 30 MI per 10 000 person-years. MI incidence did not differ between FVL carriers and noncarriers (Figure 2A; log-rank,P = .37). Age-adjusted relative risk of MI was 0.83 (95% CI, 0.58-1.20) (Table 3). No statistically significant interactions were observed.
Survival functions for FVL carriers (heterozygotes + homozygotes) and noncarriers in The Copenhagen City Heart Study.
Survival functions for FVL carriers (heterozygotes + homozygotes) and noncarriers in The Copenhagen City Heart Study.
Risk of IS (study 2 and 5)
Age-adjusted odds ratio for IS was 0.92 (95% CI, 0.56-1.53) and age-adjusted relative risk was 0.68 (95% CI, 0.45-1.04) (Table 3), with an overall incidence rate of 26 IS per 10 000 person-years. IS incidence did not differ between FVL carriers and noncarriers (Figure2B; log-rank, P = .10). FVL was associated with reduced relative risk in men, those younger than 59 years, smokers, and in individuals with low triglycerides; however, tests of interaction were not significant.
Risk of non-MI–IHD (study 3 and 6)
Age-adjusted odds ratio for non-MI–IHD was 1.01 (95% CI, 0.71-1.44) and age-adjusted relative risk was 0.97 (95% CI, 0.66-1.42) (Table 3), with an incidence rate of 24 non-MI–IHD per 10 000 person-years. Non-MI–IHD incidence did not differ between FVL carriers and noncarriers (Figure 2C; log-rank, P = .99). No statistically significant interactions were observed.
Meta-analyses
Risk of myocardial infarction.
Eighteen previous studies with 3661 MI case subjects and 8611 control subjects met the criteria for inclusion into the meta-analysis on FVL and MI. Average prevalence of FVL in included control populations and MI cases were 5.3% (range, 2.0%-10.9%) and 6.8% (range, 1.9%-21.4%). This finding corresponds to an aggregated odds ratio for MI of 1.30 (95% CI, 1.08-1.57) (Figure3). Sex-stratified analyses revealed odds ratios for MI of 1.19 (95% CI, 0.92-1.54) and 1.28 (95% CI, 0.37-4.39) in men and women, respectively. When The Copenhagen City Heart Study was included, these 3 odds ratios were 1.20 (95% CI, 1.03-1.39), 1.15 (95% CI, 0.95-1.40), and 1.07 (95% CI, 0.56-2.04), respectively.
Meta-analyses on myocardial infarction risk in FVL carriers (heterozygotes + homozygotes).
Studies are listed according to their weight. All, risk of MI including all studies; chi-square test for heterogeneity,P = .61; test for overall effect, P = .02. Men, risk of MI in men only; chi-square test for heterogeneity,P = .95; test for overall effect, P = .15. Women, risk of MI in women only; chi-square test for heterogeneity,P = .07, test for overall effect, P = .80. Premature, risk of MI before age 50 years; chi-square test for heterogeneity, P = .69; test for overall effect,P = .02.
Meta-analyses on myocardial infarction risk in FVL carriers (heterozygotes + homozygotes).
Studies are listed according to their weight. All, risk of MI including all studies; chi-square test for heterogeneity,P = .61; test for overall effect, P = .02. Men, risk of MI in men only; chi-square test for heterogeneity,P = .95; test for overall effect, P = .15. Women, risk of MI in women only; chi-square test for heterogeneity,P = .07, test for overall effect, P = .80. Premature, risk of MI before age 50 years; chi-square test for heterogeneity, P = .69; test for overall effect,P = .02.
Six previous studies with 789 MI case subjects and 1022 control subjects reported data on FVL and risk of MI before age 50 years. Aggregated odds ratio for premature MI in this subanalysis was 1.61 (95% CI, 1.09-2.38) (Figure 3). When The Copenhagen City Heart Study was included, the odds ratio was 1.54 (95% CI, 1.07-2.22).
Risk of ischemic stroke.
Eight previous studies with 1270 IS case subjects and 2269 control subjects were included in the meta-analysis on adults with IS. FVL was present in 7.2% of control subjects (range, 3.0%-8.0%) compared with 5.3% (range, 0%-9.3%) of IS patients. This finding corresponds to an aggregated odds ratio for adult IS of 1.05 (95% CI, 0.73-1.50) (Figure4). When The Copenhagen City Heart Study was included, the odds ratio was 1.00 (95% CI, 0.75-1.34).
Meta-analyses on ischemic stroke risk in FVL carriers (heterozygotes + homozygotes).
Studies are listed according to their weight. Adults, risk of IS in adults only; chi-square test for heterogeneity, P = .54; test for overall effect, P = 1.00. Children, risk of IS in children only; chi-square test for heterogeneity, P = .81; test for overall effect, P < .000 01.
Meta-analyses on ischemic stroke risk in FVL carriers (heterozygotes + homozygotes).
Studies are listed according to their weight. Adults, risk of IS in adults only; chi-square test for heterogeneity, P = .54; test for overall effect, P = 1.00. Children, risk of IS in children only; chi-square test for heterogeneity, P = .81; test for overall effect, P < .000 01.
Seven previous studies with 453 IS case subjects and 1180 control subjects were included in the meta-analysis on childhood IS. The prevalence of FVL among control subjects was 6.7% (range, 3.3%-13.8%) and 18.5% (range, 5.4%-34.9%) among case subjects. The aggregated odds ratio for IS in this analysis was 4.79 (95% CI, 3.26-7.03) (Figure 4).
Discussion
Meta-analyses of previous mainly cross-sectional studies on FVL versus noncarriers on risk of MI and adult IS produced odds ratios of 1.30 (95% CI, 1.08-1.57) and 1.05 (95% CI, 0.73-1.50). We, therefore, felt that insufficient data were available to advocate or reject FVL as a risk factor for arterial thrombosis and, consequently, undertook the present study, including prospective designs.
Myocardial infarction
Both case-control and prospective studies from The Copenhagen City Heart Study suggest that FVL is not associated with an increased risk of MI. The credibility of this conclusion is reflected in a statistical power of 95% not to overlook a 50% increase in relative risk of MI (α = .05). However, the present study achieves only 40% power (α = .05) to detect a 20% increase in relative risk.
Obviously, an overall lack of association could conceal an association present only in a specific context. Association between FVL and MI may be influenced by sex,5,9,20,23,29,52 age,21smoking,9,22,23 hypertension,9,22,30 diabetes mellitus,9,22 or hypercholesterolemia9 22; however, none of these proposed interactions have been definitively established. We, therefore, performed analyses stratified on 11 different cardiovascular risk factors and performed formal tests of multiplicative interaction. Despite this scrutiny, we did not observe evidence for the existence of interaction between FVL and sex, age, smoking, diabetes mellitus, body mass index, cholesterol, HDL cholesterol, lipoprotein(a), or fibrinogen. Although FVL was associated with an increased MI risk in those without hypertension (and not in those with hypertension) and in those with low triglycerides (and not in those with high triglycerides) in the case-control study, this evidence was not found in the prospective study. This discrepancy together with insignificant tests of multiplicative interaction suggests that these associations are chance findings.
Several factors may explain why some previous studies find context-dependent associations,21,23,24 32 whereas the present study overall fails to confirm such associations. Discrepancies may reflect different population ethnicity between studies. Furthermore, because ethnic origin strongly determines FVL prevalence, even slightly different ethnic composition of case and control groups could lead to spurious results. Because the populations studied in the present paper are ethnically homogenous, such selection bias is unlikely to seriously affect our results. Finally, several of the previously observed context-dependent associations could represent chance findings, just as we believe might be the case for our own observation of increased MI risk in FVL carriers in those without hypertension or with low triglycerides and of decreased risk of IS in FVL carriers in men, those younger than 59 years, smokers, and those with low triglycerides.
Results from the present meta-analysis on FVL and MI comprising 19 studies of approximately 17 500 individuals suggest that FVL may be associated with a 20% increase in the risk of MI. The Copenhagen City Heart Study together with the 5 largest studies previously published18,20,22,32 53 largely account for this conclusion, contributing approximately 60% to the overall result of the meta-analysis. All 6 studies demonstrate statistically insignificant odds ratios above 1.0 (range, 1.03-1.40). However, 2 findings question the conclusions of this meta-analysis: (1) results from the prospective studies from The Copenhagen City Heart Study do not support an association and (2) no significant association was demonstrated between FVL and adult IS.
Premature MI, in which the effect of prothrombotic conditions is thought to outweigh the importance of atherosclerosis, is a clinical situation that has attracted special interest with respect to FVL. In The Copenhagen City Heart Study, we did not find evidence of interaction between FVL and age and report an odds ratio for premature MI of 1.13 (range, 0.40-3.16). As an isolated finding, this conclusion should be interpreted with caution because of limited sample size. The present study thus includes only 41 cases of premature MI, of which 4 are FVL carriers. Therefore, we performed a meta-analysis on MI before age 50 years. The 5 largest studies on premature MI so far published (including our own present study)17,20,23,24 reported odds ratios for MI between 1.13 and 2.45; however, only one study reached statistical significance.23 The present meta-analysis on premature MI yielded an odds ratio of 1.54 (95% CI, 1.07-2.22).
Ischemic stroke
Both case-control and prospective studies from The Copenhagen City Heart Study argue against an association between FVL and risk of adult IS, the other major clinical presentation of arterial thrombosis. This conclusion is supported by the meta-analysis, which, contrary to the result on MI, also failed to support a positive association between FVL and adult IS. However, even when combining all available data on FVL and adult IS in the present meta-analysis, the statistical power is only 40% (α = .05) to detect a 20% risk increase.
Context-dependent associations between FVL and IS in The Copenhagen City Heart Study include reduced IS risk in men, those younger than 59 years, smokers, and individuals with low triglycerides. Because (1) this finding is in contrast with the expectation of increased risk of IS, (2) similar results are not found in the case-control study, (3) there was no evidence of multiplicative interaction and, (4) these associations have never been previously reported, we believe that these context-dependent reduced risks of IS most likely represent chance findings.
Contrary to the conclusion on FVL and adult IS, the meta-analyses on FVL and childhood IS suggest that FVL may, in fact, increase risk of IS in children. As The Copenhagen City Heart Study holds data on adults only, we are, unfortunately, not able to test this finding in a prospective manner.
Previously, a meta-analysis on FVL and risk of coronary heart disease and cerebrovascular events has been published.54 In that study, considerably fewer studies were included than in the present paper. Furthermore, the endpoints examined were heterogeneous, including both acute ischemic events and nonacute disease. Finally, individual results on cerebrovascular events appeared to be heterogeneous in the meta-analysis. With these limitations in mind, Wu and Tsongalis54 reported an odds ratio for coronary heart disease of 1.24 (95% CI, 0.96-1.60) and for adult cerebrovascular events of 1.43 (1.03-1.97).
Nonmyocardial infarction ischemic heart disease
Few studies have specifically investigated the hypothesis that FVL contributes to atherogenesis, and the results are inconclusive.55-58 Data from The Copenhagen City Heart Study, both case-control and prospective, do not support the hypothesis that FVL increases risk of non-MI–IHD.
Limitations
An objection to the prospective studies presented here is that only individuals who survived to attend the third examination were studied. Both excessive and decreased mortality among FVL carriers could influence our conclusions. There are, however, several arguments against such bias: (1) the genotype distribution in The Copenhagen City Heart Study was not different from that predicted by the Hardy-Weinberg equilibrium, (2) the prevalence of FVL carriers did not change as a function of age (data not shown), (3) limiting analyses to a follow-up period after the third Copenhagen City Heart Study examination 1991-1994 yielded results similar to those presented, and, finally, (4) FVL genotype distribution observed in The Copenhagen City Heart Study was similar to that observed among Danish newborns.59 If a higher mortality applies to FVL carriers than to controls, the present study designs would lead to conservative risk estimates.
The studies entered into the present meta-analyses were all assessed to be of adequate quality. Nevertheless, individual shortcomings may blur the overall conclusion. A major objection bears on misclassification of case and control subjects. In an attempt to minimize such bias, only studies using rather strict diagnostic criteria for cases were included (WHO criteria for MI and only strokes verified as ischemic in origin by either CT or magnetic resonance scan). Controls were, however, declared free of MI or IS by using different and often less reliable criteria. The consequence of misclassified case subjects and undiagnosed patients in control groups will, if an association does in fact exist, result in a conservative risk estimate and thus possibly a false-negative finding. Another major concern in meta-analyses is publication bias, ie, the tendency of papers (often reporting on studies of limited size) with positive associations to be published in preference to papers supportive of the null hypothesis. Plots of the standard error of the log (odds ratio) as a function of the odds ratio (funnel plots) in the present meta-analyses were symmetrical and, therefore, argue against such publication bias. Despite the reassuring funnel plots, however, publication bias cannot be ruled out completely.
We thank Hanne Damm for technical assistance and the participants of The Copenhagen City Heart Study for their willingness to participate.
The sponsors of the study are public or nonprofit organizations that support science in general. They had no role in gathering, analyzing, or interpreting the data and had no right to approve or disapprove the submitted paper.
Prepublished online as Blood First Edition Paper, April 17, 2002; DOI 10.1182/blood-2002-01-0111.
Supported by The Danish Heart Foundation, The Danish Medical Research Council, Lykfeldt's Fund, Dagmar Marshall's Fund, Wedelborg's Fund, Lily Benthine Lund's Fund, The Beckett Fund, Chief Physician Johan Boserup's and Lise Boserup's Fund, and P. Carl Petersen's Fund.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Børge G. Nordestgaard, Department of Clinical Biochemistry, Herlev University Hospital, DK-2730 Herlev, Denmark; e-mail: brno@herlevhosp.kbhamt.dk.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal