Abstract
To identify the role of iron overload in the natural history of liver fibrosis, we reviewed serial hepatic biopsy specimens taken annually from patients cured of thalassemia major by bone marrow transplantation. The patients underwent transplantation between 1983 and 1989 and did not receive any chelation or antiviral therapy. Two hundred eleven patients (mean age, 8.7 ± 4 years) were evaluated for a median follow-up of 64 months (interquartile range, 43-98 months) by a median number of 5 (interquartile range, 3-6) biopsy samples per patient. Hepatic iron concentration was stratified by tertiles (lower, 0.5-5.6 mg/g; medium, 5.7-12.7 mg/g; upper, 12.8-40.6 mg/g dry weight). Forty-six (22%) patients showed signs of liver fibrosis progression; the median time to progression was 51 months (interquartile range, 36-83 months). In a multivariate Cox proportional hazard model, the risk for fibrosis progression correlated to medium hepatic iron content (hazard rate, 1.9; 95% confidence interval [CI], 0.74-5.0), high hepatic iron content (hazard rate, 8.7; 95% CI, 3.6-21.0) and hepatitis C virus (HCV) infection (hazard rate, 3.1; 95% CI, 1.5-6.5). A striking increase in the risk for progression was found in the presence of both risk factors. None of the HCV-negative patients with hepatic iron content lower than 16 mg/g dry weight showed fibrosis progression, whereas all the HCV-positive patients with hepatic iron concentration greater than 22 mg/g dry weight had fibrosis progression in a minimum follow-up of 4 years. Thus, iron overload and HCV infection are independent risk factors for liver fibrosis progression, and their concomitant presence results in a striking increase in risk.
Introduction
Liver fibrosis and cirrhosis are well-known complications of thalassemia, but no data are available on the risk for and rate of fibrosis progression in this disease or other posttransfusional iron overload conditions.
A bone marrow transplantation (BMT) program1 for thalassemia has been operational in Pesaro, Italy since 1983. At that time there was no knowledge of the effect of iron overload and chelation therapy after BMT; therefore, watchful waiting through periodic percutaneous liver biopsy was established for all patients cured of thalassemia by BMT. These patients were affected by iron overload but did not receive any chelation therapy. Before entering an effective iron reduction program, they had stable liver iron levels and represented unique model group to study the natural history of liver fibrosis in this condition. Thus, all the hepatic biopsies performed during this watchful waiting program have been blindly re-evaluated to assess the risk for and the rate of fibrosis progression.
Patients and methods
Study population
Patients cured of thalassemia by BMT in Pesaro took part in a watchful waiting program based on yearly liver biopsy to monitor unanticipated complications of marrow transplantation and iron overload. All these patients had received unmodified bone marrow from an HLA-identical related donor. All but 4 were prepared for transplantation with busulfan (14 mg/kg) and cyclophosphamide (200 mg/kg)1 (the other 4 patients received 120 mg/kg cyclophosphamide).2 Three graft-versus-host disease (GVHD) prophylaxis regimens were used—either methotrexate or cyclosporine1 or combination cyclosporine and “short” methotrexate.2 Liver biopsy was performed before transplantation to assess each patient's risk1 and was scheduled annually thereafter.
Patients who underwent transplantation from January 1, 1983 to December 31, 1989 were evaluated. No patient underwent transfusion, chelation therapy, or antiviral therapy after recovery from transplantation, and none presented, after transplantation, risk factors for hepatitis C virus (HCV) infection or for other liver injuries. During the follow-up period, serum ferritin, serum aspartate (AST), and serum alanine (ALT) aminotransaminase levels were determined yearly by standard commercial methods. Transaminases are expressed as multiplied by the upper level of the normal range (AST, 2-37 U/L normal range; ALT, 2-41 U/L normal range). Serum ferritin is expressed as micrograms per liter (normal range, 20-200 μg/L). Transaminase and ferritin obtained in the first year after transplantation were not included in this analysis because of the effect of chemotherapy. Hepatitis B virus (HBV) status was determined before transplantation and yearly thereafter. Second-generation enzyme-linked immunosorbent assay was used from 1991 to 1992 to assess HCV status. Serum polymerase chain reaction for HCV was used from 1993 to 1994 (Roche Diagnostic Systems, Basel, Switzerland). Patients were excluded from the analyses when they entered an effective program of iron reduction3,4 or interferon therapy.5 This iron removal program was started in Pesaro in 1992 to 1993 on the basis of serum ferritin3as the hepatic iron concentration, determined later on paraffin-embedded specimens. The iron removal program has been widely applied since 1994.
After the procedure and its risks had been explained in detail, written informed consent was obtained from each patient or the patient's parents, or both, before biopsy. Protocols for transplantation and liver biopsy were approved by the institutional review board of the Pesaro Hospital. The study design was approved by the hospital review board.
Liver biopsy
Biopsy execution techniques and complications have been extensively described elsewhere.6 No fatality was registered, and only one complication (intra-abdominal bleeding not requiring surgery)6 occurred in the follow-up biopsies. Specimens obtained were processed according to standard methodology, and the following stains were used: hematoxylin-eosin, periodic acid–Schiff (PAS), diastase-PAS, Mallory trichrome stain, Gomori silver impregnation, and Prussian blue reaction. All the biopsy samples were reviewed from 1999 to 2000 by 2 pathologists (P.M. and S.T.). A biopsy specimen was considered adequate if 5 or more portal tracts were present. This was considered the absolute minimum necessary for the assessment of fibrosis and cirrhosis because the ability to identify these processes varies considerably, depending on the size of the biopsy specimen.7
Each pathologist graded the findings according to the Ishak staging score,8 which divides liver fibrosis into 7 categories: 0, no fibrosis; 1, fibrosis in some portal areas; 2, fibrosis in most portal areas; 3, portal-to-portal bridging; 4, marked fibrosis with portal-to-portal and portal-to-central bridging; 5, marked fibrosis with occasional nodules (incomplete cirrhosis); 6, definite cirrhosis. Pathologists were unaware of the patients' clinical status and date of biopsy and of the hepatic iron content of each biopsy specimen. After the completion of the initial review, the 2 pathologists conducted a consensus review in which biopsy specimens with discordant grading definition were examined jointly, and a final decision was made. Hepatic iron concentration was determined on the first posttransplantation informative, paraffin-embedded biopsy specimen by atomic absorption spectrophotometry.6,9 Hepatic iron was expressed as milligram per gram liver tissue dry weight (dw), which can be converted to micromols of iron per gram by multiplying by 17.9 and to wet weight by dividing the concentration by 3.33 on the assumption that the liver is 70% water.10 Determinations performed on liver specimens whose gross weight was less than 1 mg dw were not considered for this data analysis.11
Fibrosis progression
Clinically significant progression of fibrosis was considered to have occurred if there was a change in the histologic score from 0 (no fibrosis) to 1 or greater, from 1 or 2 to 3 or greater, or from 3 or 4 to 5 or 6. Changes in the score from 1 to 2, from 3 to 4, or from 5 to 6 were not considered clinically significant.12 This method was chosen because of the clinical significance of the appearance of fibrosis without bridging (stages 1 and 2), the development of porto-portal and porto-central bridging (stages 3 and 4), and the appearance of regenerative nodules (stages 5 and 6). Moreover, considering only easily visible major morphologic signs of progression clearly decreases the possibility of sampling variability. Patients whose initial biopsy sample showed incomplete or definite cirrhosis (n = 10) could not be evaluated for progression of fibrosis and were not entered in the study. Fibrosis regression was not recorded for this study.
Rate of progression
Rate of progression was determined by dividing the difference between the final score and the initial score by years of follow-up (Δ score/y). For the assessment of rate of progression, only patients with at least 4 years of follow-up were considered (n = 160; median follow-up, 80 months; interquartile range, 63-113 months).
Statistical analysis
For continuous variables with symmetric distribution, the results are expressed as mean ± SD, and for those with a skewed distribution, the results are expressed as median and interquartile range (25th to 75th percentiles). For the purpose of analysis, hepatic iron concentration was divided in tertiles (lower tertile: range, 0.5-5.6 mg/g dw, median, 3.8 mg/g dw; medium tertile: range, 5.7-12.7 mg/g dw, median, 8.7 mg/g dw; upper tertile: range, 12.8-40.6 mg/g dw, median, 19.2 mg/g dw).
Time to progression was estimated from the date of the first biopsy (21-28 days before transplantation) until the date of the first liver biopsy documenting the progression of fibrosis. Correlates of progression were first investigated with a series of univariate analyses by using life tables and the log-rank χ2 test. Multivariate analysis with a Cox proportional hazard model was then performed to investigate the role of potentially confounding risk factors on patient outcome. The following variables were tested in the Cox model: age (lower tertile, 1-6 years, reference category [rc]; medium tertile, 7-11 years; upper tertile, 12-17 years), gender, anti–HCV status (positive or negative), HBV status (positive or negative), GVHD prophylaxis (methotrexate [rc] or cyclosporine or cyclosporine plus methotrexate), acute GVHD (grades 0-2 or grades 3-4) chronic GVHD (yes or no), histologic hepatic GVHD (yes or no), basal fibrosis Ishak stage (0 [rc] or 1-2 or 3-4). Correlation between fibrosis progression and serum AST and ALT levels (normal range [rc], less than 2 times upper limit normal range, more than 2 times upper limit normal range) and serum ferritin (less than 1300 μg/L [rc], 1301-2500 μg/L, more than 2500 μg/L) was analyzed by using life tables and the log-rank χ2 test.
The Cox model was also used to plot estimated cumulative fibrosis progression-free functions according to HCV status and hepatic iron concentration categories. Data analysis was discontinued in the 10th year of follow-up because only a few patients had a longer period of observation.
Rates of progression according to hepatic iron concentration categories were compared by using Kruskal-Wallis one-way analysis of variance. All statistical analyses were performed using SPSS version 6.0 software (SPSS, Chicago, IL).
Results
Two hundred thirty-three consecutive thalassemia-free patients who underwent transplantation between January 1, 1983 and December 31, 1989 entered the study and were analyzed. Twenty-two patients could not be evaluated because they did not undergo at least 2 informative liver biopsies (mean age, 5 years; median fibrosis stage of the available biopsy, 1). The remaining 211 patients (91% of the eligible patients) were evaluated for a median follow-up of 64 months (interquartile range, 43-98 months). Patients' mean age was 8.7 years (range, 2-17 years); 120 were boys and 91 were girls. Median hepatic iron concentration was 8.7 mg/g dw (interquartile range, 4.7-15.5 mg/g dw). Median serum ferritin level during the follow-up was 1615 μg/L (interquartile range, 105-2684 μg/L). Median AST and ALT levels during the follow-up were 1.5 (interquartile range, 1-2.4) and 2.5 (interquartile range, 1.3-4.5) times the upper limit of the normal range, respectively. One hundred twelve patients were anti-HCV–positive, and in 81 (72%) of them a positive plasma polymerase chain reaction was subsequently demonstrated. HCV status was not determined in 10 patients. Ten patients were HBV surface antigen–positive. Basal fibrosis stage was 0 in 35 patients, 1 or 2 in 114 patients, and 3 or 4 in 62 patients. A median number of 5 evaluable biopsies per patient (interquartile range, 3-6 biopsies) were performed. Median interval between biopsies was 18 months (interquartile range, 14-25 months) in the entire group and 16 months (interquartile range, 12-21 months) in patients with fibrosis progression.
Fibrosis progression
Forty-six patients (22%) had fibrosis progression during the follow-up, and 165 (78%) had stable liver fibrosis scores (5 patients had variations from stage 1 to stage 2, and 2 patients had variations from stage 3 to stage 4, which, according to the study methods, was not considered a clinically significant progression of liver fibrosis). Median time to progression was 51 months (interquartile range, 36-83 months). Only one patient had 2-step fibrosis progression during the entire follow up. Thirteen (6%) patients acquired incomplete or definite cirrhosis; 10 of these patients were anti-HCV–positive and 3 were anti-HCV–negative, and 1 was coinfected with HCV and HBV. All but 2 had high iron levels. The 2 patients in the medium iron level were HCV-positive and HBV-negative. Median time to cirrhosis development was 50 months (interquartile range, 31-80 months).
Univariate and multivariate analyses
Several factors were analyzed as risk factors for fibrosis progression (ns = not significant): sex (P = ns), acute GVHD (P = ns), chronic GVHD (P = ns), histologic hepatic GVHD (P = ns), basal fibrosis stage (P = ns), HBV status (P = .04), GVHD prophylaxis (P = .03), age (P = .001), hepatic iron concentration (P < .001), and anti-HCV status (P = .001).
In multivariate analyses, only hepatic iron concentration and HCV status were confirmed to be statistically significant risk factors predicting fibrosis progression. Table 1reports results of the Cox regression analysis.
Results of multivariate Cox proportional hazard model
| . | Hazard rate . | 95% CI . | Two-tailed P . |
|---|---|---|---|
| Medium hepatic iron, mg/g dw (range, 5.7-12.7; median, 8.7) | 1.9 | 0.74-5.0 | .18 |
| High hepatic iron, mg/g dw (range, 12.8-40.6; median, 19.2) | 8.7 | 3.6-21.0 | < .001 |
| Anti–HCV-positive | 3.1 | 1.5-6.5 | .002 |
| . | Hazard rate . | 95% CI . | Two-tailed P . |
|---|---|---|---|
| Medium hepatic iron, mg/g dw (range, 5.7-12.7; median, 8.7) | 1.9 | 0.74-5.0 | .18 |
| High hepatic iron, mg/g dw (range, 12.8-40.6; median, 19.2) | 8.7 | 3.6-21.0 | < .001 |
| Anti–HCV-positive | 3.1 | 1.5-6.5 | .002 |
Mean ALT and AST values during the follow-up were correlated with fibrosis progression in HCV-positive patients (P = .01 andP = .05, respectively) but not in HCV-negative patients (P = .4 and P = .12, respectively). Serum ferritin correlated with fibrosis progression (P < .001) in HCV-positive and HCV-negative patients.
Hepatic iron–HCV interaction
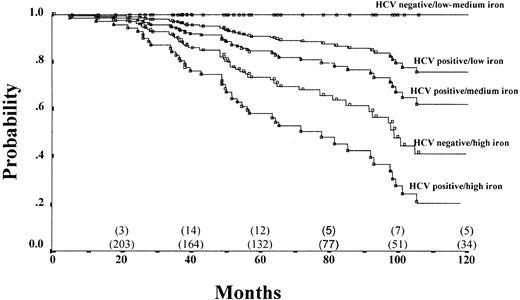
To study the interaction between hepatic iron and anti-HCV status, patients were stratified into 6 groups according to hepatic iron and HCV status. HCV-negative patients with low and medium hepatic iron levels did not show progression of fibrosis and were, therefore, grouped together. Figure 1 reports the probability of remaining free from fibrosis progression in these 5 categories of patients.
Probability of fibrosis progression-free survival by iron and HCV status after controlling for confounding variables.
Patients were stratified into 6 groups according to hepatic iron concentration and HCV status. HCV-negative patients with low and medium hepatic iron levels did not show progression of fibrosis and were grouped together: HCV-negative and low-medium iron (0.5-12.7 mg/g dw), n = 62; HCV-positive and low iron (0.5-5.6 mg/g dw), n = 32; HCV-positive and medium iron (5.7-12.7 mg/g dw), n = 43; HCV-negative and high iron (12.8-40.6 mg/g dw), n = 27; HCV-positive and high iron (12.8-40.6 mg/g dw), n = 37.
Probability of fibrosis progression-free survival by iron and HCV status after controlling for confounding variables.
Patients were stratified into 6 groups according to hepatic iron concentration and HCV status. HCV-negative patients with low and medium hepatic iron levels did not show progression of fibrosis and were grouped together: HCV-negative and low-medium iron (0.5-12.7 mg/g dw), n = 62; HCV-positive and low iron (0.5-5.6 mg/g dw), n = 32; HCV-positive and medium iron (5.7-12.7 mg/g dw), n = 43; HCV-negative and high iron (12.8-40.6 mg/g dw), n = 27; HCV-positive and high iron (12.8-40.6 mg/g dw), n = 37.
Rate of fibrosis progression
Figure 2 reports the rate of fibrosis progression in HCV-negative and in HCV-positive patients according to hepatic iron concentration (P < .001 andP = .009, respectively). In the HCV-negative group, no patient with hepatic iron content less than 16 mg/g dw showed fibrosis progression. No such threshold of fibrosis progression could be identified in HCV-positive patients. All the HCV-positive patients with hepatic iron concentration greater than 22 mg/g dw had fibrosis progression by a minimum follow-up of 4 years.
Rate of fibrosis progression in HCV-negative and HCV-positive patients according to hepatic iron concentration.
Rate of fibrosis progression was determined by dividing the difference between the final fibrosis score and the initial fibrosis score by years of follow-up (Δ score/y). Minimum follow-up, 4 years; median follow-up, 86 months; interquartile range, 63-113 months. HCV-negative, n = 67; P < .001. HCV-positive, n = 93;P = .009. In the HCV-negative group, no patient with hepatic iron content lower than 16 mg/g dw showed fibrosis progression. No such threshold for fibrosis progression could be identified in HCV-positive patients. All HCV-positive patients with hepatic iron concentration greater than 22 mg/g dw had fibrosis progression.
Rate of fibrosis progression in HCV-negative and HCV-positive patients according to hepatic iron concentration.
Rate of fibrosis progression was determined by dividing the difference between the final fibrosis score and the initial fibrosis score by years of follow-up (Δ score/y). Minimum follow-up, 4 years; median follow-up, 86 months; interquartile range, 63-113 months. HCV-negative, n = 67; P < .001. HCV-positive, n = 93;P = .009. In the HCV-negative group, no patient with hepatic iron content lower than 16 mg/g dw showed fibrosis progression. No such threshold for fibrosis progression could be identified in HCV-positive patients. All HCV-positive patients with hepatic iron concentration greater than 22 mg/g dw had fibrosis progression.
Discussion
The BMT program for thalassemia was started in Pesaro in 1983. At that time there was neither precise knowledge of the effects of iron overload and chelation on children after BMT nor knowledge of HCV infection. Patients were not treated for iron overload and viral infections, but a watchful waiting program with periodic percutaneous liver biopsies was established to monitor unanticipated complications of marrow transplantation and persistent iron overload after the cure of thalassemia. Significant progress has been made in this field in the last 2 decades, and it is now possible to identify and prevent potentially tragic late complications of persistent iron overload, including liver disease,3 heart disease,13and other organ damage. In Pesaro, beginning in 1992 to 1993, a program to reduce iron overload in patients who successfully underwent BMT was established.3 4
From the data collected in this watchful waiting program, we were able to establish that forty-six (22%) patients had worsening of hepatic fibrosis following BMT, but only one had 2-step progression. To further prove the validity of the results, we analyzed the data using 1-point Ishak score variation to define progression, which we found made no difference in the results (data not shown). Thirteen of these patients (6% of total, 28% of the progressive fibrosis group) acquired incomplete or definite cirrhosis. Of these, all but one had bridging fibrosis at the beginning of the study.
This study shows that hepatic iron overload and HCV infection are independent risk factors for hepatic fibrosis progression. None of the other analyzed risk factors resulted in significant correlation to fibrosis progression in the multivariate analyses. Even age, found to be a significant predictor of risk in the univariate analyses, failed to confirm its role in the multivariate Cox proportional hazard model. However, this study reports a homogeneous and limited age population (range, 2-17 years at the start of the study), and an adverse effect of age on fibrosis progression cannot be excluded in older patients.
In the intermediate iron group (range, 5.7 to 12.7 mg/g dw), a trend was noted to an increase in risk for fibrosis progression with increase in iron overload, which did not reach statistical significance. There is a marked increase in this risk when iron overload and HCV infection are present at the same time. It was not possible to calculate the hazard rate for the interaction between the 2 risk factors because of the lack of events in the HCV-negative, low-moderate iron group (Figure1). The role of iron overload was indirectly confirmed by serum ferritin whereas elevations in AST and ALT were found to be associated with fibrosis progression only in HCV-positive patients with concomitant inflammatory liver disease. The present data set is inadequate to study the effect of HBV because only 5% of the patients were HBV-positive. To avoid HBV interference with the results, we repeated the analyses excluding HBV-positive patients and found that the results did not change (data not shown).
As shown in Figure 1, the risk for fibrosis progression, which is a continuous phenomenon, was estimated using a time-dependent analysis. We also performed logistic regression to further validate the results. This second kind of analysis confirmed the time-dependent analysis (data not shown). The fact that biopsies were performed at predetermined intervals contributes to the time placement for the event, but, because the median interval between biopsies was 16 months, the median maximum error of the probability expressed in Figure 1 was not more than 16 months.
Our data indicate that the rate of fibrosis progression significantly correlates to the hepatic iron concentration in HCV-negative and HCV-positive patients. In HCV-negative patients, a threshold for fibrosis progression could be identified (16 mg/g dw) with follow-up from 4 to 10 years. Loreal et al14 showed in untreated patients with hereditary hemochromatosis (mean age, 44 ± 12 years) that mean hepatic iron concentration was 13 ± 6 mg/g dw in prefibrotic subjects and 18 ± 9 mg/g dw in patients with fibrosis. Despite the different ages and the fact that HCV status was not determined, in the Loreal et al14 study most of the patients in the nonalcoholic subgroup, with hepatic iron concentration less than 16 mg/g dw, did not have hepatic fibrosis. Thus, both the Loreal study and our data support the idea that in HCV-negative patients, the rate of fibrosis progression is proportional to the amount of hepatic iron for hepatic iron concentrations greater than 16 mg/g dw (Figure 2A). In HCV-positive patients, no threshold effect could be identified for progression of fibrosis, but all HCV-positive patients with hepatic iron concentrations greater than 22 mg/g dw had fibrosis progression by a minimum follow-up of 4 years (Figure 2B). Although our data do not directly address the controversies regarding the progression of liver fibrosis in patients receiving oral iron chelators,12,15 16 it seems to support the idea that in patients with liver iron loading less than 16 mg/g dw, progression of hepatic fibrosis should not simply be presumed to be caused by iron overload, especially in the presence of hepatitis C virus infection.
In immunocompromised HCV-infected patients, rapid progression toward liver failure has been reported17 and seems to be proportional to the level and duration of immunosuppression.18-20 It is possible that the period of immunosuppression associated with BMT and its complications may alter the course of chronic HCV infection, but subsequent restoration of normal immune function may prevent rapid progression to cirrhosis. However, the recovery of immunologic competence occurs 3 to 6 months after BMT,21 and the patients reported here presented favorable risk profiles for producing fast immune recovery (age, age of donor, nonmalignant disease).22 In our data set, none of the transplantation-related factors (GVHD, GVHD prophylaxis) showed a relationship to fibrosis progression, and the plot of the cumulative hazard function clearly showed that the risk for progression was constant over time, a result not expected if BMT was to modify the course of HCV disease. Although it is difficult to compare different data sets, the risk for and the rate of fibrosis progression observed in our HCV-positive patients with low iron overload do not appear to be dissimilar from those of other groups of HCV-infected patients of similar age in the absence of cofactors for liver disease.23-25
This study provides for the first time an estimate of the role of iron overload and HCV positivity in determining the risk for hepatic fibrosis progression in thalassemia patients following successful BMT. These findings will help in designing targeted treatments to prevent the development of liver damage in this group of patients and in all patients affected by transfusional iron overload with or without HCV infection.
We thank Dr G. Pupita for excellent computer assistance. We also thank Jan Mohabull for assistance in manuscript preparation.
Supported by a research grant from the Berloni Foundation Against Thalassemia.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Emanuele Angelucci, Unità Operativa di Ematologia, Ospedale A Businco, via Jenner 09121 Cagliari, Italy; e-mail: emnang@tin.it.