Abstract
Residue K5 in factor IX γ-carboxyglutamic acid (Gla) domain participates in binding endothelial cells/collagen IV. We injected recombinant factor IX containing mutations at residue 5 (K5A, K5R) into factor IX–deficient mice and compared their behavior with that of wild-type factor IX. The plasma concentration of factor IX that binds to endothelial cells/collagen IV (recombinant wild type and K5R) was consistently lower than that of the one that does not bind (K5A). Mice treated with wild type or K5R had 79% of the injected factor IX in the liver after 2 minutes, whereas 17% remained in circulation. In mice injected with K5A, 59% of the injected factor IX was found in liver and 31% was found in plasma. When we blocked the liver circulation before factor IX injection, 74% of K5A and 64% of K5R remained in the blood. When we treated the mouse with EDTA after injecting exogenous factor IX, the blood levels of factor IX that bind to endothelial cells/collagen IV increased, presumably because of release from endothelial cell/collagen IV binding sites. In contrast, the levels of the mutants that do not bind were unaffected by EDTA. In immunohistochemical studies, factor IX appears on the endothelial surfaces of mouse arteries after factor IX injection and of human arteries from surgical specimens. Thus, we have demonstrated that factor IX binds in vivo to endothelial cell–collagen IV surfaces. Our results suggest that factor IX Gla-domain mediated binding to endothelial cells/collagen IV plays a role in controlling factor IX concentration in the blood.
Introduction
Factor IX is the zymogen of a serine protease involved in blood coagulation. Activated factor IX (factor IXa) contains 4 identifiable structural domains. Starting from the N-terminus, these are the γ-carboxyglutamic acid (Gla) domain, the 2 epidermal growth factor–like domains, and the proteolytic domain. Factors IX and IXa bind to a specific site on the surface of cultured vascular endothelial cells. The binding is calcium-dependent, saturable, and reversible in vitro.1-3
Our previous studies on cultured endothelial cells demonstrate that the binding regions in factor IX are in the Gla domain.4Furthermore, mutations within the Gla domain, including lysine 5 to alanine (K5A) and valine 10 to lysine (V10K), produced factor IX that did not have measurable affinity for endothelial cells but retained normal clotting activity. In contrast, changing lysine 5 to arginine (K5R) increased factor IX affinity 3-fold for cultured bovine vascular endothelial cells.5
Cheung et al6 demonstrated that collagen IV was a strong candidate for the factor IX endothelial cell–associated binding site. Further, results from atomic force scanning microscopy indicate that factor IX molecules bind specifically to 2 sites on collagen IV, 98 and 50 nm from its C-terminus.7
Although the binding of factor IX to endothelial cells/collagen IV is well documented, little is known about the physiologic significance of binding. The hemophilia B mouse produced using gene-targeting technology8 offers a model that allows the study of factor IX pharmacokinetics and interactions with various body sites without interference from endogenous factor IX. In addition, the mouse model allows use of mutant factor IX to study the mutation's effect on clearance and other physiologic functions.
In the present study, we investigated the clearance of factor IX from blood, the location of factor IX that had disappeared from circulation, and the effect of Gla domain mutations on these parameters. In accordance with our in vitro data,5 mutations in the Gla domain (K5A and K5R) affected the rate of clearance and the amount of factor IX in the blood and in the liver.
Materials and methods
Proteins
Recombinant human factor IX and the mutant proteins K5A and K5R were constructed5,9 and purified from serum-free media of human embryo kidney 293 cells as described previously.10 11 Following purification, all proteins appeared as a single band on sodium dodecyl sulfate–polyacrylamide gel electrophoresis.
Animal studies
Factor IX–deficient mice (C57BL/6J-F9tm1Dws)8 were used in all experiments. Adult males and females (weight, 24-25 g; age, 8-10 weeks) were anesthetized by intraperitoneal injection of a mixture of domitor (Animal Health, Exton, PA) and Ketaset (Fort Dodge Laboratories, Fort Dodge, IA; 0.1 mL at 10% of each in saline). During the operation, the animal was held on a digital thermal system at 35°C, which was modified and assembled by the author (T.G.). On completion of the experiment, the animals were revived by subcutaneous injection of antisedan (0.1 mL, 10% in saline; Animal Health) after the surgical wound was closed.
Factor IX pharmacokinetic and distribution studies
We injected enough recombinant factor IX into the external jugular vein of each mouse to attain an initial concentration of 5 μg/mL. We assumed a blood volume of 0.63 mL/20 g body weight. Blood was collected from the tail tip (10-15 μL) at various times into heparinized capillary tubes (Curtin Matheson Scientific, Houston, TX). The plasma was isolated by centrifugation (700g at 4°C for 5 minutes) for subsequent analysis using enzyme-linked immunosorbent assay (ELISA). Unless stated otherwise, 3 mice were used in each study (n = 3).
In experiments to test the effect of blocking circulation to the liver on factor IX plasma concentrations, the abdominal cavity of each mouse was opened along the linea alba, and the porta hepatis, which includes the hepatic artery and portal vein, was tied with a double 3-0 silk suture before or after factor IX injection.
In studies of the effect of EDTA on circulating factor IX concentrations, 40 μL of 0.5 M EDTA (pH 7.4) was injected into the jugular vein 11 minutes or 12 hours after factor IX injection. The final sample was taken 1 minute later.
We analyzed the plasma concentration of factor IX versus time curves using WinNonlin (Pharsight, Mountain View, CA). We used a 2-compartment model to fit the data, and the calculated constants derived from individual experiments were averaged. The volumes of the 2 compartments were calculated using the total steady-state volume of distribution.
Preparation of liver for factor IX analysis
Livers were excised at 2 minutes after factor IX injection. After rinsing with saline–calcium chloride (5 mM), samples were homogenized in saline–EDTA (5 mM). Supernatants were collected by centrifugation at 5000g for 5 minutes at 4°C, and the amount of factor IX was determined by ELISA.
Determination of factor IX concentration by ELISA
Enzyme immunosorbent assay plates (Costar, Cambridge, MA) were coated overnight at 4°C with 100 μL rabbit anti-human factor IX IgG/well (1:1200 dilution in carbonate buffer; DAKO, Carpinteria, CA). Factor IX samples at 50 μL/well, including those for the standard curve (0.5-16 ng/mL) were incubated for 2 hours at 32°C. Then sheep anti-human factor IX IgG conjugated with horseradish peroxidase (HRP; Affinity Biologicals, Hamilton, Canada) (100 μL/well at 1:1200 in blocking buffer) was added and incubated for 45 minutes at room temperature. After washing, H2O2 ando-phenylene diamine were added, and color was allowed to develop for 10 minutes.12 The complex of factor IX–anti-human factor IX sheep IgG was detected by measuring absorbance at 490 nm with a Vmax plate reader (Molecular Devices, Sunnyvale, CA).
Blood vessel preparation and immunohistochemical staining of mouse aortas
Two minutes after injection of factor IX (20 μg/mL), the murine aortic arch was removed from the heart and was rinsed with saline–calcium chloride (5 mM). Samples were embedded in OCT compound (Sakura Finetek, Tokyo, Japan)–calcium chloride (5 mM), and frozen on dry ice. Tissue specimens were cut at 5 μm and mounted on glass slides (Erie Scientific, Portsmouth, NH).
Slides were stained using AEC (3-amino-9-ethylcarbazole) by a modification of the method described previously.13 Slides were treated in rabbit anti-human factor IX IgG (1:2000/4000) at 4°C overnight in saline–calcium chloride 5 mM, and further treated by goat anti-rabbit IgG conjugated with HRP (1:100), rabbit anti-goat IgG conjugated with HRP (1:100) in the same solution, and a second treatment with goat anti-rabbit IgG conjugated with HRP. The control was tissue from a hemophilia B mouse that had not been treated with factor IX but was otherwise treated similarly to the other mice. Before we treated with the primary antibody, we applied 2.5% hydrogen peroxide and 20% factor IX knockout mouse serum to block nonspecific binding. We routinely diluted the antibodies in buffer with 5% factor IX–deficient mouse serum. All antibodies were purchased from DAKO.
Microscopic images were captured from a Nikon Microphot FXA microscope (Nikon, Garden City, NY) equipped with an Optronics DEI-750 CCD Video Camera System (Optronics Engineering, Goleta, CA) interfaced to a Macintosh G3 computer using a Scion CG-7 capture card and Scion Image Software (Scion, Frederick, MD).
Immunohistochemical examination of human aortas
Immunohistochemical staining of frozen sections with Fast Red substrate and hematoxylin counterstain was carried out as previously described.14 Tissue was obtained from discarded portions of surgical specimens examined at the Durham Veterans Administration Hospital.
Results
Pharmacokinetics of clearance and distribution of factor IX
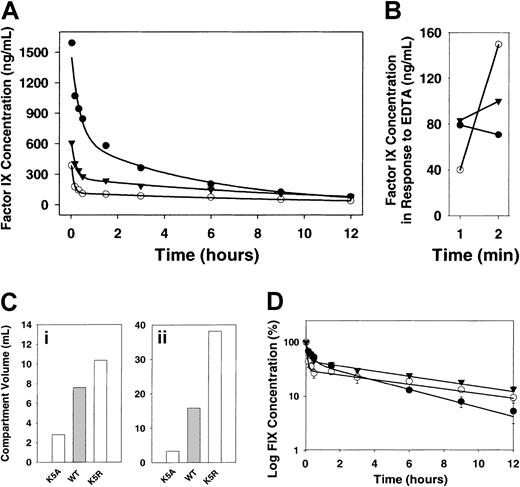
Results of an experiment designed to measure the concentration of the various factor IX mutants in the blood of hemophilia B mice for 12 hours after injection are shown in Figure1A-B,D. Data for all proteins were best fit by a bi-exponential equation. First-order rate constants for the first (α) and second (β) phases are summarized in Table1. Compared with wild-type factor IX, K5A had a lower volume of distribution (one fifth that of wild type), whereas K5R has a 2-fold higher volume (Figure 1C).
Injection and release of factor IX.
(A) Twelve-hour time-course of factor IX protein levels in the circulation. Factor IX knockout mice were injected with recombinant wild-type factor IX or its mutant with single amino acid substitutions in the Gla domain. In each case, factor IX or mutant was injected at zero time into the jugular vein to yield a concentration in plasma of 5 μg/mL. Samples were collected from the tail at 2, 10, 20, and 30 minutes and 1.5, 3, 6, 9, and 12 hours. The concentration of factor IX in plasma was determined by ELISA (n = 3). K5A (●), K5R (○), wild-type (▾). (B) Release of factor IX into circulation by EDTA. After 12 hours (A), EDTA was injected and the concentration of the factor IX was determined by ELISA (n = 3). (C) Volumes for a 2-compartment model of factor IX clearance. Data were analyzed by WinNonlin software. Ci, volume of the central compartment. Cii, volume of the second compartment. (D) Log concentration versus time for a 12-hour time-course. Time-courses of wild-type factor IX and mutant factor IX demonstrate a biphasic clearance, indicating a 2-compartment model of factor IX pharmacokinetics (symbols and data are the same as in panel A).
Injection and release of factor IX.
(A) Twelve-hour time-course of factor IX protein levels in the circulation. Factor IX knockout mice were injected with recombinant wild-type factor IX or its mutant with single amino acid substitutions in the Gla domain. In each case, factor IX or mutant was injected at zero time into the jugular vein to yield a concentration in plasma of 5 μg/mL. Samples were collected from the tail at 2, 10, 20, and 30 minutes and 1.5, 3, 6, 9, and 12 hours. The concentration of factor IX in plasma was determined by ELISA (n = 3). K5A (●), K5R (○), wild-type (▾). (B) Release of factor IX into circulation by EDTA. After 12 hours (A), EDTA was injected and the concentration of the factor IX was determined by ELISA (n = 3). (C) Volumes for a 2-compartment model of factor IX clearance. Data were analyzed by WinNonlin software. Ci, volume of the central compartment. Cii, volume of the second compartment. (D) Log concentration versus time for a 12-hour time-course. Time-courses of wild-type factor IX and mutant factor IX demonstrate a biphasic clearance, indicating a 2-compartment model of factor IX pharmacokinetics (symbols and data are the same as in panel A).
Kinetic constants of factor IX clearance from circulation of factor IX–deficient mice
| . | K5A . | K5R . | Wild type . |
|---|---|---|---|
| t1/2(hour)* | 3.3 | 7.7 | 6.9 |
| kα(hour)−1 | 4.5 | 10.8 | 5.3 |
| kβ(hour)−1 | 0.21 | 0.09 | 0.10 |
| . | K5A . | K5R . | Wild type . |
|---|---|---|---|
| t1/2(hour)* | 3.3 | 7.7 | 6.9 |
| kα(hour)−1 | 4.5 | 10.8 | 5.3 |
| kβ(hour)−1 | 0.21 | 0.09 | 0.10 |
Constants were determined using the following equation: [factor IX] = Aoe−kαt + Boe−kβt, where kα = rate constant for α phase, kβ = rate constant for β phase, Ao = Y intercept of first exponential, and Bo · = Y intercept of second exponential.
Determined using kβ.
Results are from 3 experiments.
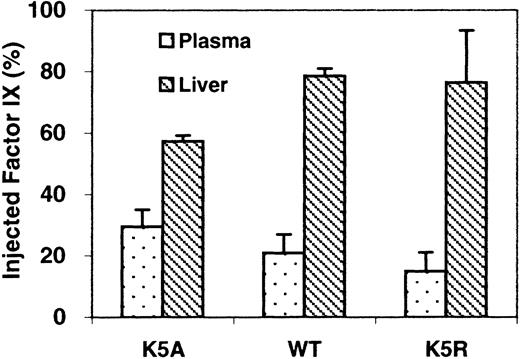
Distribution of factor IX at 2 minutes after injection is shown in Figure 2. Only 17% of wild-type factor IX was still in the blood at that point, whereas 79% of that protein was recovered from the liver. A similar distribution was observed in K5R. In contrast, approximately 31% of K5A was recovered from the blood, and less than 59% was recovered from the liver. When circulation to the liver was blocked, we found 74% of K5A and 64% of K5R in the blood after 2 minutes (Figure3). We also isolated the cellular fraction of the blood and found that after 10 minutes less than 1% of factor IX was associated with blood cells.
Amounts of factor IX relative to that injected, recovered from plasma and liver.
Samples were taken 2 minutes after injection. Protein levels were determined as described in “Materials and methods” (n = 3).
Amounts of factor IX relative to that injected, recovered from plasma and liver.
Samples were taken 2 minutes after injection. Protein levels were determined as described in “Materials and methods” (n = 3).
Effect of blocking the liver on plasma levels of wild-type factor IX, K5A, and K5R.
Experiments were performed as described in Figure 1. Except in one group of mice (n = 2), the circulation to the liver was blocked before injection of factor IX. Liver blocked (gray bars), without liver blocked (black bars).
Effect of blocking the liver on plasma levels of wild-type factor IX, K5A, and K5R.
Experiments were performed as described in Figure 1. Except in one group of mice (n = 2), the circulation to the liver was blocked before injection of factor IX. Liver blocked (gray bars), without liver blocked (black bars).
Results of experiments measuring the amount of factor IX in the circulation over a 15-minute time-course (results not shown) were consistent with the 12 hour time-course. The rate of disappearance for K5A differs from that of wild-type factor IX and K5R.
In a related experiment we injected 40 μL of 0.5 M EDTA (pH 7.4) into the jugular vein of each mouse 11 minutes after factor IX administration. Levels of K5A continued to decrease, but concentrations of K5R and wild-type factor IX increased after EDTA injection (not shown). We obtained similar results when EDTA was injected 12 hours after factor IX injection (Figure 1B). These results suggest that K5R and wild-type factor IX are released from the endothelial binding sites by EDTA.
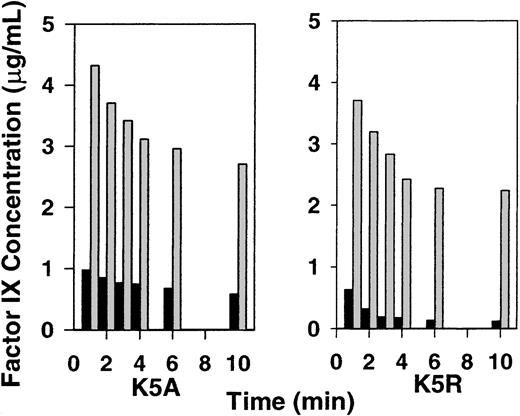
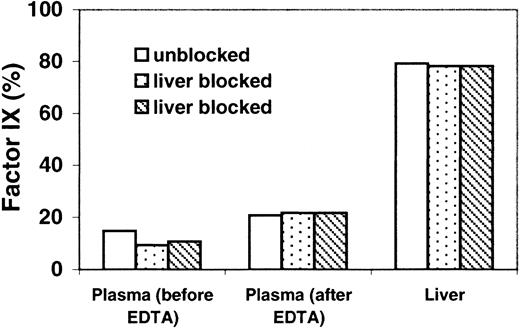
To determine whether the source of factor IX released to plasma by EDTA was the liver, we injected K5R into 2 mice, and, after 2 minutes, the liver circulation was blocked; a third mouse's liver was not blocked. At 2.5 minutes we injected EDTA in all 3 mice. The amount of factor IX released into the plasma by EDTA was not affected by liver blockage. However, the time between EDTA treatment and sample recovery was short. EDTA treatment is traumatic, and 1 minute may not be sufficient time to recover factor IX released from the liver endothelium. The amount of factor IX in the plasma of all mice doubled after EDTA treatment (Figure 4). To further investigate the source of the factor IX released by EDTA, a similar experiment was performed in which the liver was blocked at 30 minutes and EDTA was injected at 32 minutes. Results (not shown) were similar to those in Figure 4.
Relative K5R protein levels in plasma and liver; effect of liver blockage and EDTA injection.
Experiments were performed as described in the legend to Figure 1. Except at 2.4 minutes, the porta hepatis in 2 mice was blocked. A third mouse's liver was not blocked. At 2.5 minutes, EDTA was injected in all 3 mice. Plasma (before EDTA): 10-μL samples were taken from all 3 mice at 2 minutes to determine the plasma concentration of factor IX. Plasma (after EDTA), liver: samples were obtained at 3.5 minutes, 1 minute after EDTA injection.
Relative K5R protein levels in plasma and liver; effect of liver blockage and EDTA injection.
Experiments were performed as described in the legend to Figure 1. Except at 2.4 minutes, the porta hepatis in 2 mice was blocked. A third mouse's liver was not blocked. At 2.5 minutes, EDTA was injected in all 3 mice. Plasma (before EDTA): 10-μL samples were taken from all 3 mice at 2 minutes to determine the plasma concentration of factor IX. Plasma (after EDTA), liver: samples were obtained at 3.5 minutes, 1 minute after EDTA injection.
Immunohistochemical studies of mouse arteries after factor IX injection
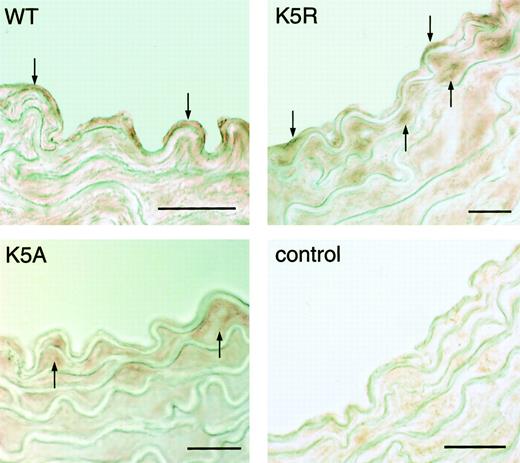
Only K5R and wild-type factor IX appeared on the surface of the aorta of the hemophilia B mouse, apparently associated with the endothelium (Figure 5). On the other hand, in all 3 mice factor IX was located beneath the endothelial layer, but K5A appeared to be present at higher concentrations.
Photomicrographs of cross-sections of murine aortas.
Samples were obtained 2 minutes after the injection of human factor IX (K5A, K5R, and wild type), and detected with anti-human factor IX IgG. K5A, K5R, and WT: identify the injected protein. Color was developed by AEC. Control: artery was isolated from a factor IX knockout mouse that had not received any factor IX. Down arrows indicate factor IX K5R and wild-type binding at the endothelial surface. Up arrows indicate factor IX in the region beneath the endothelium. Bar = 20 μm.
Photomicrographs of cross-sections of murine aortas.
Samples were obtained 2 minutes after the injection of human factor IX (K5A, K5R, and wild type), and detected with anti-human factor IX IgG. K5A, K5R, and WT: identify the injected protein. Color was developed by AEC. Control: artery was isolated from a factor IX knockout mouse that had not received any factor IX. Down arrows indicate factor IX K5R and wild-type binding at the endothelial surface. Up arrows indicate factor IX in the region beneath the endothelium. Bar = 20 μm.
Localization of human factor IX in human arteries
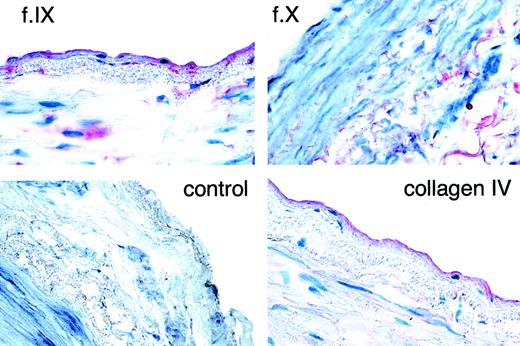
Results (Figure 6) show that both factor IX and collagen IV are localized on the endothelial surface in human vessels. Factor IX is also present in the subendothelium and the media. Control panels were performed with preimmune serum or factor X antibody. Factor X appears only in the media; thus, it is unlikely that the appearance of factor IX, which is found in the circulation at lower concentrations than factor X, is present only as a residual of plasma factor IX.
Immunohistochemical detection of factor IX and collagen IV in human popliteal artery.
Frozen sections of fresh tissue were stained with polyclonal antisera to factor IX (f.IX), factor X (f.X), collagen IV, or preimmune rabbit serum (control). In each panel, the endothelial (lumenal) surface of the vessel faces the label. Note the primary localization of red staining to the endothelial surface for the factor IX and collagen IV panels. Some faint staining is also present in the subendothelium and media. By contrast, only faint staining in the media is seen for factor X. Original magnification, 400×.
Immunohistochemical detection of factor IX and collagen IV in human popliteal artery.
Frozen sections of fresh tissue were stained with polyclonal antisera to factor IX (f.IX), factor X (f.X), collagen IV, or preimmune rabbit serum (control). In each panel, the endothelial (lumenal) surface of the vessel faces the label. Note the primary localization of red staining to the endothelial surface for the factor IX and collagen IV panels. Some faint staining is also present in the subendothelium and media. By contrast, only faint staining in the media is seen for factor X. Original magnification, 400×.
Discussion
Mutation of factor IX residue K5 to A eliminates endothelial cell/collagen IV binding but has little effect on clotting activity in vitro.5 On the other hand, mutation of residue K5 to R yields a factor IX with slightly higher affinity than wild-type factor IX for the endothelial cell/collagen IV binding site. It seemed likely that if endothelial cell/collagen IV binding has a significant physiological function, the clearance, distribution, or both of factor IX in vivo might be affected by mutation at residue K5. After intravenous administration to hemophilia B mice, we investigated the behavior of wild-type human factor IX and its 2 Gla domain mutants (K5A, K5R). Specifically, we examined the mutation's effect on factor IX pharmacokinetics and on its distribution in the liver and blood.
Our investigation revealed that most factor IX was removed from circulating blood in less than 2 minutes and that sampling at 2 minutes produced the most reliable and reproducible early time point. Our data agree with previous clearance studies that showed that the pharmacokinetics of factor IX could be divided into 2 phases.15-17 We found that the half-time of the first phase was similar to that reported by Fuchs et al18 for normal mice. Half-lives reported for the slower second phase vary considerably (5-14 hours).19 The differences in half-times probably reflect the different sampling times and species differences. For example, in our study, normal factor IX (plasma or recombinant wild-type) had a 7-hour half-life when we took the first sample at 2 minutes, but if we considered only time points taken after 30 minutes, the half-life was estimated to be 18 hours.
Although the overall biphasic shape of the clearance curves for all the factor IX species was similar, the concentration of K5A decreased more slowly during the initial phase than that of normal factor IX and K5R. This resulted in higher concentrations for this mutant over the course of most of the 12-hour experiment. However, the rate of disappearance from plasma at longer times was actually faster for K5A than for K5R and wild-type factor IX. K5R and wild-type factor IX probably disappeared faster initially because they rapidly bound to the endothelial cells/collagen IV, whereas K5A remained in circulation until clearance or other interactions, not mediated by the endothelial cell/collagen IV site, removed it from the blood. At later times factor IX bound to endothelial cells/collagen IV was gradually released and cleared slowly from the blood. On the other hand, K5A, which was not bound to the endothelial cells/collagen IV, was free to clear more rapidly in the later phase. The second clearance phase may be common to both endothelial cell/collagen-binding and nonbinding forms of factor IX.
The first, short phase in the factor IX lifetime in vivo presumably involves distribution to parts of the body, in addition to endothelial cells/collagen IV, that are freely accessible to the blood. Previous reports indicate this α phase represents diffusion of factor IX into certain tissues and extravascular fluid.16,20 For example, Fuchs et al18 showed that 80% of injected factor IX is in the liver of normal mice after 2 minutes. This is similar to our results with wild-type factor IX and K5R (Figure 2). On the other hand, only approximately 60% of K5A was in the liver at 2 minutes. As mentioned, the concentration of K5A in plasma was higher than wild-type factor IX and K5R at this point. Our results suggest that decreasing concentrations of factor IX in the blood and localization of factor IX in the liver is, at least partially, caused by endothelial cell/collagen IV binding. Therefore, because of its effect on endothelial cell/collagen IV binding, the Gla domain mutation K5A affects the rate of clearance from blood, the amount of protein remaining in circulation, and the amount found in the liver.
To further investigate the role of the liver in controlling the amount of factor IX in the blood, we blocked the hepatic artery and portal vein entering the mouse liver. Under these conditions, the level of factor IX K5R in circulation 2 minutes after injection was approximately 64% of that injected as opposed to less than 20% when the blood was free to circulate through the liver. Under similar conditions the concentration of K5A was 74% compared to approximately 30% when the liver circulation was not blocked. The higher concentration of K5A in plasma, either with or without the liver circulation blocked, suggests that K5R binds to endothelial cell/collagen IV sites in circulation, both in the liver and outside the liver, unavailable to K5A. However, the significant amount of K5A in the liver indicates that there are means of hepatic clearance or alternative binding sites not related to collagen IV.
Because endothelial cell/collagen IV factor IX binding is calcium dependent in vitro, we investigated the effect of EDTA on blood levels of factor IX in the mouse. Although we did not expect the mouse to survive for long after EDTA injection, EDTA should result in an immediate release of factor IX from the endothelial cell/collagen IV binding site into the circulation because of the disruption of the Gla domain calcium-dependent structure. The mice were first treated with factor IX as usual. Then EDTA was injected, and a sample was taken after 1 minute. Consistent with the association to a calcium-dependent endothelial binding site, we observed a significant increase in K5R and wild type in plasma after EDTA treatment even 12 hours after injection. In contrast, similar experiments with K5A showed no factor IX release after EDTA treatment. Experiments performed while the liver circulation was blocked gave similar results. This suggests the liver is not the source of released factor IX. Thus, although it is difficult to estimate the size of the EDTA-susceptible pool, given the short time allowed for release and the undoubtedly large stores available in the liver, the amount released in our studies must be considered a minimum. If one assumes a 1-mL volume for the mouse's blood, that released would be approximately 2 nM for K5R and 0.3 nM for wild-type factor IX. That the amount of K5R released is larger, both at 12 hours and 12 minutes, than the amount of wild type is consistent with our previously reported results showing that K5R has a lower Kdthan wild-type factor IX for endothelial cells/collagen IV in culture. In experiments that support the existence of an intravascular store of factor IX available for release, Stern et al21 isolated arteries from baboons treated with radiolabeled bovine factor IX. They showed that the bovine factor IX was released from the isolated arteries after treatment with EGTA.
Stern et al,21 in the same study, using an approach different from ours, reported additional results consistent with those we report here. When they injected bovine factor IX into normal baboons, the concentration of baboon factor IX increased in the blood. The amount of baboon factor IX released into the circulation is proportional to the amount of bovine factor IX injected. They concluded that the released factor IX was originally bound to endothelial cell binding sites and was displaced by the bovine factor IX. In agreement, our results also suggest that the initial rapid loss of injected factor IX from blood circulation is partially explained by factor IX binding to endothelial cells/collagen IV in the circulation. However, there must be additional storage pools because approximately 60% of the injected factor IX K5A, which does not bind endothelial cell/collagen IV sites, was distributed to the liver. Binding of factor IX to endothelial cells/collagen IV may also account for the typically poor recovery of the first dose of factor IX given during a course of replacement therapy in patients with hemophilia B. Our results are also consistent with the report that recovery of factor IX in patients with CRM-positive hemophilia B is higher than in those who are CRM negative.22 In addition, Fuchs et al18 show that radiolabeled factor IX clears more slowly in mice injected at the same time with unlabeled factor IX.
Although our pharmacokinetic data were reasonably fit by the sum of 2 exponentials, it was of interest to determine the parameters defined by a 2-compartment pharmacokinetic model.23 The most informative of these values, calculated using the plasma concentration versus time curve, is the volume of distribution of the 2 compartments. This parameter is generally interpreted to indicate the extent of distribution of the protein in the body (Figure 1C). Values for K5R and wild type are significantly larger than those for nonbinding mutants (Figure 1C). This is consistent with a system in which proteins of smaller volume do not distribute to certain tissues or spaces (perhaps endothelial cells or collagen IV) available to molecules of larger volume.
Also consistent with our kinetic studies are the results of histologic experiments designed to visualize factor IX on the endothelium or the subendothelium. After injection of factor IX into the hemophilic mice, as expected wild-type factor IX and K5R were detected by an anti-human factor IX antibody on the lumen of vessels, but K5A was not. In addition, all species of factor IX appeared in the subendothelial layer (Figure 5). In contrast K5A, which does not bind to endothelial cell/collagen IV, did not appear on the endothelial cell surface but was detected under the endothelium. The significance or mechanism of the transport of factor IX to the subendothelium is unknown, but it is consistent with the rapid disappearance of K5A. It is possible that it is an artifact of tissue processing, but this seems unlikely because K5A does not appear on the endothelial surface where we know it does not bind. Thus, transport from the vasculature to the tissues is apparently not mediated by the endothelial cell/collagen IV binding site.
In further histologic experiments using immunologic detection, we looked for factor IX and collagen IV on human arteries from surgical specimens. Consistent with factor IX binding to endothelial cells (collagen IV), antibodies to factor IX and collagen IV both appeared on the endothelial surface in these specimens (Figure 6). In addition, we detected factor IX in the basement membrane. It is unlikely that the factor IX detected in these histologic sections was the result of nonspecifically bound factor IX, because factor X, which is present in the blood at a higher concentration but binds with lower affinity than does factor IX to endothelium,1,24 is detected only in the subendothelium. In addition to their being consistent with our previous results showing collagen IV is the likely binding site for factor IX, our present results are consistent with those of Herzog et al,25 which showed in mouse gene therapy studies that when the vector carrying factor IX was injected into muscle, the synthesized factor IX appeared where collagen IV was also detected. These results are all consistent with a binding site associated with the endothelium, most likely collagen IV.
Our data show that Gla domain binding to the endothelial cell/collagen IV site not only partially mediated the initial, rapid phase of factor IX distribution, it also mediated factor IX maintenance in the blood circulation in the second clearance phase. In hopes of further understanding the physiological function of this interaction, we are characterizing a mouse in which factor IX was replaced by factor IX K5A.
We thank Dr Gary M. Pollack (School of Pharmacy, University of North Carolina at Chapel Hill) for help with analysis of the pharmacokinetic data.
Supported by National Institutes of Health grant HL06350.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Darrel W. Stafford, Department of Biology, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599-3280; e-mail: dws@emailunc.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal