Key Points
Isatuximab in relapsed and/or refractory AL amyloidosis resulted in an overall hematologic response rate of 77%.
The high rates of hematologic responses translated into organ responses.
Visual Abstract
Isatuximab is an immunoglobulin G1κ monoclonal antibody that binds with high affinity to CD38 expressed on plasma cells. Anti-CD38 antibodies have shown efficacy as monotherapy and in combination in a variety of settings for patients with multiple myeloma and light chain (AL) amyloidosis. This multicenter, cooperative group phase 2 trial was designed to evaluate hematologic response, organ response, and safety of isatuximab monotherapy for the treatment of relapsed AL amyloidosis. Isatuximab at 20 mg/kg was administered IV weekly during the first 28-day cycle, and then every other week during cycles 2 to 24. Forty-three patients were registered, with 35 patients being evaluable for response. The overall hematologic response rate was 77.1%, with 57% of patients achieving a very good partial response (VGPR) or better. The median time to partial response (PR) or better was 1.1 months. Renal response occurred in 50% (7/14) of patients with renal involvement, and cardiac response occurred in 57% (8/14) of patients who were evaluable utilizing N-terminal pro b-type natriuretic peptide (NT-proBNP) with cardiac involvement. The most common treatment-related grade ≥3 adverse events included lymphopenia (n = 3, 8.5%) and infection (n = 2, 6%). Isatuximab demonstrated substantial efficacy in previously treated patients with AL amyloidosis, and was associated with a good safety profile. This trial was registered at www.clinicaltrials.gov as #NCT03499808.
Medscape Continuing Medical Education online

In support of improving patient care, this activity has been planned and implemented by Medscape, LLC and the American Society of Hematology. Medscape, LLC is jointly accredited with commendation by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 1.0 MOC points in the American Board of Internal Medicine's (ABIM) Maintenance of Certification (MOC) program. Participants will earn MOC points equivalent to the amount of CME credits claimed for the activity. It is the CME activity provider's responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at https://www.medscape.org/journal/blood; and (4) view/print the certificate. For CME questions, see page 2615.
Disclosures
CME questions author Laurie Barclay, freelance writer and reviewer, Medscape, LLC, declares no competing financial interests.
Learning objectives
Upon completion of this activity, participants will:
Describe the efficacy of isatuximab monotherapy for the treatment of relapsed AL amyloidosis, based on findings of a multicenter, cooperative group phase 2 trial
Describe safety and tolerability of isatuximab monotherapy for the treatment of relapsed AL amyloidosis, based on findings of a multicenter, cooperative group phase 2 trial
Describe the clinical implications of the efficacy and safety of isatuximab monotherapy for the treatment of relapsed AL amyloidosis, based on findings of a multicenter, cooperative group phase 2 trial
Release date: November 20, 2025; Expiration date: November 20, 2026
Introduction
Systemic light chain (AL) amyloidosis is a monoclonal plasma cell disease in which misfolded light chains spontaneously aggregate into soluble oligomers and insoluble fibrils that may deposit in different organs, leading to organ dysfunction and clinical symptoms.1,2 Treatments for AL amyloidosis are derived from established therapies for multiple myeloma (MM). Upfront treatment has historically included high-dose melphalan and autologous stem cell transplant (ASCT), oral melphalan and dexamethasone, and a combination of cyclophosphamide, bortezomib, and dexamethasone.3-5 Despite these initial therapies, many patients will have relapsed and/or refractory disease.6,7 Treatment at the time of relapse is based upon the patient’s prior treatment(s), comorbidities, and organ function. Immunomodulatory agents and proteasome inhibitors (PIs) have been studied in the relapsed setting, with varying results. The overall hematologic response rates have ranged from 44% to 67% for lenalidomide and pomalidomide.8-13 Similarly, overall hematologic response rates for bortezomib and ixazomib have ranged from 45% to 59%.14-16 Therapies with improved efficacy and toxicity profiles are needed for patients with relapsed disease that can be particularly challenging to manage in the setting of compromised organ function.
More recently, daratumumab, a human immunoglobulin G1κ monoclonal antibody targeting the CD38 surface antigen on plasma cells, has been evaluated in both newly diagnosed AL amyloidosis and relapsed/refractory disease.17-21 In the relapsed/refractory setting, single-agent daratumumab achieved an overall hematologic response rate ranging from 55% to 86% in 2 prospective clinical trials, and was well tolerated.22,23 In 2021, daratumumab received approval in combination with bortezomib, cyclophosphamide, and dexamethasone for newly diagnosed AL amyloidosis based upon the ANDROMEDA trial. The primary end point for that study was a hematologic complete response (CR), achieved in 53% of patients receiving the daratumumab-based regimen.21
Isatuximab is another human immunoglobulin G1κ monoclonal antibody that binds with high affinity to a unique epitope on CD38 compared with daratumumab. It has been shown to be efficacious and well tolerated in relapsed/refractory MM when used as a single agent, as well as in various combinations with other agents.24-26 However, clinical trials evaluating isatuximab in AL amyloidosis are lacking. It was hypothesized that the efficacy and tolerability would be similar to that of daratumumab in relapsed/refractory AL amyloidosis. Therefore, a clinical trial was conducted to study the impact of isatuximab in patients with relapsed/refractory AL amyloidosis. The primary objective was to assess the efficacy as measured by the confirmed overall hematologic response rate. The secondary objectives were to evaluate toxicities per the National Cancer Institute Common Terminology Criteria for Adverse Events, version 5, time to hematologic response, duration of response, progression-free survival (PFS), and overall survival (OS).
Patients and methods
Eligibility criteria
Patients with a diagnosis of relapsed systemic AL amyloidosis that was histologically confirmed by positive Congo red staining with green birefringence on polarized light microscopy, or characteristic appearance by electron microscopy and confirmatory AL typing were enrolled. Patients were required to have measurable disease, defined as a presence of a monoclonal protein on serum and/or urine by immunofixation electrophoresis (IFE) and/or serum free light chain (FLC) ratio outside of the normal range (0.25-1.65). The difference in the involved serum FLC vs the uninvolved serum FLC (dFLC) had to be ≥4.5 mg/dL (45 mg/L).
Objective organ involvement of at least 1 organ was required at the time of enrollment.
All patients were aged ≥18 years. Patients had to have received ≥1 prior line of therapy, and completed prior systemic therapy ≥14 days, investigational therapy ≥28 days, surgery ≥21 days, and ASCT ≥100 days prior to registration. Patients were not allowed to receive any supplements known to have potential antiamyloidogenic effect within 14 days of registration. Importantly, prior daratumumab treatment was allowed if it was >56 days prior to registration and the patient was not refractory to daratumumab.
Other key eligibility criteria included a total bilirubin ≤2.0 × institutional upper limit of normal (<3 mg/dL if Gilbert syndrome), aspartate aminotransferase and alanine aminotransferase ≤4.0 × institutional upper limit of normal, creatinine clearance ≥25 mL/min as measured by 24-hour urine collection or estimated by the Cockcroft and Gault formula, adequate bone marrow function defined as an absolute neutrophil count ≥1000 cell per μL without growth factor support, platelets ≥75 × 103/μL, hemoglobin ≥8 g/dL, New York Heart Association class <IV heart failure, left ventricular ejection fracture by echocardiography ≥35%, and N-terminal pro b-type natriuretic peptide (NT-proBNP) ≤8500 pg/mL.
Patients with coexisting active symptomatic MM, defined by the 2014 International Myeloma Working Group criteria, were excluded.27
Treatment protocol
All patients received treatment with isatuximab 20 mg/kg IV on days 1, 8, 15, and 22 of cycle 1, followed by days 1 and 15 for cycles 2 to 24, with cycle length of 28 days. All patients received premedication with diphenhydramine 25 to 50 mg IV or orally, methylprednisolone 100 mg IV, ranitidine 50 mg IV, and acetaminophen 650 to 1000 mg orally 15 to 30 minutes prior to treatment. Of note, ranitidine is no longer available in the United States as of 2020. Patients who did not experience infusion-related reactions (IRRs) during the first 4 isatuximab infusions could have the premedications reduced or discontinued at the discretion of the treating physician in consultation with the study chairs to avoid unnecessary sedation during subsequent infusions. This included methylprednisolone as the steroid was used as a premedication only and not considered part of the treatment. All patients received antiviral prophylaxis, such as acyclovir 800 mg orally daily, or an equivalent throughout the duration of the study, and were recommended to receive a proton pump inhibitor such as lansoprazole 15 mg orally daily, or an equivalent.
Dose modifications
Dose modifications were not permitted on the trial. Dose delays were allowed for treatment-related adverse events, and a cycle could be delayed up to 14 days. Isatuximab was held for grade ≥3 neutropenia, and all other grade 2 or 3 treatment-related adverse events. Treatment could be resumed once the adverse event improved to grade <2, or grade ≤2 for neutropenia. If a patient experienced a grade 2 IRR, treatment was interrupted and subsequently resumed at half of the initial infusion rate under close monitoring once the reaction improved to grade ≤1. Patients who experienced grade 3 or 4 IRRs were required to have isatuximab permanently discontinued.
Toxicity and response criteria
The primary objective of hematologic response rate was assessed based upon criteria defined by consensus opinion from the International Society of Amyloidosis (ISA).28 A hematologic CR was defined as a normal serum FLC ratio (0.25-1.65), and absence of a monoclonal protein in serum and urine by IFE. The ISA subsequently proposed that the 2012 response criteria be expanded to redefine hematologic CR, characterized by the absence of amyloidogenic light chains as suggested by the absence of monoclonal protein on both serum and urine IFE, and a FLC ratio that is either within the reference range or abnormally inverted due to the uninvolved nonamyloidogenic FLC concentration being greater than the involved FLC concentration.29 A very good partial response (VGPR) was defined as a dFLC of <4.0 mg/dL, and a partial response (PR) was defined as a dFLC decrease of >50% but remaining ≥4 mg/dL. Hematologic response was assessed every 28 days, and 2 consecutive measurements were required to confirm response.
Organ responses were defined based on functional improvement in one or more involved organ systems. Response criteria were based on consensus guidelines, and utilized biomarker measurements and clinical assessments.28,30 Only 1 parameter was required to satisfy the organ response criteria, and a second consecutive assessment at least 4 weeks after the first observation of response was required for confirmation. Cardiac responses were assessed every 28 days, while the remainder were assessed every 56 days. The time to hematologic response was defined as the date from registration to the date of first documentation of hematologic response. OS was calculated from the date of registration to date of death due to any cause. PFS was a composite end point of clinically observable end points, and was estimated from the date of registration to date of first documentation of any of the following: hematologic progression, symptomatic deterioration, or death due to any cause. Symptomatic deterioration was defined as the global deterioration of health status requiring discontinuation of treatment without objective evidence of progression.
Toxicities were evaluated based upon the National Cancer Institute Common Terminology Criteria for Adverse Events, version 5.
Statistical design and analysis
The study was a multicenter, single-arm phase 2 trial that used a 2-stage design for patient accrual, with an estimate of enrolling 39 patients for a total of 35 eligible patients evaluable for hematologic response. Initially, 20 evaluable patients were accrued. If 2 or more confirmed, partial, or better responses were observed, then an additional 15 evaluable patients were to be accrued.
The first 10 patients enrolled were monitored for adverse events of special interest during the first cycle of treatment. Per protocol, if ≤2 patients experienced any toxicities, then accrual would continue as planned. The adverse events of special interest included grade 3 and 4 IRRs, grade 4 cardiac events, grade 4 neutropenia or thrombocytopenia that did not resolve to grade <3 within 10 days, grade 4 infection, grade 4 gastrointestinal ileus or obstruction, and grade 4 liver function test abnormalities.
All eligible patients who had received at least 1 dose of isatuximab were evaluable for response and included in the analysis.
The Kaplan-Meier method was used to estimate OS, PFS, and the duration of response. Median follow-up was estimated using the reverse Kaplan-Meier method. The cumulative incidence competing risks method was used to estimate time to hematologic response and time to organ response, with death prior to response modeled as a competing risk in both cases. Confidence intervals (CIs) for binary proportions were computed using the Clopper-Pearson method. The exact binomial test was used to assess whether the proportion of patients having a binomially distributed feature of interest was different than a hypothesized value.
The protocol was reviewed and approved by the Central Institutional Review Board for the National Cancer Institute, National Institutes of Health.
Results
Patient characteristics
From March 2018 to September 2019, 43 patients with relapsed/refractory AL amyloidosis were registered to the trial across 20 institutions. There were 36 patients eligible to receive treatment, and 35 patients who had received at least 1 dose of isatuximab. One eligible patient who could not receive any doses of study drug was deemed not analyzable for all end points. Of the 7 ineligible patients, 4 did not complete all required baseline assessments, and 3 had baseline assessments outside of the study-specific window. Response is indeterminable in 2 patients who had discontinued treatment and lacked disease assessment follow-up.
The median age of the evaluable patients was 70 years (range, 40.1-79.5 years), with 19 females and 16 males. Most patients (66% [n = 23]) had a lambda clonal plasma cell dyscrasia. The median dFLC level was 10.8 mg/dL (range, 4.6-92.4). The median number of prior treatments was 1 (range, 1-10). Most patients had received a PI-based therapy, and one-half had undergone ASCT previously. Two patients had received prior daratumumab treatment. The most common cytogenetic abnormality using fluorescence in situ hybridization was translocation (11;14), observed in 10 patients (29%). Eleven patients (31%) had other cytogenetic abnormalities, including 3 patients (8.5%) with gain 1q. Eighteen patients (51%) had single organ involvement, and 17 (49%) patients had multiple organs involved. The distribution of organ involvement was typical for AL amyloidosis, with cardiac involvement in 25 patients (71%), and renal involvement in 14 patients (40%). Patients were staged, using the European 2015 modification of the Mayo 2004 Staging System (stage I-IIIb; Table 1).31,32
For patients with cardiac involvement, the median concentration of NT-proBNP was 764 pg/mL (range, 57-6267). Most patients were New York Heart Association class I (36%, n = 9) and II (56%, n = 14). For those patients with renal involvement, 7 patients (50%) were renal stage I, 6 (43%) were stage II, and 1 (7%) was stage III (Table 2).
Treatment
Of 35 evaluable patients, 17 patients (49%) completed the planned 24 cycles of treatment. Five patients (14%) discontinued treatment secondary to an adverse event, which included a grade 4 IRR, skin infection, worsening fatigue/hypoxia, dehydration, and subsequently diagnosed prostate cancer. Four patients (11%) discontinued treatment for reasons not specified in the protocol, including suboptimal response, decision to undergo ASCT, physician decision, and concerns surrounding COVID-19. Four patients (11%) discontinued treatment secondary to disease progression, and another 4 patients (11%) discontinued treatment secondary to refusal unrelated to an adverse event. There was 1 death related to congestive heart failure that was not deemed treatment related.
The median time on study was 21.1 months (range, 0.7-24.4 months). The median follow-up was 21.7 months (95% CI, 21.6-22.1).
Hematologic responses
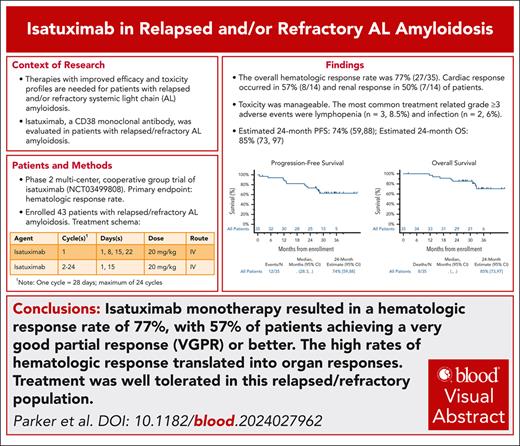
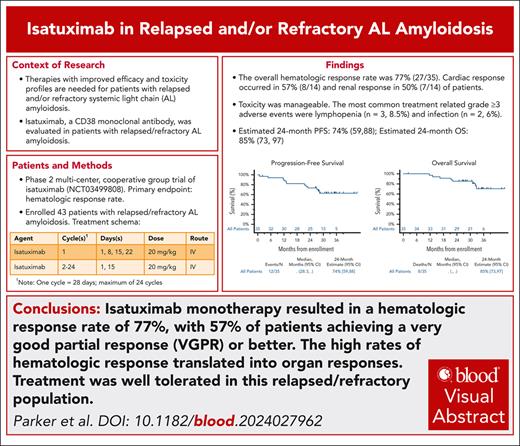
The overall hematologic response rate was 77% (98% CI, 57-91; n = 27); this observed rate of hematologic response was significantly greater than the rate for comparison in the null hypothesis hematologic response rate of 10% when tested at the 0.02 significance level (P value .0001). Two patients (6%) achieved a hematologic CR, 18 patients (51%) had a VGPR, and 7 patients (20%) had a PR. Another 4 patients (11%) had no response (Figure 1). These results utilize the 2012 ISA response criteria, and did not consider a false positive IFE secondary to isatuximab administration. Upon utilizing the updated ISA response criteria and accounting for a change in the FLC on serum IFE from lambda to kappa, the hematologic CR rate would increase from 6% to 17% (6 patients). The median time to a PR or better was 1.1 months (range 1-3 months). The median time to VGPR or better was 3 months. At 24 months, the rate of patients still in hematologic response was estimated at 81% (95% CI, 67-96). The 24-month estimate for those achieving a VGPR or better was 85% (95% CI, 69-100). Among the 10 patients with translocation (11;14), 9 patients (90%) achieved at least a PR, with 7 (70%) achieving a VGPR. The results were similar for those with normal cytogenetics, as 6 of 7 patients (86%) achieved a PR or better. Only 4 patients (57%) achieved a VGPR or better in this subgroup. All 3 patients with translocation (4;14) achieved at least a PR, with 2 (67%) having a VGPR or better. Of the 3 patients with gain 1q, 2 attained VGPR and 1 had a PR.
Hematologic response. The 2012 ISA response criteria were utilized. ORR, overall response rate.
Hematologic response. The 2012 ISA response criteria were utilized. ORR, overall response rate.
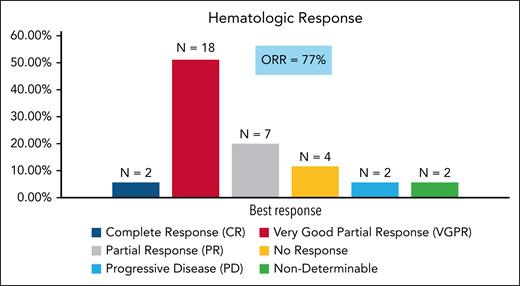
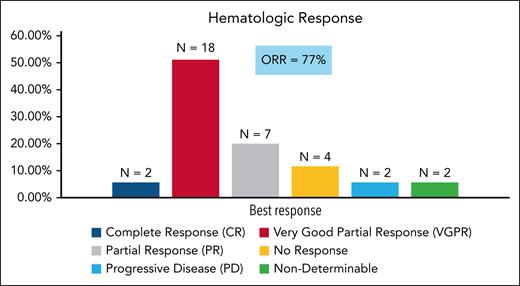
At the time of data cutoff (30 December 2022), 9 patients had died. The 24-month estimated OS rate was 85% (95% CI, 73-97). The 24-month estimated PFS was 74% (95% CI, 59-88; Figure 2).
OS and PFS. (A) Kaplan-Meier estimate of OS. (B) Kaplan-Meier estimate of PFS. PFS is defined as the date of registration to date of first documentation of hematologic progression, symptomatic deterioration defined as global deterioration of health status requiring discontinuation of treatment without objective evidence of progression, or death due to any cause.
OS and PFS. (A) Kaplan-Meier estimate of OS. (B) Kaplan-Meier estimate of PFS. PFS is defined as the date of registration to date of first documentation of hematologic progression, symptomatic deterioration defined as global deterioration of health status requiring discontinuation of treatment without objective evidence of progression, or death due to any cause.
The 2 patients who had prior daratumumab exposure did not respond to subsequent isatuximab treatment. The time from the last daratumumab exposure to the first isatuximab treatment in these 2 patients was 2.3 and 6.2 months, respectively. For those patients with prior PI exposure (n = 33), 19 patients (58%) achieved a VGPR or better. Of the 16 patients with previous ASCT, 9 (56%) had a VGPR or better.
Organ responses
Response to therapy was defined based upon functional improvement in 1 or more involved organ systems utilizing consensus criteria, with confirmation on a second consecutive assessment at least 4 weeks after the first observation of response.28,30
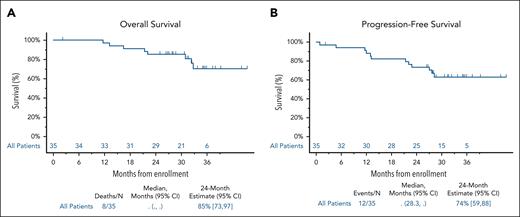
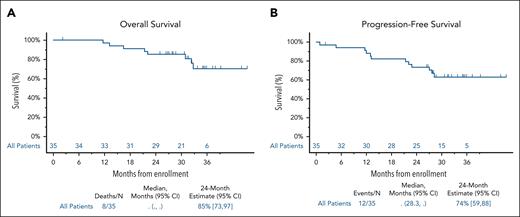
Of 25 patients with cardiac involvement, 14 had a baseline NT-proBNP ≥650 pg/mL, and were evaluable for response. Cardiac response occurred in 8 of 14 patients (57%). Among the 25 patients with cardiac involvement, 13 (53%) achieved a hematologic response of VGPR or better.
Renal responses occurred in 7 of 14 patients (50%). The median time to renal response was 18.5 months (95% CI, 3.9, NR). Among the 14 patients with renal involvement, 8 (57%) had a hematologic response of VGPR or better. Organ responses were observed in both patients with gastrointestinal involvement and the 1 patient with liver involvement (Figure 3).33,34
Organ response. (A) Overall organ response. A cardiac response was defined as either a reduction in the NT-proBNP of >30% and at least a 300 pg/mL decrease from the baseline result if the NT-proBNP was ≥650 pg/mL at baseline, or improvement in New York Heart Association (NYHA) class by 1 class if NYHA class 1 or 2, and a 2-class improvement if NYHA class 3 at baseline.28 A renal response was defined as either a 30% decrease in urine total albumin or a decrease to <500 mg per 24 hours urine total protein without renal progression.30 A gastrointestinal response was defined as either a reduction in 24-hour fecal fat excretion by ≥50% in patients with steatorrhea or a ≥50% reduction in the number of loose stools per day in patients with diarrhea. A liver response was defined as either a 50% decrease in or normalization of the alkaline phosphatase level or a reduction in the span of the liver by at least 2 cm by radiographic determination. Of the 25 patients with cardiac involvement, 14 had a baseline NT-proBNP ≥650 pg/mL, and were evaluable for cardiac response based upon the NT-proBNP. One patient with an NT-proBNP <650 pg/mL was classified as a response based upon improvement in NYHA class. This patient is not depicted in the graph. (B) Graded cardiac/renal organ response. Cardiac and renal responses were retrospectively calculated. One patient with a renal response was not evaluable for grading given a baseline 24-hour proteinuria level <500 mg per 24 hours. The 14 patients with a NT-proBNP ≥650 pg/mL were evaluable for graded cardiac responses.33,34
Organ response. (A) Overall organ response. A cardiac response was defined as either a reduction in the NT-proBNP of >30% and at least a 300 pg/mL decrease from the baseline result if the NT-proBNP was ≥650 pg/mL at baseline, or improvement in New York Heart Association (NYHA) class by 1 class if NYHA class 1 or 2, and a 2-class improvement if NYHA class 3 at baseline.28 A renal response was defined as either a 30% decrease in urine total albumin or a decrease to <500 mg per 24 hours urine total protein without renal progression.30 A gastrointestinal response was defined as either a reduction in 24-hour fecal fat excretion by ≥50% in patients with steatorrhea or a ≥50% reduction in the number of loose stools per day in patients with diarrhea. A liver response was defined as either a 50% decrease in or normalization of the alkaline phosphatase level or a reduction in the span of the liver by at least 2 cm by radiographic determination. Of the 25 patients with cardiac involvement, 14 had a baseline NT-proBNP ≥650 pg/mL, and were evaluable for cardiac response based upon the NT-proBNP. One patient with an NT-proBNP <650 pg/mL was classified as a response based upon improvement in NYHA class. This patient is not depicted in the graph. (B) Graded cardiac/renal organ response. Cardiac and renal responses were retrospectively calculated. One patient with a renal response was not evaluable for grading given a baseline 24-hour proteinuria level <500 mg per 24 hours. The 14 patients with a NT-proBNP ≥650 pg/mL were evaluable for graded cardiac responses.33,34
Toxicity/treatment-related adverse events
Grade 3 or 4 treatment-related adverse events were noted in 8 (23%) of the 35 evaluable patients. Six patients experienced a grade 3 treatment-related adverse event, and 2 experienced a grade 4 event. The 2 grade 4 events were 1 each of lymphopenia and an infusion-associated reaction. The patient who experienced the grade 4 IRR had isatuximab permanently discontinued. IRRs occurred in 17 patients (49%), with most being grade 1 (n = 1) or 2 (n = 15). No IRR occurred beyond cycle 1 of treatment.
The most common treatment-related hematologic adverse events were lymphopenia (26%) and anemia (23%). There were no cases of neutropenia. The most common treatment-related nonhematologic adverse events were IRRs, followed by fatigue and diarrhea in 7 patients (20%) each. One patient (3%) had grade 3 fatigue, whereas most (n = 5; 14%) experienced grade 1 fatigue. Upper respiratory tract infections occurred in 6 patients (17%), and all were grade 2. Two patients (6%) had a grade 3 lung infection, identified as pneumonia (Table 3). The immunoglobulin G (IgG) level of the 2 patients with grade 3 lung infection was <400 mg/dL prior to the occurrence of the adverse event. A total of 8 patients had IgG levels ≤400 mg/dL at baseline disease assessment, and an additional 14 patients developed hypogammaglobulinemia (IgG ≤ 400 mg/dL) during treatment. It is not known if these patients were receiving prophylactic IV immunoglobulin.
Discussion
In summary, a hematologic response of VGPR or better was achieved in 57% of patients receiving single-agent isatuximab for relapsed AL amyloidosis, with a median time to VGPR or better of 3 months, demonstrating both high and rapidity of response, attributes that critically affect outcomes in patients with AL amyloidosis. The hematologic response rate of VGPR or better observed with isatuximab is comparable to the reported VGPR or better hematologic response rate in the 2 prior published prospective clinical trials with single-agent daratumumab in relapsed/refractory amyloidosis. The response rates for VGPR or better in these 2 trials in which the investigators evaluated 6 and 24 cycles of daratumumab ranged from 47.5% to 86%.22,23 A prior study examining the impact of daratumumab treatment duration on outcomes showed that patients who received >12 cycles of daratumumab had a longer modified PFS and OS.35 Notably, 17 patients in the current study completed the planned 24 cycles of isatuximab, and the median duration on study for the entire cohort was 21.1 months, suggesting high tolerability. The patient population was relatively fit, with 94% (n = 32) having an Eastern Cooperative Oncology Group performance score of 0 to 1, and most being Mayo stage I or II (n = 29; 83%), which may have contributed to the high tolerability and survival.
The number of patients with specific cytogenetic abnormalities is small in the trial, which limits subgroup analyses. Of 10 patients with translocation (11;14), 7 (70%) had achieved a VGPR or better. This result is consistent with responses that have been reported with daratumumab. Besides, in prior studies with daratumumab, translocation (11;14) was associated with better hematologic event-free survival and trended toward better OS.36 Only 3 patients were noted to have a gain 1q, and 2 of these patients achieved a hematologic VGPR. Prior studies have identified gain 1q as an independent predictor of adverse outcomes to daratumumab.35 Subgroup analyses from ICARIA-MM and IKEMA examined primary outcomes by 1q21+ status for patients with relapsed/refractory MM who were treated with isatuximab. The addition of isatuximab to a standard of care backbone of either pomalidomide or carfilzomib improved PFS for patients with 1q21+.37 Additional investigation in studies with a greater number of patients is needed to determine the impact of isatuximab among patients with AL amyloidosis that harbor gain 1q signature.
Organ responses were observed in all 3 patients with gastrointestinal and/or liver involvement, recognizing the small sample size of 3 patients. Renal responses occurred in 50% of patients, while 57% with cardiac involvement who were evaluable by NT-proBNP had an organ response. These response rates are comparable to those reported in the 2 prior prospective clinical trials with daratumumab that demonstrated renal response rates of 31% and 67%, respectively, and cardiac response rates of 25% and 50%, respectively.22,23 Organ responses were assessed at the 3-month mark or at the completion of therapy, but the median time to organ response was not reported for those receiving 6 cycles of treatment.23 In the prospective trial that evaluated 24 cycles of daratumumab, the median time to best renal response was 54 weeks, and best cardiac response was 44 weeks.22
Treatment with isatuximab was well tolerated, with only 1 grade 4 IRR observed. The remainder of the IRRs were grade 1 and 2. While IRRs occurred in 49% of patients, this finding is comparable to 38% to 56% that have been reported in clinical trials with isatuximab in relapsed/refractory MM,24,25,38 and 22% to 60% in the 2 published prospective trials with daratumumab in relapsed AL amyloidosis.22,23 This trial and the 2 prior prospective trials with daratumumab utilized IV administration. In the ANDROMEDA trial and in clinical practice, daratumumab is being administered as a subcutaneous (SC) injection. Lower rates of systemic reactions have been observed with daratumumab SC dosing.39 SC administration of isatuximab by an on-body delivery system is currently being investigated. In the initial report, IRRs were not observed in the on-body delivery system cohort. Utilizing this system in the future may therefore eliminate or substantially reduce this treatment-related adverse event.40
Other treatment-related adverse events of interest included grade 3 lung infections observed in 2 (6%) patients, while the remaining cases of the upper respiratory infections (n = 6, 17%) were grade 2. The rates of infection are lower in our study than reported in the 2 prospective trials with daratumumab, as respiratory infections were reported in 59% and 32% of patients.22,23 The 2 patients who experienced a grade 3 lung infection were severely hypogammaglobulinemic, with IgG level <400 mg/dL, suggesting that in such patient populations, supportive care in the form of IV immunoglobulin could be considered to help prevent infection going forward.
The current clinical trial was designed and conducted prior to the approval of daratumumab in combination with cyclophosphamide, bortezomib, and dexamethasone in the front-line setting. With this approval, most patients are now exposed to an anti-CD38 monoclonal antibody, which was not the case in the current trial as only 2 patients here had received prior treatment with daratumumab. Importantly, these 2 patients did not achieve a hematologic response. These findings are at odds with the retrospective review in refractory MM that showed an overall response rate of 46.2% among patients who received isatuximab after daratumumab treatment.41 In the ANDROMEDA trial, patients received up to 24 cycles of treatment prior to discontinuation. As continuous maintenance therapy is not typically given in AL amyloidosis, there may be a role for re-treatment with an anti-CD38 monoclonal antibody at the time of progression. Additional studies are needed to determine the efficacy of isatuximab in AL amyloidosis following daratumumab exposure.
More recently, isatuximab has been combined with pomalidomide in relapsed AL amyloidosis. Similar to the patient population presented here, only 3 patients were daratumumab exposed in the combination trial. The combination demonstrated rapid hematologic responses with a median time to VGPR of 4 weeks, with 80% of patients having achieved a VGPR or better after 6 cycles.42 The percentage of patients achieving a VGPR or better is higher with the combination compared with the 57% seen here with single-agent isatuximab. Four patients in the current trial had previously seen treatment with pomalidomide. The use of combinations going forward will be individualized based on prior treatments and residual adverse events. Additional trials are needed to determine the optimal combination and sequencing in relapsed AL amyloidosis.
In summary, isatuximab monotherapy resulted in high rates of hematologic response in relapsed AL amyloidosis, which translated into organ responses. Treatment appears safe and was well tolerated. Ongoing follow-up is needed to determine its full impact on outcomes. The outstanding safety profile of isatuximab for patients with AL amyloidosis demonstrated in this trial also opens doors to examine its efficacy and tolerability in potentially more effective combination regimens.
Acknowledgments
This work was supported by the National Cancer Institute (NCI)/National Institutes of Health (NIH)/National Clinical Trials Network grant awards U10CA180888, U10CA180819, CA180820, and CA180821, and, in part, by Sanofi-Aventis (Sanofi US).
H.J.L. acknowledges support from the NCI/NIH Cancer Center Support Grant P30 CA008748. R.Z.O. ,the Florence Maude Thomas Cancer Research Professor, acknowledges support from the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation, the Riney Family Multiple Myeloma Research Fund at MD Anderson from the Paula and Rodger Riney Foundation, and the Myeloma Solutions Fund.
Authorship
Contribution: T.L.P. and V.S. led the phase 2 clinical trial, interpreted the data, and wrote the manuscript; A.R. and A.H. performed the statistical analysis and edited the manuscript; H.J.L., E.L.C., P.K., N.N., S.G., P.H., B.G.M.D., and R.Z.O. enrolled patients, performed clinical analysis, and/or edited the manuscript; and E.C.S. codesigned the phase 2 clinical trial and edited the manuscript.
Conflict-of-interest disclosure: P.H. serves on the speaker’s bureau for Sanofi. P.K. is the principal investigator of trials for which Mayo Clinic has received research funding from Amgen, Regeneron, Bristol Myers Squibb (BMS), Loxo Pharmaceuticals, Ichnos, Karyopharm, Sanofi, AbbVie, and GlaxoSmithKline; has received honorarium from Keosys; and has served on the advisory boards of BeiGene, Mustang Bio, Janssen, Pharmacyclics, ×4 Pharmaceuticals, Kite, Oncopeptides, Ascentage, Angitia Bio, GlaxoSmithKline (GSK), Sanofi, and AbbVie. N.N. is the principal investigator of trials for which Yale has received research funding from Janssen and GSK; and is a consultant for Cellsbin Inc. H.J.L. has received research funding from Janssen, AbbVie, Alexion, Prothena, and Protego; and serves on the advisory boards of Pfizer, Nexcella, AbbVie, BMS, Alexion, Arcellx, and Karyopharm. V.S. has received research funding from Celgene, Millennium-Takeda, Janssen, Prothena, Sorrento, Karyopharm, Oncopeptide, Caelum, and Alexion; serves as a consultant for Pfizer, Janssen, Attralus, GateBio, AbbVie, and BridgeBio; and serves on the advisory boards of Proclara, Caelum, AbbVie, Janssen, Regeneron, Protego, Pharmatrace, Telix, Prothena, AstraZeneca, Nexcella, Alexion, and GSK. The remaining authors declare no competing financial interests.
Brian G. M. Durie died on 12 October 2025.
Correspondence: Terri Parker, Department of Internal Medicine, Section of Medical Oncology and Hematology, Yale University School of Medicine, 333 Cedar St, New Haven, CT 06510; email: terri.parker@yale.edu.
References
Author notes
T.L.P. and V.S. contributed equally to this study.
Data are available on request from the corresponding author, Terri Parker (terri.parker@yale.edu).
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.