Abstract
A patient with type II cryoglobulinemic vasculitis and hepatitis C virus (HCV) infection presented with a leukemiclike proliferation of B cells bearing marginal zone B-cell phenotypic markers. A partial trisomy 3 (bands 3q11–29) and overexpression of Bcl-2 without t(14;18) translocation was detected in the monoclonal B cells that were classic rheumatoid factor–producing B cells bearing the WA cross-idiotype. Treatment with interferon-α produced a complete clinical remission and synchronous marked decreases in viremia and monoclonal B-cell prevalence. This is the first report of partial trisomy 3 and Bcl-2 overexpression in type II cryoglobulinemic vasculitis associated with HCV infection. Further studies of HCV-infected patients with and without type II cryoglobulinemia are required to determine the prevalence and possible physiologic and/or pathophysiologic significance of these findings.
Introduction
Type II cryoglobulinemia, a lymphoproliferative disorder characterized by cold-precipitable immune complexes composed of polyclonal immunoglobulin (Ig)–G and monoclonal IgM rheumatoid factor (mRF), predominantly occurs secondary to hepatitis C virus (HCV) infection (HCV–type II cryogloblulinemia). The concentration of HCV in type II cryoglobulins and the presence of a highly restricted mRF bearing the WA cross-idiotype in approximately 80% of patients lead to the hypothesis that proliferation of the WA mRF-producing cells was driven by HCV.1,2 Moreover, recent data demonstrated that contrary to the premise that type II cryoglobulinemia is a low-grade malignancy,3 the oligoclonal B-cell expansion frequently present in the bone marrow of these patients showed no evidence of malignancy.4 In this study, we present a patient with HCV–type II cryoglobulinemia, a leukemiclike monoclonal B-cell proliferation bearing marginal zone B-cell (MZBC) phenotypic markers without definitive evidence of malignancy, Bcl-2 overexpression, and partial trisomy 3. The course of the patient's disease is informative because this is the first report of partial trisomy 3 and Bcl-2 overexpression in HCV–type II cryoglobulemia, and there was a synchronous decrease in B-cell proliferation and viremia with interferon-α therapy.
Study design
Index case
A 57-year-old white male with HCV–type II cryoglobulinemia in clinical remission for 10 years relapsed in March 1998 and presented with palpable purpura, cryoglobulinemia (4% cryocrit), marked leukocytosis (white blood cell [WBC] count, 35.7 × 109/L), and splenomegaly. Other notable laboratory findings were the following: positive HCV serology and HCV RNA; anemia (hemoglobin 116 g/L); normal platelet count (151 × 109/L); low C4 component of complement (7 mg/dL; normal range, 17-45 mg/dL); high lactate dehydrogenase (LDH) (843 IU/mL; normal range, 325-685 IU/mL); high IgM (3.05 g/L; normal range, 0.5-2 g/L) with a monoclonal IgMκ; low IgG (3.64 g/L; normal range, 7.5-14 g/L); but normal IgA (1.55 g/L; normal range, 0.75-3.1 g/L). There was no clinical evidence of renal or liver disease. A chest and abdominal computed tomography (CT) scan showed a normal liver, a 20.6 × 11.2 cm spleen, and no adenopathy. Bone marrow examination showed multifocal clusters of small atypical lymphocytes suspicious for lymphoproliferative disorder. Lymphocyte phenotypes for peripheral blood and bone marrow were identical: IgM+, κ+, IgD−, CD19+, CD20+, CD22+, CD5−, CD10−, CD23−; villous lymphocytes were not present. Treatment with interferon (IFN) was initiated. Longitudinal studies from April 8, 1998, to September 24, 1999, are shown in Figure 1. Subsequently, cryoglobulinemia remained in remission with no clinical evidence of malignancy. CT scans performed in February and May 2001 showed no lymphadenopathy and spleen measurements of 14 × 6 cm and 13 × 6 cm, respectively. The patient expired in June 2001 from complications of atherosclerotic peripheral vascular disease. An autopsy was not performed. These retrospective studies were approved by the institutional review board of the Lahey Clinic, Burlington, MA.
An 18-month longitudinal study of the index case demonstrating the effect of IFN therapy on viral titers and the prevalence of IgMκ B cells.
Prelevalence of IgMκ (percentage of lymphocytes) with HCV viremia genomic equivalents (gEs) per milliliter during treatment with IFN (3 MU given daily [qd] or 3 times weekly [tiw] as previously described5) are demonstrated. Relapse was associated with marked lymphocytosis (lymphocyte count of 44 × 109/L [44 000/μL]), which was due predominantly to IgMκ B cells; a high level of viremia (7.3 × 107gE/mL), and a cryocrit of 4%. WBCs declined from 53.4 × 109/L to 5.0 × 109/L and lymphocytosis from 82% (lymphocyte count of 44 × 109/L) to 38% (lymphocyte count of 1.9 × 109/L) with IFN therapy. Decreases in IFN therapy from daily to 3 times weekly administration and interruption of therapy were accompanied by parallel increases in viremia and monoclonal B cells. Cryoglobulinemia fell to 1% cryocrit after 1 month of therapy and remained at trace levels (less than 1%) for the next 17 months. LDH returned to normal in May 1998 and remained within normal range throughout the remainder of the patient's course of treatment.
An 18-month longitudinal study of the index case demonstrating the effect of IFN therapy on viral titers and the prevalence of IgMκ B cells.
Prelevalence of IgMκ (percentage of lymphocytes) with HCV viremia genomic equivalents (gEs) per milliliter during treatment with IFN (3 MU given daily [qd] or 3 times weekly [tiw] as previously described5) are demonstrated. Relapse was associated with marked lymphocytosis (lymphocyte count of 44 × 109/L [44 000/μL]), which was due predominantly to IgMκ B cells; a high level of viremia (7.3 × 107gE/mL), and a cryocrit of 4%. WBCs declined from 53.4 × 109/L to 5.0 × 109/L and lymphocytosis from 82% (lymphocyte count of 44 × 109/L) to 38% (lymphocyte count of 1.9 × 109/L) with IFN therapy. Decreases in IFN therapy from daily to 3 times weekly administration and interruption of therapy were accompanied by parallel increases in viremia and monoclonal B cells. Cryoglobulinemia fell to 1% cryocrit after 1 month of therapy and remained at trace levels (less than 1%) for the next 17 months. LDH returned to normal in May 1998 and remained within normal range throughout the remainder of the patient's course of treatment.
Methods
Comparative genomic hybridization (CGH) was performed on DNA extracted from the patient's July 1,1998, peripheral blood mononuclear cells (PBMCs) as previously described with a slight modification.6,7 Fluorescent in situ hybridization (FISH) experiments were performed on interphase nuclei from the same blood specimen, in routine fashion, with an alpha-satellite probe, pα3.5, for the centromeric region of chromosome (kindly provided by M. Stul, University of Leuven, Belgium), and a mixture of BACs for MLF1 gene at 3q25 (kindly provided by S. Morris, Memphis, TN). The presence of t(14;18) translocation was determined by real-time Taqman reverse-transcriptase–polymerase chain reaction (RT-PCR) employing primers and probes specific for the major and minor breakpoints.8 DNA samples from 2 patients with follicular lymphoma served as the positive controls for t(14;18) translocation. The amount of DNA in each sample was normalized to the actin DNA present in the sample. Bcl-2 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) messenger RNA levels were determined by real-time Taqman RT-PCR in triplicate. Standard curves were prepared using human control RNA. Bcl-2/GAPDH ratios were calculated and normalized to normal lymphocytes. Clonal expansion analysis and sequencing were performed by means of routine methodology but with primers designed to amplify VH1 region or Vκ3 complementarity determining region (CDR3) of WA mRF.
Results and discussion
An 18-month longitudinal study of the index case following relapse of HCV infection and cryoglobulinemia is shown in Figure 1. IFN therapy was accompanied by a marked decrease in lymphocytosis, monoclonal IgMκ B cells, and viremia. The close correlation of the prevalence of monoclonal B cells with IFN-induced decrease in viremia is consistent with the hypothesis that the HCV infection drove the proliferation of the monoclonal B cells but does not constitute direct evidence since IFN has both antiviral and immunomodulatory effects.
A WA mRF was demonstrated in the cryoglobulins by detection of the WA cross-idiotype on serologic analysis1 of the isolated mRF (data not shown). The presence of the WA mRF was confirmed by performing PCR B-cell clonal expansion analysis of the PBMCs and detecting and sequencing monoclonal bands corresponding to the VH1 region and VK3 CDR3. The sequencing analyses (Gen-Bank accession nos. AY059634 and AY059635) demonstrated the typical WA RF sequence.9
There was no evidence of t(14;18) translocation in the patient's PBMCs; with the use of comparable levels of actin DNA in the test and control samples, breakpoint amplification occurred only in the positive control samples. There was, however, a 20-fold overexpression of Bcl-2 in the patient's PBMCs compared with normal PBMCs. The patient's PBMCs had a Bcl-2–to–GAPDH ratio of 18:8; normal lymphocytes had a ratio of 0:94. Overexpression of Bcl-2 in the absence of t(14;18) translocation has been noted in PBMCs and cloned WA B cells from patients with HCV–type II cryoglobulinemia in a preliminary report.10
CGH and interphase FISH results confirmed a partial trisomy of chromosome 3 (bands 3q11–29), without any involvement of the centromeric region (Figure 2). The overrepresented region corresponds to the region commonly involved in MZBC lymphoma.11 The existence of the partial trisomy 3 in the monoclonal B cells was deduced from the overlapping prevalence of monoclonal B cells determined by immunophenotyping (85%), and the partial trisomy 3 was determined by FISH studies performed on the same PBMC sample (80%). Unfortunately, since material was not available for further metaphase analysis, additional structural chromosomal abnormalities that may have been associated with the partial trisomy 3 in this patient could not be excluded although the t(14;18) translocation was excluded by the studies described above. The marked lymphocytosis that occurred with relapse of disease in the index case is unusual in type II cryoglobulinemia. Our findings suggest that (1) the duplicated genes on the third chromosome were involved in enhanced proliferation of the monoclonal B cells that resulted in the lymphocytosis and (2) the partial trisomy 3 did not produce loss of antigen dependence in those cells.
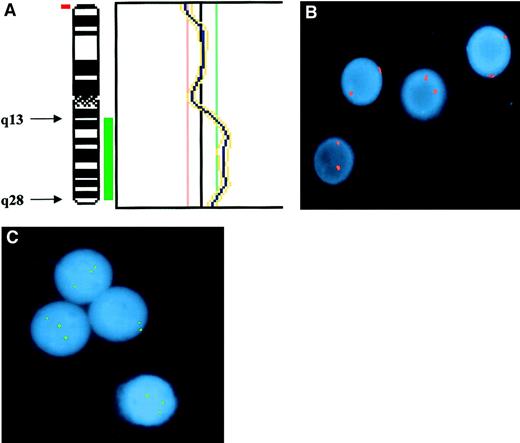
Demonstration of partial trisomy 3 in the PBMCs of the index case.
(A) CGH. Partial profile of chromosome 3 showing the region of duplication involving the long(q) arm. Chromosomal regions were considered overrepresented when the corresponding green-to-red ratio exceeded 1:18 and underrepresented when the ratio was below 0:83. Thresholds were fixed on profiles obtained from hybridization of 2 differently labeled normal DNAs extracted from healthy donors. Telomeric and centromeric regions as well as heterochromatic regions of chromosome 1, 9, 16, and Y were excluded from analysis, because of possibly false-positive results. Results were confirmed by means of a 99% confidence interval, with 1% error probability. (B) Interphase FISH with probe for centromeric sequences of chromosome 3 showing only disomic nuclei. Signals were evaluated on 600 interphase cells, of which 2.6% showed 1 spot; 97.1%, 2 spots; and 0.3%, 3 spots. (C) Interphase FISH with the locus-specific probe for MLF1 gene (3q25) showing 3 spots in 3 of 4 nuclei. Signals were evaluated in 100 interphase cells, of which 20% showed 2 spots and 80% showed 3 spots.
Demonstration of partial trisomy 3 in the PBMCs of the index case.
(A) CGH. Partial profile of chromosome 3 showing the region of duplication involving the long(q) arm. Chromosomal regions were considered overrepresented when the corresponding green-to-red ratio exceeded 1:18 and underrepresented when the ratio was below 0:83. Thresholds were fixed on profiles obtained from hybridization of 2 differently labeled normal DNAs extracted from healthy donors. Telomeric and centromeric regions as well as heterochromatic regions of chromosome 1, 9, 16, and Y were excluded from analysis, because of possibly false-positive results. Results were confirmed by means of a 99% confidence interval, with 1% error probability. (B) Interphase FISH with probe for centromeric sequences of chromosome 3 showing only disomic nuclei. Signals were evaluated on 600 interphase cells, of which 2.6% showed 1 spot; 97.1%, 2 spots; and 0.3%, 3 spots. (C) Interphase FISH with the locus-specific probe for MLF1 gene (3q25) showing 3 spots in 3 of 4 nuclei. Signals were evaluated in 100 interphase cells, of which 20% showed 2 spots and 80% showed 3 spots.
The data presented have conflicting interpretations and are insufficient to determine whether a malignant or benign lymphoproliferation was present in the index case. The lymphoproliferation had characteristics common to both the B-cell lymphocytosis associated with partial trisomy 3 and very low grade subtypes of MZBC lymphomas.12-14 The splenomegaly and elevated LDH that responded to IFN therapy were suggestive of splenic lymphoma. The monoclonal B cells had MZBC markers and were WA+; 25% or more of B-cell lymphomas associated with HCV–type II cryoglobulinemia arise from WA+ B cells although correlation with MZBC markers has not been assessed.15,16 However, neither MZBCs nor splenic lymphomas are commonly associated with HCV–type II cryoglobulinemia. Only 5% to 10% of these patients develop overt B-cell malignancies17,18 that are predominantly lymphoplasmacytoid lymphoma/immunocytoma.3,19 Moreover, the correlation of regression of the putative HCV-driven B-cell proliferation with IFN therapy is against a malignant lypmphoproliferative process. However, antigen-driven lymphoproliferation has been reported in some low-grade extranodal marginal zone lymphomas.20 Since there is evidence that the monoclonal B cells in type II cryoglobulinemia arise predominantly in the pseudofollicle in the liver,21 22 the lymphoproliferation in the index case could be an HCV-driven very-low-grade extranodal marginal zone lymphoma.
Overexpression of Bcl-2 has heretofore not been associated with duplication of 3q11-29.11-13 Further studies are needed to determine the prevalance of these abnormalities in HCV infection with and without type II cryoglobulimenia and their possible physiologic and/or pathophysiologic implications.
We thank Dr K. M. Steve Lo, Hematology Oncology, Stamford Health System, Stamford, CT, and Dr Kristan D. Zimmermann, The Elliot & Roslyn Jaffe Diagnostic Imaging Center, Stamford, CT, for providing clinical data on the index case, and Dr Nicolas Chiorrazi for reviewing the manuscript.
Supported by grants from the Italian National Research Council (C.Me.); the Italian National Association for Cancer Research (C. Me.); the Italian Ministry for University and Scientific Research (MURST) (C.Me., M.F.); the Robert E Wise, MD, Research and Education Institute, Lahey Clinic, Burlington, MA (V.A.); grant RO1 AI 40672-02 from the National Institute of Allergy and Infectious Diseases (V.A.); the Veterans Affairs Administration (V.A.); the Istituto Pasteur Fondazione Cenci Bolognetti at the University of Roma La Sapienza (M.F.); and MURST COFIN 2000 (M.F.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Vincent Agnello, Lahey Clinic, 41 Mall Rd, Burlington, MA 01805-0001; e-mail: vincent.agnello@lahey.org.

![Fig. 1. An 18-month longitudinal study of the index case demonstrating the effect of IFN therapy on viral titers and the prevalence of IgMκ B cells. / Prelevalence of IgMκ (percentage of lymphocytes) with HCV viremia genomic equivalents (gEs) per milliliter during treatment with IFN (3 MU given daily [qd] or 3 times weekly [tiw] as previously described5) are demonstrated. Relapse was associated with marked lymphocytosis (lymphocyte count of 44 × 109/L [44 000/μL]), which was due predominantly to IgMκ B cells; a high level of viremia (7.3 × 107gE/mL), and a cryocrit of 4%. WBCs declined from 53.4 × 109/L to 5.0 × 109/L and lymphocytosis from 82% (lymphocyte count of 44 × 109/L) to 38% (lymphocyte count of 1.9 × 109/L) with IFN therapy. Decreases in IFN therapy from daily to 3 times weekly administration and interruption of therapy were accompanied by parallel increases in viremia and monoclonal B cells. Cryoglobulinemia fell to 1% cryocrit after 1 month of therapy and remained at trace levels (less than 1%) for the next 17 months. LDH returned to normal in May 1998 and remained within normal range throughout the remainder of the patient's course of treatment.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/6/10.1182_blood.v99.6.2259/6/m_h80622276001.jpeg?Expires=1769228507&Signature=ejURPQD-tlWZjgcgYekc0E18nFL81u3dyOrHScd5XHObr~isHIcWQjWpwzYBYZY-FDX66bWZWW9RklpTn5uOCyQ9Ue~9MIn1u6cfqlRIxy-nJmDyKlK74ng7EL-flI8ydfElbJXo~TKTw9czHfE~ke7lmBK-Qc5RSW5zhikQBH8crE8Bi7k7dSYRsOUP2qnSJeuuLdLZRxr4rCN1hs65T1ab7goptWPKzTcRkerJBWbsU2Lzb98d-3McgbXjqOCMPO9-JiXzXMRA77~d9lFmfEYPagK7wt0e-aWMMUYV9YaKqeqQfJiCgq3nyEugA18k90UjUwsMXwD4fhA92Hn77A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)