In their work recently published in Blood, Wierda and coworkers1 demonstrated that adenovirus-mediated gene transfer of CD154 into chronic lymphocytic leukemia (CLL) B cells and the retransfer of these cells into the bloodstream of CLL patients resulted in increased or de novo expression of immune accessory molecules, such as CD54, CD95, CD80, and CD86, on CLL bystander cells and restored the capacity of CLL cells to stimulate autologous T cells. As a result, rising plasma levels of Thl-type cytokines and increased absolute numbers of blood T cells were observed, as well as a T-cell subset reactive against autologous CLL cells. These findings correlated with a clinical response manifested as a reduction in absolute lymphocyte count and lymph node size.
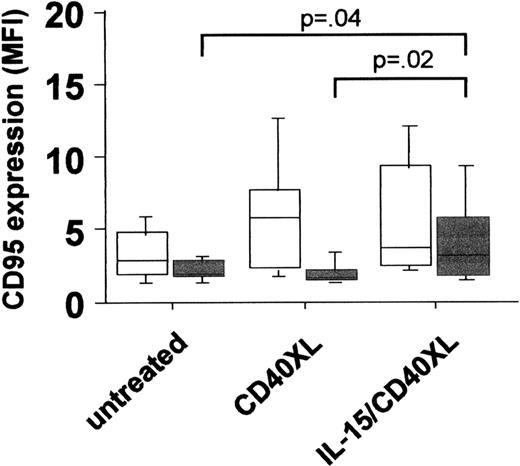
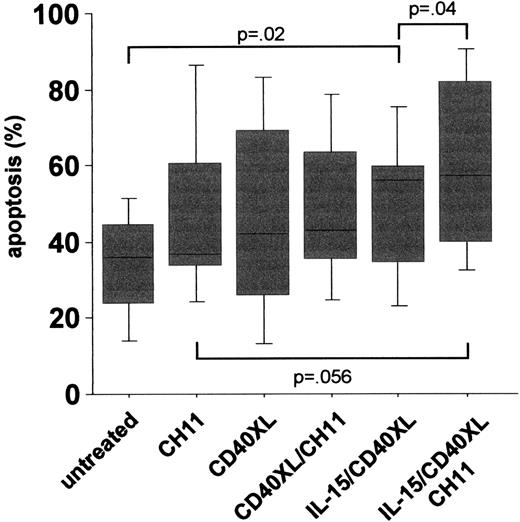
The idea behind a CD154 gene therapy protocol was based on an earlier study in which an impaired intrinsic CD40 activation of CLL cells was observed and contributed to a suppression of CD154 expression on T cells by B-CLL cells.2 In the present study, we found significantly lower basal CD40 expression levels in CLL compared to B cells from age- and sex-matched healthy donors (mean specific fluorescence intensity [MFI] CLL (n = 41): mean ± SEM 2.7 ± .22 vs healthy controls (n = 41): 4.6 ± .26,P < .000l). Besides lower expression levels, the CD40-mediated signal transduction was impaired, as an up-regulation of CD95 expression following CD40 activation could be observed only in normal but not in CLL B-cells (Figure 1). From our results, we conclude that not only aberrant regulation of CD154, but also deficiencies in CD40 expression and/or signal transduction might contribute to the impaired T-cell activation in CLL. In an attempt to increase CD40 expression and signaling in CLL cells, we tested the effect of interleukin-15 (IL-15), a known T- and natural killer (NK)–cell activator. Although in vitro experiments suggested an increase in [3H]-thymidine uptake in CLL cells triggered by IL-15,3 such a tumor-promoting effect might be counteracted by the induction of a successful T-cell response. In fact, this cytokine has been shown to up-regulate costimulatory molecules in hematopoietic cell lines.4 Our results demonstrated that stimulation of B-CLL cells by IL-15 enhances CD40 expression significantly (MFI untreated: 3.9 ± .19 vs IL-15-treated: 5.4 ± .38, P < .002, n = 14), accompanied by a reconstitution of CD40 signaling capacity, as shown by the up-regulation of CD95 following costimulation of CLL cells with IL-l5 and also by CD40 cross-linking (IL-I5/CD40XL) (Figure 1). Wierda et al demonstrated that CD154-mediated CD40 activation led to the stimulation of T cells reactive against CLL cells.1 We observed that IL-15 was a prerequisite for the induction of apoptosis in CLL cells via the CD40 pathway (Figure 2). It has been shown that the killing of target cells by cytotoxic T cells is mediated, at least in part, via the activation of CD95.5Since we observed the up-regulation of CD9S on CLL cells activated by IL-l5 and CD40, we tested whether this increases the sensitivity of CLL cells toward CD95 ligation following the addition of an agonistic anti-CD95 antibody (CHll, 250 ng/mL for 3 days). As also observed for spontaneous apoptosis, CD40 activation following IL-15 prestimulation resulted in an increased number of apoptotic CLL cells (Figure 2). From these data it can be concluded that the combination of IL-15 and CD40 stimulation renders B-CLL cells more sensitive to FasL+cells, which are either cytotoxic T cells or themselves B-CLL cells.6
PBMC of 20 CLL patients (gray squares) and of 24 healthy donors (white squares) were left untreated or stimulated with IL-15 (50 ng/mL, 7 days) or activated via CD40 cross-linking (CD40XL, ie, treated with 1 μg/mL anti-human CD40 mab followed by a 5-day incubation with an antimouse mab).
The expression of CD95 was determined by staining of cells with a specific FITC-labeled antihuman CD95 mab or a relevant isotype-specific control.
PBMC of 20 CLL patients (gray squares) and of 24 healthy donors (white squares) were left untreated or stimulated with IL-15 (50 ng/mL, 7 days) or activated via CD40 cross-linking (CD40XL, ie, treated with 1 μg/mL anti-human CD40 mab followed by a 5-day incubation with an antimouse mab).
The expression of CD95 was determined by staining of cells with a specific FITC-labeled antihuman CD95 mab or a relevant isotype-specific control.
PBMCs of 18 CLL patients were left untreated or stimulated with IL-15 (7 days), activated via CD40XL (5 days), or stimulated with an anti-CD95 mab (CH11, 250 ng/mL, 3 days).
Apoptosis was measured by using an annexin V/PI assay. All statistics were done using ANOVA tests.
PBMCs of 18 CLL patients were left untreated or stimulated with IL-15 (7 days), activated via CD40XL (5 days), or stimulated with an anti-CD95 mab (CH11, 250 ng/mL, 3 days).
Apoptosis was measured by using an annexin V/PI assay. All statistics were done using ANOVA tests.
In conclusion, we wonder whether autologous IL-l5 contributes to some of the immunological effects observed during treatment with adenovirus CD154-modified B-CLL cells and suggest that the cytokine might qualify as an adjuvant augmenting the therapeutic efficacy by rendering B-CLL cells more susceptible to killing by tumor-specific T cells.
Supported by Oesterreichische Nationalbank, grant no. 8222 (R.G., G.A.); Austrian Science Foundation, grant no. T95-Pat (I.T.); and Tyrolean Cancer Aid (R.G., I.M.).