Abstract
Hypercalcemia is one of the most frequent and serious complications in patients with adult T-cell leukemia (ATL) and is due to marked bone resorption by accumulation of osteoclasts (OCLs). Although several cytokines such as interleukin 1 and parathyroid hormone–related protein are thought to be involved in the development of high serum Ca++ levels, its precise underlying mechanism remains unknown. This study analyzed the expression of various genes that are thought to regulate serum Ca++ levels in ATL and showed that the overexpression of the receptor activator of nuclear factor κB (RANK) ligand gene correlated with hypercalcemia. ATL cells from patients with hypercalcemia, which highly expressed the transcripts of the RANK ligand (RANKL) gene, induced the differentiation of human hematopoietic precursor cells (HPCs) into OCLs in vitro in the presence of macrophage colony-stimulating factor (M-CSF). In contrast, ATL cells from patients without hypercalcemia did not induce such differentiation, suggesting that the induction of the differentiation correlated with the expression of the RANKL gene in ATL cells. Cell differentiation was suppressed by osteoprotegerin/Fc, an inhibitor of RANKL, indicating that such differentiation occurred through the RANK-RANKL pathway. In addition, direct contact between ATL cells and HPCs was essential for the differentiation, suggesting that not the soluble form but membrane-bound RANKL played a role in this process. These results strongly suggested that ATL cells induce the differentiation of HPCs to OCLs through RANKL expressed on their surface, in cooperation with M-CSF, and ultimately cause hypercalcemia.
Introduction
Adult T-cell leukemia (ATL) is a highly aggressive neoplastic disease of peripheral helper T lymphocytes that is etiologically associated with human T-cell leukemia virus type I (HTLV-I).1-7 There are 4 clinical subtypes of ATL: smoldering, chronic, lymphoma-type, and acute ATL.8 Of these subtypes, patients with smoldering and chronic ATL usually have an indolent clinical course; however, most patients progress to acute or lymphoma-type ATL several years later. Despite the use of aggressive chemotherapy, survival of patients with acute or lymphoma-type ATL has not improved; the mean survival period of patients with acute ATL is still less than 1 year. The main challenges for a successful treatment of ATL are drug resistance of ATL cells, high frequency of opportunistic infections, and hypercalcemia.9
The high frequency of hypercalcemia is the most striking feature of ATL; about 70% of ATL patients have high serum Ca++levels during the clinical course of the disease, particularly during the aggressive stage of ATL.10 Such a frequency is the highest among hematologic malignancies, and hypercalcemia is more severe in patients with ATL than in those with other hematologic malignancies.11 In those patients, serum Ca++levels are often more than 20 mg/mL, and accordingly, most patients are in a state of coma.
Several pathologic studies of ATL patients with hypercalcemia have indicated that high serum Ca++ levels are due to an increased number of osteoclasts (OCLs) and accelerated bone resorption.10,12,13 Several cytokines, such as interleukin-1 (IL-1),14 transforming growth factor β,15 and parathyroid hormone–related protein (PTH-rP)16 have been implicated in ATL-associated hypercalcemia. Among these factors, PTH-rP is considered to play an important role by stimulating OCLs, resulting in increased bone resorption. Immunodeficient mice implanted with leukemic cells from patients with ATL exhibited hypercalcemia and overexpressed PTH-rP.17 However, PTH-rP cannot directly induce the differentiation of hematopoietic precursor cells (HPCs) to OCLs.18 Furthermore, high levels of PTH-rP in the serum are not always associated with hypercalcemia in patients with ATL, suggesting that another factor is involved in the pathogenesis of hypercalcemia.19
Bone is constitutively remodeled by osteoblasts (the synthesis of matrix) and OCLs (bone resorption). OCLs are derived from HPCs and belong to the monocyte macrophage lineage. During differentiation of OCLs, precursor cells sequentially express c-Fms followed by receptor activator of nuclear factor κB (RANK).20 Macrophage colony-stimulating factor (M-CSF) and RANK ligand (RANKL) are critical factors for the differentiation of OCLs, which are physiologically produced by stromal cells and osteoblasts.21 The RANK-RANKL system has also been demonstrated to play an important role in the immune system,22 and the loss of the expression of RANKL gene results in impaired formation of lymphoid organs.23 Recently, Kong et al24 identified abnormal expression of RANKL on activated T lymphocytes and postulated that this gene is associated with bone loss and joint destruction in inflammatory arthritis.
In the present study, we demonstrate that ATL cells derived from patients with hypercalcemia express RANKL messenger RNA and induce the development of OCLs from HPCs in vitro, indicating that RANKL is critically involved in the pathogenesis of hypercalcemia in ATL.
Patients, materials, and methods
Patients
Fifteen patients with ATL (12 cases with acute ATL and 3 with chronic ATL) were examined in this study. They had a diagnosis of ATL confirmed by the monoclonal integration of HTLV-I provirus in the host genome by the Southern blot method. Clinical characteristics of these patients are shown in Table1. Eight of these patients had hypercalcemia, and 5 exhibited infiltration of leukemic cells into the bone marrow. When leukemic cells constituted more than 10% of nuclear cells in the bone marrow, it was considered being bone marrow invasion by ATL cells (Table 2).
Semiquantitative reverse transcriptase–polymerase chain reaction
Transcripts of PTH-rP, M-CSF, RANKL, osteoprotegerin (OPG), and tumor necrosis factor α (TNF-α) were quantified by using semiquantitative reverse transcriptase–polymerase chain reaction (RT-PCR) as described previously.25 Total RNA was extracted from each sample using TRIZOL (Life Technologies, Paisley, United Kingdom), and complementary DNA (cDNA) was synthesized from 1 μg total RNA using the Superscript Preamplification System (Life Technologies) according to the protocol recommended by the manufacturer. PCR was performed by using 1 μL reverse transcriptase reaction sample mixed with 50 μL PCR reaction buffer containing 0.1 mM each of deoxynucleotide triphosphates, 1.5 mM MgCl2, 2.5 U Taq polymerase, and 20 pmol of each primer. Primers were 5′-GCAAGCTTGAAGCTCAGCCT-3′ (forward) and 5′-CCTCTCCAGACCGTAACTTA-3′ (reverse) for RANKL, 5′-TGTGCAATGCAAGGAAGGG-3′ (forward) and 5′-CTTTAGTGCGTGCATTAGGC-3′ (reverse) for OPG, 5′-ATCACCGAGGAGGTGTCGGAGTA-3′ (forward) and 5′-GCTGGAGCATTCAGCAAGCTGT-3′ (reverse) for M-CSF, 5′-TCTTCTGCTGCACTTTGG-3′ (forward) and 5′-ATCTCTCAGCTCCACGCATTG-3′ (reverse) for TNF-α, and 5′-CAGCGGAGACTGGTTCAGCA- GTG-3′ (forward) and 5′-ACCAGGCAGAGCGAGTTCGGAGTA-3′ (reverse) for PTH-rP in PC-960G gradient thermal cycler (COSMO BIO, Tokyo, Japan) under the following conditions: RANKL, for 3 minutes at 94°C, for 26 cycles for 30 seconds at 94°C, 30 seconds at 57°C, and 30 seconds at 72°C; OPG, for 3 minutes at 94°C, for 35 cycles of 30 seconds at 94°C, 30 seconds at 60°C, and 30 seconds at 72°C; M-CSF, for 3 minutes at 94°C, for 26 cycles for 30 seconds at 94°C, 30 seconds at 57°C, and 30 seconds at 72°C; TNF-α, for 3 minutes at 94°C, for 26 cycles for 30 seconds at 94°C, 30 seconds at 57°C, and 30 seconds at 72°C; and PTH-rP, for 3 minutes at 94°C, for 26 cycles for 30 seconds at 94°C, 30 seconds at 55°C, and 30 seconds at 72°C. PCR products were detected by ethidium bromide staining in 2% agarose gel. PCR was performed at the 7 different cycles (22, 25, 27, 30, 33, 35, and 37 cycles) to determine the linear amplification for quantification. The intensity of PCR-amplified band was quantified using ATTO densitograph 4.0 (ATTO, Tokyo, Japan).
Measurement of serum concentrations of M-CSF, TNF-α, and PTH-rP
Serum concentrations of M-CSF and TNF-α were measured by the sandwich enzyme–linked immunosorbent assay method using Quantikine Human M-CSF immunoassay and Quantikine Human TNF-α immunoassay (R&D Systems, Minneapolis, MN), according to the protocol provided by the manufacturer. The normal ranges of M-CSF and TNF-α were 102 to 765 (n = 30) and less than 15.6 pg/mL, respectively. Serum levels of PTH-rP were measured by SRL (Tokyo, Japan) using the C-PTH-rP Kit (Daiichi Radioisotope Lab, Tokyo, Japan). The normal range of serum PTH-rP was 13.8 to 55.3 pmol/L.
Flow-activated cell sorter analysis and cell sorting
Human bone marrow cells were harvested from healthy volunteers and layered onto a Ficoll-Paque (Amersham Pharmacia Biotech, Uppsala, Sweden) gradient and centrifuged for 30 minutes at 400g. Informed consent was obtained from all patients before bone marrow aspiration. After centrifugation, mononuclear cells were collected from the upper layer, and cells were suspended in 5% fetal bovine serum (JRH Bioscience, Lenexa, KS) that contained phosphate-buffered saline (staining buffer) and were incubated with 5 μg/mL mouse immunoglobulin G2a, κ (antitrinitrophenol) for 30 minutes on ice to block Fc receptors. Subsequently, cells were incubated with fluorescein isothiocyanate (FITC)–conjugated anti-CD34 antibody (NU4A1) and R-phycoerythrin (R-PE)–conjugated anti-c-Kit antibody (NU-c-Kit) (both Nichirei, Tokyo, Japan) for 30 minutes on ice. After 2 washes, cells were suspended with staining buffer, and cell sorting was performed using a flow-activated cell sorter (FACS) Calibur (Becton Dickinson Immunocytometry Systems, San Jose, CA). Data were analyzed using Cell Quest software (Becton Dickinson Immunocytometry Systems).
OCL differentiation in vitro
In these experiments, 1 × 103 sorted cells (CD34+c-Kit+ cells) were plated on 96-well culture plates (PRIMARIA; Falcon 3872; Becton Dickinson Labware, Lincoln Park, NJ) and cultured in α minimum essential medium (GIBCO-BRL, Gaithersburg, MD) containing 10% fetal bovine serum with 50 ng/mL recombinant human M-CSF (R&D Systems) alone or together with 25 ng/mL recombinant human RANKL (sRANKL; Peprotech EC, London, United Kingdom) and 50 U/mL human IL-2 for 7 days. For blocking the effect of sRANKL, 10 μg/mL recombinant human RANK/Fc or OPG/Fc chimeras (R&D Systems) was added to the culture, and human sTNF RI (R&D Systems) was used to block the function of TNF-α. In some experiments, 1 × 105 peripheral blood mononuclear cells (PBMCs) from patients with ATL were added to the culture instead of sRANKL. Cultured cells were subjected to tartrate-resistant acid phosphatase (TRAP)–staining or TRAP-solution assay as described previously.20 To study the osteoclastogenic ability of PTH-rP, sorted 1 × 103 CD34+c-Kit+ cells were cultured in α-minimum essential medium containing 10% fetal calf serum with human M-CSF and 10 μg/mL human PTH-rP 7-34 Amide (Peninsula Lab, San Carlos, CA).
To investigate whether direct interaction between OCL precursor cells and ATL cells is required for OCL formation, a culture insert (0.4 μm; Iwaki Glass, Chiba, Japan) was used. The insert was placed in each well of 24-well culture plates (PRIMARIA, Becton Dickinson), and ATL cells and OCL precursor cells were cultured separated from each other by a membrane filter in the presence of M-CSF and IL-2. In this system, 5 × 103 CD34+c-Kit+cells were cultured in the bottom with or without 1 × 104 ATL cells on the culture insert. TRAP activity was measured after 7 days of cultivation.
Statistical analysis
Differences of cytokines between hypercalcemic and normocalcemic patients were examined for statistical significance using the nonparametric Mann-Whitney U test. A P value < .05 denoted the presence of statistically significant difference.
Results
Expression of RANKL messenger RNA in ATL cells
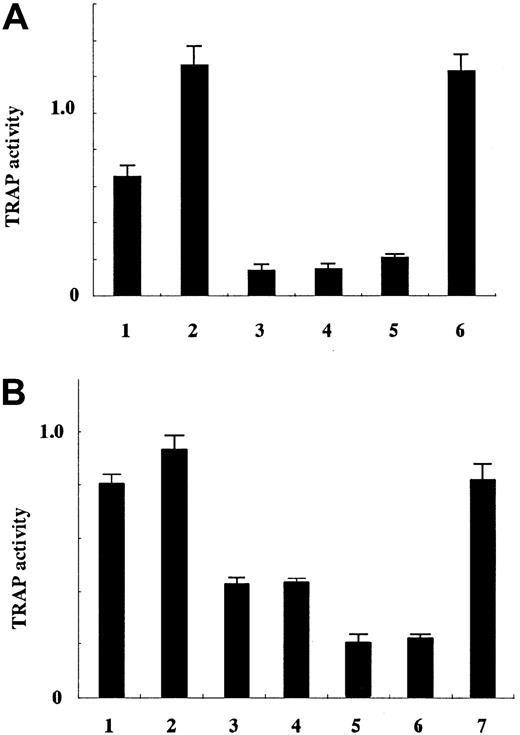
An increased number of OCLs in bones, causing bone resorption, is the causative factor of hypercalcemia in ATL. Differentiation of HPCs to OCLs is controlled by several cytokines. Among them, RANKL and M-CSF have been shown to play a major role in the development of OCLs.21 To identify the molecules involved in ATL-associated hypercalcemia, leukemic cells from ATL patients with (8 cases) or without hypercalcemia (7 cases) were studied for the transcriptions and serum levels of various cytokines. The expressions of RANKL, M-CSF, TNF-α, and OPG genes was analyzed using semiquantitative PCR (Figure 1), and serum levels of M-CSF, TNF-α, and PTH-rP were measured (Table 2). As shown in Figure 1A, the expression of M-CSF gene transcripts varied among patients with hypercalcemia and those without hypercalcemia (P = .42, by Mann-Whitney). However, serum levels of M-CSF were elevated in both groups (Table 2) as reported previously.26 The level of M-CSF in the sera of HTLV-I carriers (341.5 ± 24.9) was not different from that of uninfected individuals (256.8 ± 51.2) (P = .12, by Mann-Whitney), suggesting that ATL cells not present in the peripheral blood were the source of increased serum M-CSF levels. Thus, serum concentrations of M-CSF were elevated in patients without hypercalcemia, suggesting that M-CSF alone is not likely to be responsible for hypercalcemia.
Semiquantitative analysis of transcription of various genes associated with hypercalcemia.
Transcripts of various cytokine genes [(A) M-CSF, (B) PTH-rP, (C) RANKL, (D) TNF-α, and (E) OPG] were quantified and compared with the expression of glyceraldehydes-3-phosphate dehydrogenase (GAPDH) as described. U indicates unstimulated PBMCs; s, phytohemagglutinin-stimulated PBMCs; and 1-15 represent the number of patients shown in Tables 1 and 2.
Semiquantitative analysis of transcription of various genes associated with hypercalcemia.
Transcripts of various cytokine genes [(A) M-CSF, (B) PTH-rP, (C) RANKL, (D) TNF-α, and (E) OPG] were quantified and compared with the expression of glyceraldehydes-3-phosphate dehydrogenase (GAPDH) as described. U indicates unstimulated PBMCs; s, phytohemagglutinin-stimulated PBMCs; and 1-15 represent the number of patients shown in Tables 1 and 2.
The expression of RANKL gene was higher in ATL cells of patients with hypercalcemia than of those without hypercalcemia and phytohemagglutinin-stimulated PBMCs (Figure 1C; cases 1-7) (P = .012, by Mann-Whitney). However, PTH-rP was elevated in sera of both groups, and its transcription levels were comparable without regard to the presence of hypercalcemia (Figure 1B, Table 2) (P = .20, by Mann-Whitney). For example, PTH-rP was elevated in the serum (253 and 199 pmol/L) and the PTH-rP gene was highly transcribed in leukemic cells of cases 10 and 12, although both patients had normal levels of serum Ca++. In case 8 (with hypercalcemia), serum PTH-rP was elevated (1160 pmol/L), but RANKL expression was not elevated, indicating that markedly increased PTH-rP could cause hypercalcemia. It is possible that elevated PTH-rP induced the expression of RANKL on osteoblasts, resulting in increased OCLs. However, elevated RANKL gene transcription correlated well with hypercalcemia in most hypercalcemic patients.
TNF-α is known to induce the differentiation of HPCs to OCLs in cooperation with M-CSF as well as RANKL.27 Therefore, we studied the transcription of the TNF-α gene and determined its serum levels (Figure 1D, Table 2). The amount of TNF-α transcripts and serum levels did not correlate with the presence of hypercalcemia (NS, by Mann-Whitney), indicating that TNF-α is not a contributing factor to hypercalcemia in ATL.
OPG is the decoy receptor for RANKL,28 which inhibits the function of RANKL and prevents osteoporosis.29 OPG production may abrogate overproduction of RANKL. In our patients, transcription of the OPG gene was observed in ATL cells of cases 3, 4, and 5 (Figure 1E). However, clinical features of these patients were not different from those who did not express OPG.
ATL cells from hypercalcemic patients can induce differentiation of OCLs
To examine whether ATL cells can induce the differentiation of HPCs to OCLs, PBMCs of ATL patients with or without hypercalcemia were cocultured with CD34+c-Kit+ bone marrow cells along with IL-2 and M-CSF for 7 days. Flow cytometric analysis showed that more than 95% of PBMCs were leukemic cells in these patients. Serum levels of M-CSF were usually elevated in patients with ATL in spite of its transcription in ATL cells (Table 2), suggesting that not only ATL cells but also normal parenchymal cells were responsible for the elevated serum levels of M-CSF. Therefore, we added M-CSF at a concentration of 50 ng/mL, which is compatible to that observed in patients. In addition, fresh ATL cells isolated from the peripheral blood tend to undergo spontaneous apoptosis. Because IL-2 did not cause the proliferation of ATL cells in most cases but inhibited apoptosis of ATL cells, we used this cytokine (50 U/mL) to prevent apoptosis.30 For evaluation of the differentiation and activation of OCLs, TRAP assay was performed. The assay showed positive reaction of TRAP in OCLs but not in macrophages.
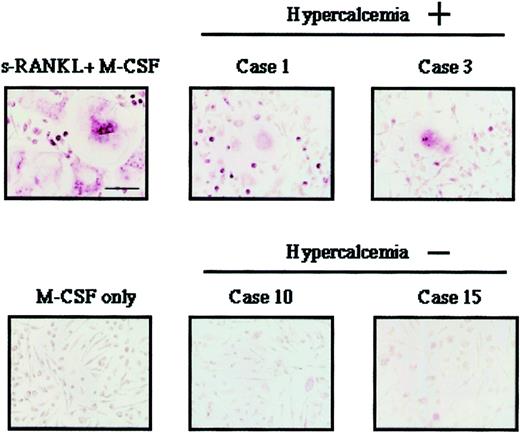
As a positive control, we used human soluble RANKL and human M-CSF, which induced differentiation of HPCs into OCLs in vitro (Figure2). Induction of differentiation of HPCs into OCLs did not occur when these cells were cultured with M-CSF alone or with normal CD3+ T cells and M-CSF (Figure 2A, 4 and 5; 2B, 5 and 6). ATL cells of patients with hypercalcemia (cases 1 and 3), but not those of patients without hypercalcemia (cases 10 and 15), induced the differentiation of HPCs into OCLs (Figure 2A,B). The morphologic features of induced OCLs are shown in Figure3. Large multinucleated cells were observed when HPCs were cocultured with ATL cells from cases 1 and 3 (with hypercalcemia). However, such OCLs could not be found when cocultured with leukemic cells from cases 10 and 15 (without hypercalcemia).
Osteoclastogenesis induced by ATL cells.
HPCs were cocultured with ATL cells of patients with hypercalcemia (cases 1 and 3 in Tables 1 and 2), and those without hypercalcemia (cases 10 and 15). Experiments were performed by using HPCs from different healthy volunteers (A and B). (A) 1 indicates case 1; 2, case 3; 3, Case 15; 4, CD3+ T-lymphocytes from a healthy volunteer; 5, M-CSF only; and 6, sRANKL + M-CSF. (B) 1 indicates case 1; 2, case 3; 3, case 10; 4, case 15; 5, CD3+ lymphocytes from a healthy volunteer; 6, M-CSF only; and 7, sRANKL + M-CSF.
Osteoclastogenesis induced by ATL cells.
HPCs were cocultured with ATL cells of patients with hypercalcemia (cases 1 and 3 in Tables 1 and 2), and those without hypercalcemia (cases 10 and 15). Experiments were performed by using HPCs from different healthy volunteers (A and B). (A) 1 indicates case 1; 2, case 3; 3, Case 15; 4, CD3+ T-lymphocytes from a healthy volunteer; 5, M-CSF only; and 6, sRANKL + M-CSF. (B) 1 indicates case 1; 2, case 3; 3, case 10; 4, case 15; 5, CD3+ lymphocytes from a healthy volunteer; 6, M-CSF only; and 7, sRANKL + M-CSF.
Morphology of OCLs induced by coculture of HPCs with ATL cells.
Giant multinucleated cells were observed when HPCs were cocultured with ATL cells from patients with hypercalcemia (cases 1 and 3). In contrast, such OCLs were not observed when HPCs were cocultured with ATL cells from patients without hypercalcemia (cases 10 and 15).
Morphology of OCLs induced by coculture of HPCs with ATL cells.
Giant multinucleated cells were observed when HPCs were cocultured with ATL cells from patients with hypercalcemia (cases 1 and 3). In contrast, such OCLs were not observed when HPCs were cocultured with ATL cells from patients without hypercalcemia (cases 10 and 15).
Inhibition of ATL-induced differentiation of HPCs to OCLs
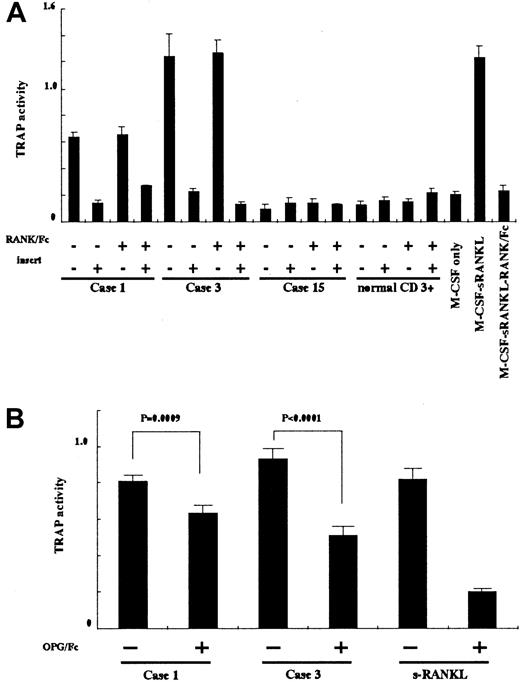
To clarify the mechanism of ATL-induced differentiation of HPCs to OCLs, we used several inhibitors against RANKL and TNF-α in coculture experiments. To block the action of RANKL, we used RANK/Fc and OPG/Fc. Although both molecules can bind to RANKL, the inhibitory activity of OPG/Fc is much stronger than that of RANK/Fc. To inhibit the action of TNF-α, we used soluble TNF R1. These inhibitors were added to the media during coculture of ATL cells and HPCs. As shown in Figure4A, RANK/Fc failed to suppress ATL-induced differentiation of HPCs to OCLs, and sTNF R1 had no effects on TRAP assay (data not shown). However, OPG/Fc successfully suppressed TRAP activities in cases 1 and 3 compared with controls, indicating that ATL-induced differentiation of HPCs to OCLs was mediated by interaction between RANKL and RANK (Figure 4B).
Inhibition of osteoclastogenesis.
(A) HPCs were cocultured with ATL cells and soluble RANKL with or without RANK/Fc in culture dishes with inserts to prevent direct interaction in the presence of M-CSF and IL-2. Suppression of TRAP activity indicated differentiation of cells into OCLs. (B) HPCs were cocultured with ATL cells and soluble RANKL with or without OPG/Fc. Suppression of TRAP activity indicated differentiation of cells to OCLs. Suppression caused by OPG/Fc was statistically significant. Statistical analysis was performed by using the Studentt test.
Inhibition of osteoclastogenesis.
(A) HPCs were cocultured with ATL cells and soluble RANKL with or without RANK/Fc in culture dishes with inserts to prevent direct interaction in the presence of M-CSF and IL-2. Suppression of TRAP activity indicated differentiation of cells into OCLs. (B) HPCs were cocultured with ATL cells and soluble RANKL with or without OPG/Fc. Suppression of TRAP activity indicated differentiation of cells to OCLs. Suppression caused by OPG/Fc was statistically significant. Statistical analysis was performed by using the Studentt test.
Because the soluble forms of RANKL might play a role in the differentiation of OCLs, we examined this possibility by culturing HPCs with ATL cells from patients with hypercalcemia (cases 1 and 3), which were separated by a filter membrane to prevent direct contact (Figure4A). Inhibition of direct interaction between ATL cells and HPCs resulted in no differentiation into OCLs, suggesting the involvement of cell-bound RANKL and its direct interaction with RANK on HPCs in hypercalcemia.
Discussion
Mechanism of hypercalcemia in patients with ATL
The frequency of hypercalcemia in patients with ATL is markedly high compared with other hematologic malignancies, such as malignant lymphoma (< 10%), acute leukemia (< 1%), and multiple myeloma (20%-40%).11 Its frequency in patients with ATL is reported at about 70% during the whole clinical course, although it tends to be more frequent in those patients with clinically aggressive ATL,10 suggesting that molecules expressed or secreted by ATL cells play an important role in the induction of hypercalcemia. Previous studies have shown an increased number of activated OCLs in the bone of hypercalcemic ATL patients, which resulted in generalized decalcification and hypercalcemia.10 Among several cytokines that are associated with hypercalcemia, PTH-rP has emerged as an important factor in the pathogenesis of high serum Ca++.16 In the present study, case 8, which had high levels of PTH-rP but no increased expression of RANKL, was hypercalcemic, indicating that increased PTH-rP could cause hypercalcemia. Because PTH-rP did not induce the differentiation of HPCs to OCLs in vitro (data not shown), it is possible that increased PTH-rP induced the expression of RANKL on osteoblasts as reported previously31 and indirectly stimulated the differentiation of HPCs into OCLs. However, PTH-rP was also elevated in patients without hypercalcemia, suggesting that elevated PTH-rP alone does not always explain the mechanism of hypercalcemia in most patients with ATL, as reported previously.16 19 However, most patients with hypercalcemia showed increased transcription of RANKL gene, and in vitro coculture experiments demonstrated ATL cell-induced differentiation of HPCs into OCLs. Taken together, increased RANKL expression is thought to be the most important factor in the pathogenesis of ATL-associated hypercalcemia.
In this study, coculture of ATL cells with HPCs for 7 days in the presence of IL-2, which might induce the expression of Tax, altered the gene expression. We have previously reported a defective virus that lacked 5′-LTR and internal viral sequences and did not produce Tax because of deletion of the promoter.32 ATL cells in case 1 had this type of HTLV-I provirus, which did not express Tax even after stimulation with phorbol ester (data not shown). This finding proved that Tax expression is not associated with the expression of RANKL gene. Furthermore, we studied the expression of RANKL gene in various HTLV-I–infected cell lines and found that Tax expression did not always correlate with transcription of RANKL gene. In addition, induced expression of Tax in Jurkat cells did not increase the expression of RANKL gene, indicating that Tax expression is not associated with transcription of RANKL gene (our unpublished observation, February 2000).
Factors influencing hypercalcemia in ATL patients
Our study showed that RANKL on ATL cells plays a critical role in the differentiation of OCLs in patients with ATL, and blocking experiments of direct interaction between ATL cells and HPCs revealed that not a soluble form but a membrane-bound form of RANKL is essential for the differentiation of HPCs to OCLs. In this regard, it is noteworthy that infiltration of ATL cells into the bone marrow was observed in all patients with hypercalcemia in whom bone marrow samples were aspirated (Table 2). Because invasion of ATL cells into the bone marrow is frequently observed during the aggressive stage, it is speculated that certain adhesion molecules expressed on ATL cells enhance such an infiltration process. When such infiltrating ATL cells expressed RANKL on the surface, they induced the differentiation of HPCs to OCLs, resulting in hypercalcemia. Infiltration into the bone marrow by ATL cells without expression RANKL gene did not cause hypercalcemia as observed in case 10 (Table 2). Thus, elevated serum M-CSF levels, expression of RANKL, and ATL infiltration into the bone marrow are critical factors in the pathogenesis of hypercalcemia. PTH-rP secreted from ATL cells might be an additional factor that induces the expression of RANKL on stromal-activated OCLs and exacerbates hypercalcemia.
Pathologic role of RANKL
Activated T cells regulate bone loss by increased expression of RANKL in inflammatory arthritis.24 Furthermore, in Paget disease,33 increased local expression of RANKL on stromal cells is responsible for accumulation of OCLs in local tissues and subsequent bone resorption, but the expression of RANKL was not sufficient to cause hypercalcemia. However, patients with ATL overexpressed RANKL, which resulted in accumulation of OCLs in the bones in cooperation with M-CSF. It can be explained by several reasons; because ATL is a neoplastic proliferation, RANKL-expressing cells are extremely more abundant than the inflammatory disease.
Because ATL cells are derived from activated helper T lymphocytes, secreted cytokines can modify the clinical features. For example, ATL cell–produced IL-5 caused eosinophilia, and granulocyte-monocyte colony-stimulating factor increased the number of neutrophils.34 Monocytosis is one of the clinical features of ATL, in which increased M-CSF should be implicated in the pathogenesis.35 M-CSF increased precursor cells, which supports the differentiation into monocytes and OCLs. Without RANKL, these precursor cells differentiate into monocytes, but once the expression of RANKL is induced in ATL cells, and such leukemic cells infiltrate the bone marrow, these precursor cells differentiate into OCLs. Activated T lymphocytes are known to induce differentiation of cells into OCLs in vitro possibly through RANKL expression.36 In this study, we showed that ATL cells of patients with normal serum Ca++ levels, which exhibit activated phenotypes such as expression of activated antigens like CD25, did not induce differentiation of cells to OCLs. These results clearly show that not the activated phenotype but the expression of RANKL is critical for elevation of serum Ca++levels.
Our study emphasized the significance of RANKL in ATL-associated hypercalcemia. This finding may allow the development of new strategies for the treatment of hypercalcemia and this aggressive disease.
We thank Jun-ichiro Yasunaga and Mika Yoshida for valuable suggestions. We also thank Dr F. G. Issa (word-medex.com.au) for the careful reading and editing of the manuscript.
Supported by a grant-in-aid for Scientific Research from the Ministry of Education, Science, Sports, Culture and Technology in Japan and by grants from Welfide Medicinal Research Foundation and Haraguchi Memorial Cancer Research Fund.
K.N. and T.M. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Masao Matsuoka, Laboratory of Virus Immunology, Institute of Virus Research, Kyoto University, Kyoto 606-8507, Japan; e-mail: mmatsuok@virus.kyoto-u.ac.jp.

![Fig. 1. Semiquantitative analysis of transcription of various genes associated with hypercalcemia. / Transcripts of various cytokine genes [(A) M-CSF, (B) PTH-rP, (C) RANKL, (D) TNF-α, and (E) OPG] were quantified and compared with the expression of glyceraldehydes-3-phosphate dehydrogenase (GAPDH) as described. U indicates unstimulated PBMCs; s, phytohemagglutinin-stimulated PBMCs; and 1-15 represent the number of patients shown in Tables 1 and 2.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/2/10.1182_blood.v99.2.634/6/m_h80222006001.jpeg?Expires=1765918630&Signature=ZC9lRWy-ndKblRHetkN-dEej-AJaTF1jeRS0uB4hyRFjlhdmX6darjFvRyGWzso4Tq4dFeNbiaSRmb5VqZy42naNfrpuW38ANy2QGzwG3vUW6lePaVdN5VT5JCzM21-U0GncISUAQdyvNMvZK83QxJDapxe4o~ZLzIkriU9oV3CFwStz9VJU6k5ip5x3xLDvwP5VSL16v6xqGpq4RWM22A6wN0eumhV2HapvEGZfXbgDDDuCJYfuUmWAaYplUUhMNw5VbnbuB-~dlzOC4j4r4JsOwCVUB6jv4HdKHJuTnLszQvRNea4F5kGHkaWmP39hahH3MQq5IH-K-zUblBj6Tw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)