P-selectin glycoprotein ligand–1 (PSGL-1) serves as the leukocyte ligand for P-selectin, and many of the structural features of its ectodomain required for interactions with P-selectin have been uncovered. In contrast, the function of the highly conserved PSGL-1 cytoplasmic domain has not been explored. Stable transfectants expressing similar levels of either wild-type PSGL-1 or truncated PSGL-1 in which only 4 cytoplasmic residues were retained (designated PSGL-1Δcyto), were analyzed. Transfectants expressing full-length PSGL-1 rolled well on P-selectin. In contrast, rolling was almost completely absent in cells transfected with PSGL-1Δcyto, even at low shear. Importantly, cells expressing truncated PSGL-1 were able to bind soluble P-selectin and to bind COS cells overexpressing P-selectin, demonstrating that the P-selectin binding site on the PSGL-1Δcyto transfectants was intact and was capable of recognizing P-selectin. Impaired rolling by PSGL-1Δcyto transfectants was not due to alterations in subcellular localization because both wild-type and truncated PSGL-1 had similar surface distributions on K562 transfectants. Treatment of cells expressing native PSGL-1 with actin cytoskeletal toxins inhibited adhesion in a dose-dependent way. PSGL-1 was associated with the actin cytoskeleton, and this interaction was greatly impaired in PSGL-1Δcyto– expressing cells. The PSGL-1 cytoplasmic domain interacted selectively with the ezrin/radixin/moesin (ERM) protein moesin, but not with other ERM proteins or several other cytoskeletal linker proteins. Pharmacologic disruption of interactions between moesin and F-actin in cells expressing PSGL-1 resulted in a dose-dependent inhibition of rolling on P-selectin. Thus, attachment of PSGL-1 to the leukocyte cortical cytoskeleton is essential for leukocyte rolling on P-selectin.
Introduction
Leukocyte migration is mediated by several groups of adhesion molecules, including the selectin family. Expression of L-selectin on leukocytes is constitutive, whereas expression of E-selectin on endothelium, and P-selectin on endothelium and platelets, requires specific activators for surface expression.1 The selectins recognize a heterogeneous array of glycoprotein ligands including the homodimeric mucin P-selectin glycoprotein ligand–1 (PSGL-1).2 PSGL-1 interacts with both P-selectin and L-selectin, and thus mediates leukocyte adhesion to endothelium, platelets, and other leukocytes.3,4 Recognition of selectins requires that specific ectodomain residues of PSGL-1 be enzymatically modified,5,6 and dimerization through a single extracellular cysteine residue is also required for leukocyte rolling on P-selectin.7 To date, most research has concentrated on essential modifications of the extracellular portions of PSGL-1, but the role of the highly conserved PSGL-1 cytoplasmic tail in leukocyte rolling has not been investigated.
The cytoplasmic domains of many adhesion molecules are known to play an essential role in adhesive events by serving as sites for structural and functional linkages between cell surface molecules and cytoskeletal components. These interactions are involved in cell-cell and cell-matrix adhesion, as well as receptor-ligand interactions, receptor internalization, redistribution, shedding, endocytic sorting, and signal transduction. Among leukocyte adhesion molecules, leukocyte function-associated antigen–1 (LFA-1), very late activation–4 (VLA-4), intercellular adhesion molecule–1 (ICAM-1), L-selectin, and E-selectin have been shown to interact with the cytoskeleton, and these interactions are mediated through a series of cytoplasmic linker proteins, including α-actinin, paxillin, vinculin, and talin.8-12 In contrast, linkage between CD44, CD43, ICAM-1, or ICAM-2 and the cytoskeleton is mediated through the ezrin/radixin/moesin (ERM) family of proteins.13-15 The ERM proteins are part of the band 4.1 superfamily of proteins and are closely related structurally (∼75% identity at the amino acid level, rising to ∼85% in their amino terminal half). Although they are often coexpressed, these proteins differ in tissue and cellular distribution and functional properties.16,17 ERM proteins have both plasma membrane– and actin filament–binding domains that allow them to function as linker proteins between the membrane and the actin cytoskeleton.18 Although these associations have been biochemically documented, their functional consequences are not well understood.
In this study, we investigated the role of the highly conserved cytoplasmic tail of PSGL-1. The results indicate that interaction of the PSGL-1 cytoplasmic domain with the actin cytoskeleton is essential for rolling on P-selectin, and thereby suggest a novel paradigm for adhesion receptors that mediate leukocyte rolling under flow.
Materials and methods
Generation of PSGL-1 complementary DNA truncated in the cytoplasmic domain
Introduction of a stop codon at position 1053 (residue 348) of the human PSGL-1 complementary DNA (cDNA) by polymerase chain reaction (PCR)–based site directed mutagenesis truncated the 69–amino acid cytoplasmic domain to 4 residues, RLSR. Amplification used an antisense oligonucleotide 5′ TT GGTACC GGG GTA CAT GTG TCA CTT GCG GGA GAG 3′, which inserted a stop codon at position 348 and maintained a unique KpnI site (underlined) just downstream from the newly generated stop codon, and a sense oligonucleotide 5′ AAT TTG TCC GTCAAC TAC CCA GTG 3′, which incorporated a unique HincII site (underlined) just upstream from the cytoplasmic tail. The 156-bp PCR-generated fragment was gel purified, ligated into theHincII/KpnI site of the pBS.KS/PSGL-1 vector19 and sequenced to confirm the fidelity of the mutagenesis. The mutant cDNA was subcloned into a modified SP65/RSV vector containing a puromycin-resistant cassette and used to transfect K562/FucT-VII and BJAB-FucT-VII cells.
Generation and verification of stable transfectants in K562/FucT-VII cells
The generation of both K562 and BJAB cells stably expressing FucT-VII has been described.19,20 Wild-type and PSGL-1Δcyto cDNAs were used to stably transfect K562/FucT-VII and BJAB/FucT-VII by electroporation. Bulk transfectants were drug selected in media containing 2.5 μg/mL puromycin, screened by flow cytometry with the anti–PSGL-1 monoclonal antibody (mAb) KPL1,21and cloned by limiting dilution. Individual clones were screened by flow cytometry with KPL1, and with HECA-452, which recognizes a reporter epitope associated with FucT-VII enzymatic activity.20,22 FACS analysis was performed on a Becton Dickinson FACSCalibur, and data were analyzed with CellQuest software (Mountain View, CA). Clones that had equivalent surface staining of both these mAbs were tested by semiquantitative reverse transcription-PCR (RT-PCR) analysis7 20 for core-2 β1,6 N-acetyglucosaminyltransferase-I (C2GlcNAcT-I) and α1,3-fucosyltransferase-VII (FucT-VII) messenger RNA (mRNA) expression to ensure that the selected clones had equivalent levels of FucT-VII and C2GlcNAcT-I mRNA. Western blotting of 1% Triton X-100 whole-cell lysates (WCLs, see below) were generated from each transfectant, run on 6% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gels, transferred to nitrocellulose, probed with KPL1 followed by goat antimouse–horseradish peroxidase (Biosource International, Camarillon, CA), and developed as described below. Data in this report are representative of multiple experiments with numerous clones from multiple independent transfections.
Soluble P-selectin binding
HL60 cells or transfectants were incubated with purified mouse P-selectin–human IgG fusion proteins (referred to as P-RIg; BD Pharmingen, San Diego, CA) for 20 minutes at 4°C, washed, and incubated with goat antihuman IgG fluorescein isothiocyanate (FITC; Biosource International) for an additional 20 minutes. Samples were analyzed on a Becton Dickinson FACSCalibur as described above.
Low-shear COS cell adhesion assay
The COS cells were transfected with P-selectin by the diethylaminoethyl-dextran method in 100-mm tissue culture–grade Petri dishes, replated on 35-mm dishes (assay plates), and allowed to readhere overnight. The next day, each assay plate was washed 3 times and either 2 × 106 HL60 cells or transfectants (in a final volume of 0.6 mL) were gently added. Assay plates with added cells were incubated on a constantly rocking platform for 15 minutes at 4°C, plates were washed 5 times followed by fixation with cold 0.37% formaldehyde. Mean number of cells bound per COS cell was determined by counting approximately 100 COS cells in multiple 40 × fields using a standard inverted light microscope.
Parallel-plate flow assay
Rolling of HL60 cells and transfectants on monolayers of stably transfected Chinese hamster ovary (CHO) cells expressing human P-selectin (referred to as CHO/P) was analyzed.22 CHO/P cells were grown to confluence in 35-mm culture dishes and positioned on a 0.0254-cm–thick gasket of a flow chamber (Glycotech, Rockville, MD), with the CHO/P monolayer serving as the bottom floor of the flow chamber. A constant flow level was maintained by drawing media containing cells at 0.5 × 106 cells/mL through the chamber using a PHD 2000 programmable syringe pump (Harvard Apparatus, Holliston, MA). The flow chamber was mounted on an inverted Eclipse TE300 microscope (Nikon, Melville, NY) with Hoffman interference contrast objectives (Modulation Optics, Greenvale, NY) and rolling data were collected with a video camera. Data were analyzed with Celltrak software (Compix, Cranberry Township, PA). Sequential images of tracked cells were digitized and matched on the basis of trajectories and morphology every 2 seconds. Interactive “events,” which correspond to rolling cells, were collected for 50 sequential paired images. In each experiment, each cell line or transfectant was analyzed twice. HL60 cells were included as a control in all experiments because they roll very well on P-selectin. In some experiments, cells were preincubated for 20 minutes with various concentrations of purified KPL1, washed, resuspended, and analyzed for rolling on CHO/P monolayers. In other experiments cells were pretreated for 30 to 60 minutes at 37°C with indicated doses of either latrunculin B (Calbiochem, La Jolla, CA), cytochalasin B (Sigma, St. Louis, MO), or staurosporine (Sigma), washed, resuspended, and analyzed for rolling on P-selectin.
FACS analysis for cytoskeletal linkage of transmembrane receptors
Minor modifications were made to previously published protocols.23-25 Cells were added to FACS tubes precoated with 1% bovine serum albumin (BSA) in phosphate-buffered saline (PBS) and incubated for 15 minutes with either purified KPL1 or an isotype-matched control mAb. Cells were washed and incubated with goat antimouse IgG FITC (Biosource) for an additional 15 minutes, pelleted, and the pellet was gently resuspended in buffer consisting of 0.5% Triton X-100, 13 mM Tris-HCl (pH 8.0), 50 mM NaCl, 2 mM MgCl2, 0.2 mM EGTA, 2% fetal bovine serum (FBS), and 1 mM each phenylmethylsulfonyl fluoride (PMSF), leupeptin, pepstatin A, and aprotinin, incubated at room temperature for 20 minutes, and washed with 3 mL of the above buffer without Triton X-100. Pellets (containing the insoluble fraction including the actin cytoskeleton and proteins linked to it) were gently resuspended in PBS/1% formaldehyde and analyzed by flow cytometry. Cells not treated with detergent were included as a control for the setting of data collection gates.
Affinity capture assay and Western blotting
The GST fusion proteins containing all 69 cytoplasmic tail residues of PSGL-1 (GST/PSGL-1) or GST alone (GST only) were generated using pGEX-2T by standard methods, coupled to glutathione-Sepharose, and used as an affinity matrix to capture cytoplasmic proteins from HL60 WCLs. HL60 cells were washed twice in PBS and resuspended at 1 × 107 cells/mL in lysis buffer (1% Triton X-100, 150 mM NaCl, 10 mM Tris-HCl (pH 8.0), and 1 mM each CaCl2, MgC12, aprotinin, leupeptin, pepstatin A, and PMSF). Glutathione-Sepharose beads with the indicated fusion proteins were incubated with WCLs for 2 hours at 4°C, washed with lysis buffer containing 0.1% Triton X-100, eluted with SDS-PAGE sample buffer, run on a 10% polyacrylamide gel, and transferred to nitrocellulose. Individual blots were probed with a mAb or pAb to several cytoskeletal proteins, including α-actinin (Sigma), vinculin (Sigma), talin (Sigma), moesin (mAb from Transduction Laboratories, San Diego, CA and pAb provided by Dr A. Bretscher, Cornell University, Ithaca, NY) and ezrin (pAb to ezrin provided by Dr A. Bretscher, Cornell University). Blocking, incubations, washes, development, and visualization by enhanced chemiluminescence (ECL) was carried out as previously described.7 19
Immunoprecipitation
The PSGL-1 and PSGL-1Δcyto transfectants were resuspended at 1 × 107 cells/mL in lysis buffer (0.5% Triton X-100, 50 mM Hepes, 500 mM NaCl, and 1 mM each aprotinin, leupeptin, pepstatin A, and PMSF) and incubated on ice for 45 minutes. Extracts were clarified by centrifugation at 13 000g for 20 minutes at 4°C, and precleared with 50 μL of a 50% solution (wt/vol) of Affigel coupled to an irrelevant isotype-matched control mAb for 60 minutes with rotation at 4°C. Precleared lysates were incubated with KPL1-coupled Affigel as described above. Immunoprecipitates were washed twice in lysis buffer, boiled in SDS-PAGE sample buffer, run on a 7.5% polyacrylamide gel, and transferred to nitrocellulose. Blots were probed with either a mAb to moesin, vinculin, talin, ezrin, α-actinin or PSGL-1, or HECA-452 culture supernatants.
Scanning immunoelectron microscopy
The 106 transfectants expressing either PSGL-1 or PSGL-1Δcyto were resuspended in 100 μL PBS plus 5% goat serum containing KPL1, incubated on ice for 15 minutes, rinsed twice, and resuspended in 100 μL PBS plus 5% goat serum containing goat antimouse IgG conjugated to 12 nm gold (Jackson Immunoresearch Laboratory, West Grove, PA). Following a 15-minute incubation on ice, cells were rinsed twice in PBS plus 0.2% BSA (BSA Fraction V, Sigma), and resuspended in 100 μL PBS plus 0.2% BSA. Approximately 50 μL of the cell suspension was applied to degreased glass chips precoated with 0.1% poly-L-lysine (Sigma), the cells were allowed to settle on the chips for 10 minutes at room temperature, and the entire chip was transferred to a Petri dish containing 3% electron microscopic grade glutaraldehyde (Electron Microscopy Services, Fort Washington, PA) in 0.1 M sodium cacodylate (Sigma) containing 7.5% sucrose for overnight fixation. Samples were assigned a code number and analyzed for either PSGL-1 or PSGL-1Δcyto distribution using a Hitachi S-900 LVSEM equipped with a stereoviewer for enhanced spatial resolution.
Statistics
For statistical analysis of leukocyte rolling assays, the Student t test was used. Differences were considered statistically significant with P < .05.
Results
Generation and characterization of stably transfected K562/FucT-VII cells expressing PSGL-1 or PSGL-1Δcyto
Stable transfectants expressing either wild-type PSGL-1 or PSGL-1Δcyto were generated in K562/FucT-VII cells, a cell line that we have previously shown does not express PSGL-1, but does express all the glycosyltransferases required for appropriate enzymatic modification of PSGL-1.7,19,20 Wild-type PSGL-1 and PSGL-1Δcyto were expressed at similar levels on the surface of the transfected cells, although wild-type PSGL-1 was expressed approximately 2-fold higher compared to PSGL-1Δcyto expression (Figure 1A, left panels). Both transfectants stained equivalently with HECA-452 (Figure 1A, right panels). HL60 cells were included as a positive control because they endogenously express both PSGL-1 and HECA-452 and roll extremely well on P-selectin even though they express approximately 10-fold lower levels of PSGL-1 compared to both K562 transfectants. Western blotting of WCLs generated from either native PSGL-1 or PSGL-1Δcyto transfectants showed the expected increase in mobility consistent with the loss of 65 amino acids (Figure 1B). Individual clones were also analyzed by semiquantitative RT-PCR using PCR cycles titered to be below plateau phase,7 20 and showed equivalent levels of FucT-VII and C2GlcNAcT-I mRNA (data not shown).
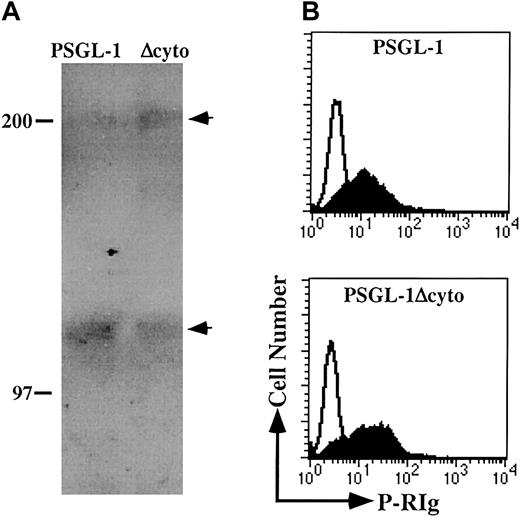
Flow cytometric analysis and Western blotting of PSGL-1 and PSGL-1Δcyto transfectants.
(A) HL60 cells (top panels) or K562/FucT-VII cells transfected with either PSGL-1 (middle panels) or PSGL-1Δcyto (bottom panels) were stained with either an isotype-matched negative control (open histograms), the anti-PSGL-1 mAb, KPL1 (left panels, filled histograms), or HECA-452, an antibody that recognizes a reporter epitope associated with FucT-VII activity (right panels, filled histograms). (B) Western blotting of WCLs made from K562/FucT-VII transfectants expressing either PSGL-1 (lane 1) or PSGL-1Δcyto (lane 2). Bands of the appropriate molecular weight were seen in lysates from PSGL-1 (lane 1), and a slight increase in electrophoretic mobility was associated with truncation of 65 amino acids (lane 2).
Flow cytometric analysis and Western blotting of PSGL-1 and PSGL-1Δcyto transfectants.
(A) HL60 cells (top panels) or K562/FucT-VII cells transfected with either PSGL-1 (middle panels) or PSGL-1Δcyto (bottom panels) were stained with either an isotype-matched negative control (open histograms), the anti-PSGL-1 mAb, KPL1 (left panels, filled histograms), or HECA-452, an antibody that recognizes a reporter epitope associated with FucT-VII activity (right panels, filled histograms). (B) Western blotting of WCLs made from K562/FucT-VII transfectants expressing either PSGL-1 (lane 1) or PSGL-1Δcyto (lane 2). Bands of the appropriate molecular weight were seen in lysates from PSGL-1 (lane 1), and a slight increase in electrophoretic mobility was associated with truncation of 65 amino acids (lane 2).
In addition to the decrease in the apparent molecular mass of PSGL-1Δcyto, Western blotting also showed a less heterogeneous banding pattern for both the monomeric and dimeric forms of PSGL-1Δcyto compared to wild-type (Figure 1B), suggesting possible differences in glycosylation. To further investigate the glycosylation of these molecules, WCLs generated from K562/FucT-VII transfectants expressing either PSGL-1 or PSGL-1Δcyto were immunoprecipitated with KPL1 followed by Western blotting with HECA-452 (Figure2A). Both PSGL-1 and PSGL-1Δcyto were recognized by HECA-452, suggesting that the glycosylation of PSGL-1Δcyto by FucT-VII is not altered by truncation of the cytoplasmic tail.
PSGL-1 and PSGL-1Δcyto transfectants are appropriately posttranslationally modified and can recognize soluble P-selectin.
(A) WCLs were generated from PSGL-1 (lane 1) or PSGL-1Δcyto (lane 2) transfectants and immunoprecipitated with the anti-PSGL-1 mAb KPL1 coupled to Affigel followed by Western blotting with HECA-452. Arrows on right indicate dimeric (top arrow) and monomeric (lower arrow) forms of PSGL-1. Both full-length and truncated PSGL-1 were recognized by HECA-452. (B) PSGL-1 and PSGL-1Δcyto transfectants were incubated with P-RIg (filled histograms) or second stage only (open histograms) and analyzed by flow cytometry as described in “Materials and methods.” Both transfectants were capable of binding soluble P-selectin.
PSGL-1 and PSGL-1Δcyto transfectants are appropriately posttranslationally modified and can recognize soluble P-selectin.
(A) WCLs were generated from PSGL-1 (lane 1) or PSGL-1Δcyto (lane 2) transfectants and immunoprecipitated with the anti-PSGL-1 mAb KPL1 coupled to Affigel followed by Western blotting with HECA-452. Arrows on right indicate dimeric (top arrow) and monomeric (lower arrow) forms of PSGL-1. Both full-length and truncated PSGL-1 were recognized by HECA-452. (B) PSGL-1 and PSGL-1Δcyto transfectants were incubated with P-RIg (filled histograms) or second stage only (open histograms) and analyzed by flow cytometry as described in “Materials and methods.” Both transfectants were capable of binding soluble P-selectin.
To confirm that the functionally relevant modifications of the PSGL-1 ectodomain were not affected by truncation of the cytoplasmic domain, the ability of recombinant soluble P-RIg fusion proteins to recognize PSGL-1Δcyto transfectants was examined. K562 transfectants were incubated with P-RIg and analyzed by flow cytometry (Figure 2B). Under these conditions, both wild-type and truncated PSGL-1 bound P-RIg, with PSGL-1Δcyto transfectants actually exhibiting slightly higher levels of P-RIg binding. These data show that, as expected, the P-selectin binding site is intact on PSGL-1Δcyto.
Rolling of PSGL-1 or PSGL-1Δcyto transfectants on P-selectin
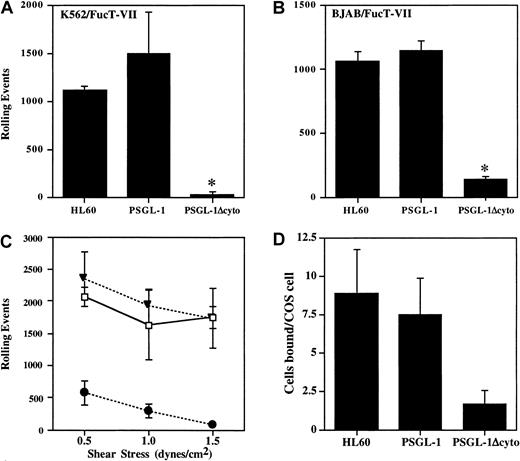
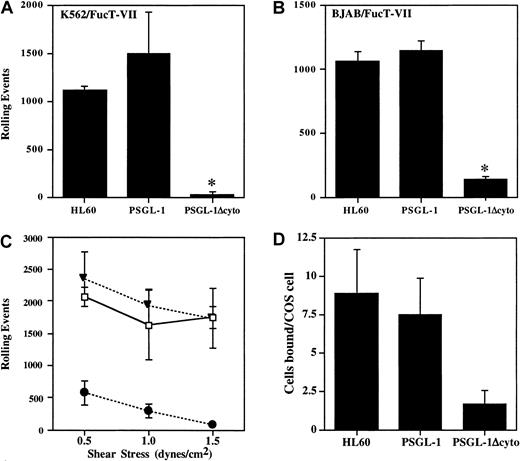
Cells were analyzed in a parallel-plate flow system for rolling on P-selectin. At a shear stress of 1.9 dynes/cm2, cells expressing PSGL-1 rolled extremely well on CHO/P monolayers, comparable with HL60 cells, with more than 1000 rolling events (Figure3A). In contrast, PSGL-1Δcyto cells rolled at greatly reduced levels in all experiments (7.3% ± 6.9% of control PSGL-1 transfectants, n = 7, P < .05). To confirm that this dramatic loss of rolling was not cell type specific, the PSGL-1 or PSGL-1Δcyto cDNAs were stably transfected into the BJAB/FucT-VII cell line, another PSGL-1− cell line containing all of the enzymes required for proper modification of PSGL-1. Transfectants were generated, cloned, and characterized as described above (data not shown). Rolling behavior of the BJAB cell lines on CHO/P was quite similar to the K562 transfectants for cells transfected with PSGL-1Δcyto compared to BJAB cells transfected with wild-type PSGL-1 (11.7% ± 10% of control PSGL-1 transfectants, n = 7, P < .05; Figure 3B). Thus, truncation of the PSGL-1 cytoplasmic tail blocks nearly all rolling on P-selectin.
Rolling and adhesion of PSGL-1Δcyto transfectants on P-selectin is significantly reduced.
Total rolling events in 50 sequential paired images were acquired, recorded, and analyzed as described in “Materials and methods” for K562/FucT-VII cells (A, 1 of 7 experiments) or BJAB/FucT-VII cells (B, 1 of 7 experiments) expressing either full-length or truncated PSGL-1. High numbers of rolling events were recorded for HL60 cells and PSGL-1 transfectants, but rolling was greatly reduced in both PSGL-1Δcyto transfectants. *P < .05 versus PSGL-1 transfectants. (C) Total rolling events were collected as described for panels A and B, but rolling was analyzed at 3 different shear stresses: 1.5, 1.0, and 0.5 dynes/cm2. Total rolling events in 50 fields (1 of 3 experiments) were recorded for HL60 cells (open boxes), K562/FucT-VII/PSGL-1 transfectants (closed triangles), and K562/FucT-VII/PSGL-1Δcyto transfectants (closed circles). High numbers of rolling events were recorded for HL60 cells and PSGL-1 transfectants. In contrast, rolling events were greatly reduced for PSGL-1Δcyto transfectants at the tested shear rates. (D) HL60 (8.8 ± 2.9 cells/COS cell) and PSGL-1 transfectants (7.4 ± 2.32 cells/COS cell) bound at similar levels to COS cells transfected with P-selectin, whereas PSGL-1Δcyto transfectants bound at lower levels (1.7 ± 0.9 cells/COS cell).
Rolling and adhesion of PSGL-1Δcyto transfectants on P-selectin is significantly reduced.
Total rolling events in 50 sequential paired images were acquired, recorded, and analyzed as described in “Materials and methods” for K562/FucT-VII cells (A, 1 of 7 experiments) or BJAB/FucT-VII cells (B, 1 of 7 experiments) expressing either full-length or truncated PSGL-1. High numbers of rolling events were recorded for HL60 cells and PSGL-1 transfectants, but rolling was greatly reduced in both PSGL-1Δcyto transfectants. *P < .05 versus PSGL-1 transfectants. (C) Total rolling events were collected as described for panels A and B, but rolling was analyzed at 3 different shear stresses: 1.5, 1.0, and 0.5 dynes/cm2. Total rolling events in 50 fields (1 of 3 experiments) were recorded for HL60 cells (open boxes), K562/FucT-VII/PSGL-1 transfectants (closed triangles), and K562/FucT-VII/PSGL-1Δcyto transfectants (closed circles). High numbers of rolling events were recorded for HL60 cells and PSGL-1 transfectants. In contrast, rolling events were greatly reduced for PSGL-1Δcyto transfectants at the tested shear rates. (D) HL60 (8.8 ± 2.9 cells/COS cell) and PSGL-1 transfectants (7.4 ± 2.32 cells/COS cell) bound at similar levels to COS cells transfected with P-selectin, whereas PSGL-1Δcyto transfectants bound at lower levels (1.7 ± 0.9 cells/COS cell).
To confirm that the low residual rolling observed in PSGL-1Δcyto transfectants was due to interactions between PSGL-1 and P-selectin, PSGL-1Δcyto transfectants were preincubated with either KPL1 or an isotype control mAb prior to rolling on CHO/P. Preincubation of PSGL-1Δcyto transfectants with KPL1 almost completely inhibited this low level of rolling on P-selectin (126 ± 46 events in the absence of KPL1 versus 3 ± 1 in the presence of 2.5 μg/mL KPL1).
To determine if the remaining low rolling activity seen with PSGL-1Δcyto cells was a function of shear stress, transfectants were analyzed in a parallel-plate flow system as described above, but the shear stress was adjusted to either 0.5, 1.0, or 1.5 dynes/cm2. At all 3 shear rates, cells expressing full-length PSGL-1 rolled extremely well on P-selectin, with total events slightly increasing at the lowest shear rate (Figure 3C). In contrast, PSGL-1Δcyto cells rolled at greatly reduced levels even at the lowest shear of 0.5 dynes/cm2. Total events for PSGL-1Δcyto cells at the lowest shear level were an average of 572 events (± 184) compared to 2343 events (± 427) for transfectants expressing PSGL-1. Similar results were obtained with BJAB/FucT-VII transfectants expressing either PSGL-1 or PSGL-1Δcyto (data not shown).
In addition, PSGL-1Δcyto transfectants rolled with faster velocities than PSGL-1 transfectants. PSGL-1 transfectants rolled with very low velocities at all 3 shears, with mean velocities decreasing slightly as the shear stress decreased, as expected (ranging from 2.68 μm/s at 0.5 dynes/cm2 to 4.21 μm/s at 1.5 dynes/cm2). In contrast, PSGL-1Δcyto mean velocities were significantly higher than PSGL-1 transfectants. At a shear stress of 0.5 dynes/cm,2 PSGL-1Δcyto transfectants rolled with a mean velocity of 23.31 μm/s (± 12.76). In addition to these large differences in rolling velocities, the rolling patterns of these 2 cell lines were very distinct. PSGL-1 transfectants attached to the monolayer and rolled at a consistent low velocity across the entire filed of view. In contrast, PSGL-1Δcyto transfectants attached briefly, rolled with one or 2 long “steps,” and detached. In additional experiments, the shear stress was turned off and PSGL-1Δcyto cells were allowed to settle on the CHO/P monolayer. When the shear stress was turned back on, the settled PSGL-1Δcyto transfectants still rapidly detached without rolling (data not shown). These data show that the rolling defect associated with truncation of the PSGL-1 cytoplasmic tail is not a function of the level of shear stress (0.5-1.9 dynes/cm2), and cannot be overcome by eliminating the attachment step.
To further characterize interactions between PSGL-1Δcyto and P-selectin, binding of the transfectants to COS cells overexpressing P-selectin was evaluated. Interactions between PSGL-1Δcyto and P-selectin were easily detectable in this low shear assay, but at levels approximately 4-fold lower compared to wild-type PSGL-1 (Figure3D). These data recapitulate the low level of activity observed at 0.5 dynes/cm2 in the flow assay (Figure 3C), and indicate that the P-selectin binding domain of PSGL-1Δcyto transfectants can recognize P-selectin under low shear conditions, but that these interactions are compromised to a greater degree under the more physiologic conditions of the parallel-plate flow assay.
Interactions between the actin cytoskeleton and the cytoplasmic tail of PSGL-1
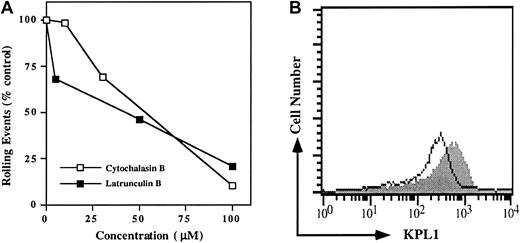
Because interactions between the cytoplasmic domains of many adhesion molecules and the actin cytoskeleton are essential for cell adhesion, we sought to determine if an intact actin cytoskeleton was required for PSGL-1 interactions with P-selectin. PSGL-1 transfectants were preincubated with increasing doses of either latrunculin B or cytochalasin B and washed; rolling was analyzed in the parallel-plate flow chamber. Rolling events were decreased in a dose-dependent manner (Figure 4A). Low doses sufficient to prevent de novo actin polymerization had only modest effects on rolling, whereas high doses capable of disrupting the pre-existing actin cytoskeleton sharply reduced rolling (Figure 4A). Importantly, surface expression of the PSGL-1 glycoprotein was only modestly reduced by the cytoskeletal inhibitors (Figure 4B), indicating that disruption of the actin cytoskeleton does not induce significant shedding or internalization of PSGL-1.
Disruption of the actin cytoskeleton in PSGL-1 transfectants blocks rolling on P-selectin.
(A) PSGL-1 transfectants were treated with either 10, 30, or 100 μM cytochalasin B (open squares), or 5, 50, or 100 μM latrunculin B (filled squares) and analyzed for rolling on CHO/P. To facilitate comparison between experiments, rolling events were normalized to the control group, which consisted of cells treated with vehicle alone (dimethyl sulfoxide). One of 3 similar experiments. (B) Expression of PSGL-1 on cells treated with 50 μM latrunculin B (open histogram) and untreated (filled histogram) PSGL-1 transfectants. Similar results were obtained when cells were treated with 100 μM cytochalasin B (data not shown).
Disruption of the actin cytoskeleton in PSGL-1 transfectants blocks rolling on P-selectin.
(A) PSGL-1 transfectants were treated with either 10, 30, or 100 μM cytochalasin B (open squares), or 5, 50, or 100 μM latrunculin B (filled squares) and analyzed for rolling on CHO/P. To facilitate comparison between experiments, rolling events were normalized to the control group, which consisted of cells treated with vehicle alone (dimethyl sulfoxide). One of 3 similar experiments. (B) Expression of PSGL-1 on cells treated with 50 μM latrunculin B (open histogram) and untreated (filled histogram) PSGL-1 transfectants. Similar results were obtained when cells were treated with 100 μM cytochalasin B (data not shown).
Because the pharmacologic disruption of F-actin gave similar rolling results to that of PSGL-1Δcyto transfectants, we hypothesized that truncation of the cytoplasmic tail disrupted essential interactions with the actin cytoskeleton. We used a combined detergent fractionation and flow cytometric assay, which has previously been used to characterize interactions between the cytoskeleton and L-selectin, major histocompatibility complex class II, and several other cell surface molecules,23-25 to determine if PSGL-1 was physically associated with the actin cytoskeleton. After exposure to detergent containing buffer, high levels of KPL1 staining were largely retained on cells transfected with PSGL-1, demonstrating that the cytoplasmic tail of PSGL-1 interacts with the actin cytoskeleton (Figure 5, left side). In contrast, sharply reduced staining was observed for PSGL-1Δcyto transfectants, indicating that interactions with the actin cytoskeleton were greatly compromised (Figure 5, right side). Thus, the PSGL-1 cytoplasmic tail interacts with the actin cytoskeleton, and the elimination of these interactions, either by truncation of the PSGL-1 cytoplasmic domain or by pharmacologic disruption of the actin cytoskeleton, dramatically reduced rolling on P-selectin.
Association of the cytoplasmic domain of PSGL-1 with the actin cytoskeleton is disrupted in PSGL-1Δcyto transfectants.
The detergent fractionation assay was performed as described in “Materials and methods.” High levels of KPL1 staining remained in the detergent-insoluble pellet associated with PSGL-1 transfectants (left panels), but this staining was greatly reduced in PSGL-1Δcyto cells (right panels). Top, untreated cells; bottom, detergent-treated cells.
Association of the cytoplasmic domain of PSGL-1 with the actin cytoskeleton is disrupted in PSGL-1Δcyto transfectants.
The detergent fractionation assay was performed as described in “Materials and methods.” High levels of KPL1 staining remained in the detergent-insoluble pellet associated with PSGL-1 transfectants (left panels), but this staining was greatly reduced in PSGL-1Δcyto cells (right panels). Top, untreated cells; bottom, detergent-treated cells.
Identification of a cytoskeletal linker protein that interacts with PSGL-1
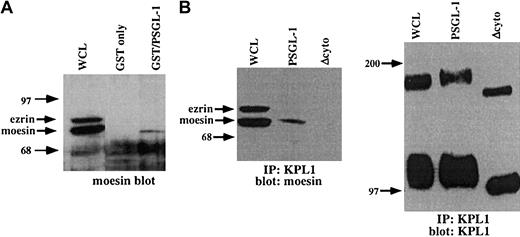
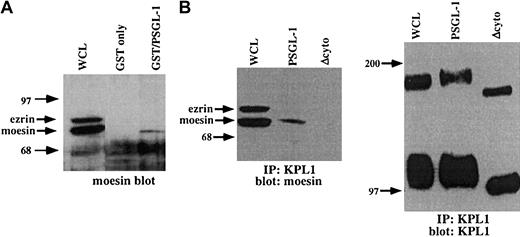
The above observation documents a functional link between PSGL-1 and the actin cytoskeleton, but does not identify the molecular basis for this interaction. To search for potential cytoplasmic mediators of this interaction, a GST fusion protein incorporating the entire PSGL-1 cytoplasmic domain was used as an affinity capture matrix to isolate cytoplasmic proteins from WCLs capable of interacting with the PSGL-1 cytoplasmic tail. Bound material was eluted, electrophoresed on SDS-PAGE gels, transferred to nitrocellulose, and probed with antibodies to α-actinin, vinculin, talin, moesin, or ezrin. Under these conditions, specific interactions between the cytoplasmic domain of PSGL-1 and moesin but not these other proteins were detected (Figure6A and data not shown). To confirm these results, coimmunoprecipitation studies with PSGL-1 and PSGL-1Δcyto transfectants were carried out. Immunoprecipitations were performed with the anti-PSGL-1 mAb KPL1 coupled to Affigel followed by Western blotting with an antimoesin mAb. Under these conditions, interactions with moesin were detected in WCLs from PSGL-1 transfectants but not PSGL-1Δcyto transfectants (Figure 6B). Controls showed that equivalent amounts of PSGL-1 were immunoprecipitated (Figure 6B, right panel). These findings confirm and extend previous observations in which the cytoplasmic domain of PSGL-1 was shown to interact with the N-terminal domain of either recombinant or in vitro–translated moesin (residues 1-310), or moesin in HL60 lysates.26 Thus, the cytoplasmic domain of PSGL-1 interacts specifically and selectively with the ERM protein moesin, at least in vitro under the condition tested, suggesting an important role for moesin in leukocyte adhesion to P-selectin.
The PSGL-1 cytoplasmic tail interacts with moesin.
(A) GST fusion proteins were generated, which incorporated the PSGL-1 cytoplasmic domain (lane 3), or GST only (negative control, lane 2), and were incubated with HL60 WCLs. The blot was probed with a rabbit pAb to moesin, which also recognizes ezrin. GST fusion proteins expressing the cytoplasmic tail of PSGL-1 (lane 3) affinity captured moesin, but not ezrin. (B) Coimmunoprecipitations of WCLs from PSGL-1 or PSGL-1Δcyto transfectants were performed with the anti-PSGL-1 mAb KPL1 coupled to Affigel followed by Western blotting with an antimoesin mAb (left panel) or KPL-1 (right panel). Interactions with moesin were detected in either untreated WCLs (left panel, lane 1) or immunoprecipitates from PSGL-1 transfectants (left panel, lane 2), but not PSGL-1Δcyto transfectants (left panel, lane 3). Probing of either untreated WCLs (right panel, lane 1) or immunoprecipitates from PSGL-1 (right panel, lane 2) or PSGL-1Δcyto (right panel, lane 3) with KPL1 revealed either full-length (lane 1 and lane 2) or truncated (lane 3) PSGL-1.
The PSGL-1 cytoplasmic tail interacts with moesin.
(A) GST fusion proteins were generated, which incorporated the PSGL-1 cytoplasmic domain (lane 3), or GST only (negative control, lane 2), and were incubated with HL60 WCLs. The blot was probed with a rabbit pAb to moesin, which also recognizes ezrin. GST fusion proteins expressing the cytoplasmic tail of PSGL-1 (lane 3) affinity captured moesin, but not ezrin. (B) Coimmunoprecipitations of WCLs from PSGL-1 or PSGL-1Δcyto transfectants were performed with the anti-PSGL-1 mAb KPL1 coupled to Affigel followed by Western blotting with an antimoesin mAb (left panel) or KPL-1 (right panel). Interactions with moesin were detected in either untreated WCLs (left panel, lane 1) or immunoprecipitates from PSGL-1 transfectants (left panel, lane 2), but not PSGL-1Δcyto transfectants (left panel, lane 3). Probing of either untreated WCLs (right panel, lane 1) or immunoprecipitates from PSGL-1 (right panel, lane 2) or PSGL-1Δcyto (right panel, lane 3) with KPL1 revealed either full-length (lane 1 and lane 2) or truncated (lane 3) PSGL-1.
Rolling on P-selectin is blocked after disruption of interactions between moesin and F-actin
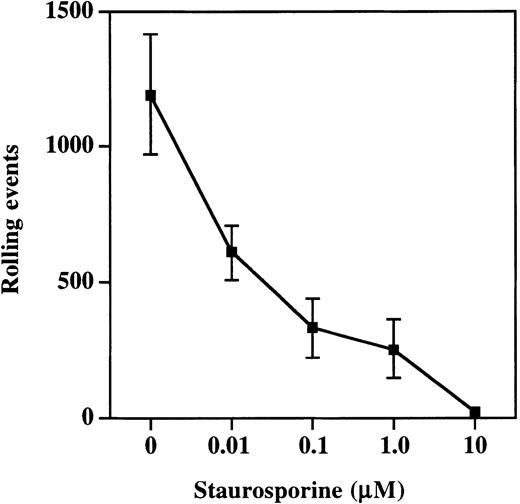
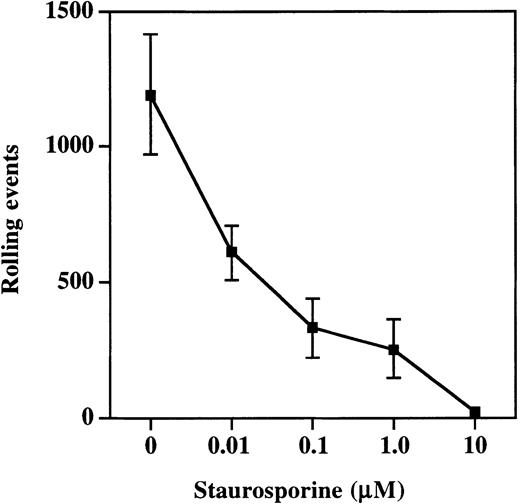
Moesin exists in both an active and inactive state within cells, with inactivation the result of intramolecular associations between the C- and N-terminal halves of the molecule.27-29 The active cross-linking form of moesin is generated and maintained by the phosphorylation of Thr558 by Rho kinase.30,31Phosphorylation at this site can be prevented by treatment of cells with staurosporine, and this treatment prevents moesin from interacting with F-actin.32 33 Therefore, to determine the importance of interactions between PSGL-1, moesin, and F-actin, HL60 cells were incubated with increasing concentrations of staurosporine and then analyzed in the parallel plate flow assay for rolling on P-selectin (Figure 7). Rolling was significantly compromised at staurosporine concentrations as low as 0.01 μM (608 ± 101 events versus 1190 ± 220 in vehicle-treated control HL60 cells) and nearly completely eliminated at concentrations of 10 μM (16 ± 9 events). Thus, pharmacologic disruption of interactions between moesin and F-actin resulted in decreased rolling on P-selectin, indicating that interactions between PSGL-1, moesin, and F-actin are essential for rolling on P-selectin.
Treatment of cells with staurosporine inhibits rolling on P-selectin.
HL60 cells were incubated with increasing concentrations of staurosporine as described in “Materials and methods.” Total rolling events at 1.9 dynes/cm2 were collected and analyzed as described for Figure 2. Staurosporine caused a dose-dependent decrease in rolling on P-selectin (1 of 3 experiments).
Treatment of cells with staurosporine inhibits rolling on P-selectin.
HL60 cells were incubated with increasing concentrations of staurosporine as described in “Materials and methods.” Total rolling events at 1.9 dynes/cm2 were collected and analyzed as described for Figure 2. Staurosporine caused a dose-dependent decrease in rolling on P-selectin (1 of 3 experiments).
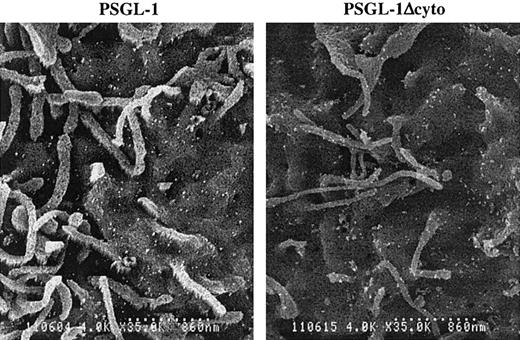
Subcellular localization of PSGL-1 and PSGL-1Δcyto
It has been reported that PSGL-1 is localized to the microvilli of normal human neutrophils.34 To determine if truncation of the PSGL-1 cytoplasmic domain altered its distribution on K562 cells, we examined PSGL-1 and PSGL-1Δcyto transfectants by scanning immunoelectron microscopy. Unlike freshly isolated human neutrophils, PSGL-1 on K562 transfectants showed a random pattern of distribution, with large numbers of PSGL-1 molecules on both the microvilli and the planar surface of the cell (Figure 8, left panel), as we showed previously.7 K562 cells transfected with PSGL-1Δcyto also showed this uniform distribution of PSGL-1 on both the microvilli and the cell body (Figure 8, right panel). Therefore, functional differences between PSGL-1 and PSGL-1Δcyto cells cannot be explained by altered subcellular localization of the mutant.
Cell surface distribution of PSGL-1 and PSGL-1Δcyto on transfected K562 cells is indistinguishable.
High-power (original magnification × 8000) photomicrographs of PSGL-1 transfectants (left panel) and PSGL-1Δcyto transfectants (right panel). Both transfectants showed a similar and uniform distribution of KPL1 immunogold particles on the microvilli and cell body. Scale bar is 860 nm.
Cell surface distribution of PSGL-1 and PSGL-1Δcyto on transfected K562 cells is indistinguishable.
High-power (original magnification × 8000) photomicrographs of PSGL-1 transfectants (left panel) and PSGL-1Δcyto transfectants (right panel). Both transfectants showed a similar and uniform distribution of KPL1 immunogold particles on the microvilli and cell body. Scale bar is 860 nm.
Discussion
Much research has been directed at the structure and function of the extracellular domain of PSGL-1. In contrast, considerably less is known about the function of the PSGL-1 cytoplasmic tail. The strong conservation between mouse35 and human36sequences in this region, which exceeds that of the extracellular region, implies an important functional role for this domain. Other features of PSGL-1, including localization to microvilli, activation-induced surface redistribution, tyrosine phosphorylation of cytoplasmic proteins after ligand engagement, secretion of specific cytokines following adhesion to P-selectin, and suppression of proliferation by hematopoietic progenitors after ligation of PSGL-1, also suggest an essential role for the PSGL-1 cytoplasmic domain.34 37-40 In this report, we present evidence indicating that attachment of the PSGL-1 cytoplasmic domain to the actin cytoskeleton is crucial for rolling on P-selectin.
Multiple observations support this hypothesis. Transfectants expressing PSGL-1Δcyto bound poorly to COS cells overexpressing P-selectin in low-shear assays and rolled poorly on P-selectin under defined shear flow compared to PSGL-1 transfectants. This impaired adhesion was not due to alterations in the PSGL-1 ectodomain, because soluble P-selectin and HECA-452 recognized PSGL-1Δcyto as well as full-length PSGL-1, indicating that the P-selectin binding site was intact. Full-length PSGL-1 was physically associated with the actin cytoskeleton, and these interactions were greatly reduced in PSGL-1Δcyto transfectants. Both pharmacologic disruption of the pre-existing actin cytoskeleton and pharmacologic inhibition of interactions between moesin and F-actin disrupted the rolling of cells expressing wild-type PSGL-1 in a dose-dependent way. Collectively, these data indicate that the highly conserved PSGL-1 cytoplasmic tail interacts with the pre-existing intact actin cytoskeleton, and that these associations are essential for rolling on P-selectin.
Interactions between PSGL-1 and the actin cytoskeleton have previously been implicated in receptor localization or redistribution.37 PSGL-1 undergoes rapid surface redistribution to the uropod of activated, surface-bound neutrophils, and this redistribution of PSGL-1 is associated with decreased adhesion to P-selectin.37 Both the redistribution of PSGL-1 and the associated reduction in adhesion were prevented by a brief pretreatment of cells with 2 μM cytochalasin D, indicating a requirement for de novo actin polymerization in these processes.37 In contrast, we observed no significant decrease in rolling on P-selectin after preincubation with low-dose (10 μM) cytochalasin B and only modest effects with low dose (5 μM) latrunculin B, indicating that de novo actin polymerization is not essential for rolling of PSGL-1 transfectants. However, progressively higher doses of either actin cytoskeleton inhibitor dramatically reduced rolling on P-selectin in a dose-dependent fashion, implying that disruption of the pre-existing actin cytoskeletal network impaired PSGL-1–mediated rolling (Figure4A). Importantly, these high doses did not cause significant decreases in surface expression of PSGL-1, indicating that treatment with cytoskeletal inhibitors did not cause shedding or internalization of the molecule (Figure 4B). That structurally dissimilar compounds with distinct mechanisms of action on actin29,41 42 exhibited very similar, dose-dependent inhibition of rolling on P-selectin argues against any nonspecific activity by these drugs. Thus, inhibition of de novo actin polymerization by low doses of inhibitors had little or no effect on PSGL-1–mediated rolling, whereas disruption of the intact actin cytoskeleton and inhibition of its repolymerization by high concentrations of actin inhibitors dramatically decreased rolling. These data suggest that a pre-existing actin cytoskeletal network, and linkages between this network and PSGL-1, are required for rolling on P-selectin.
Treatment of L-selectin transfectants with high doses of cytochalasin B (100 μM) eliminated adhesion to high endothelial venules and rolling in vivo, indicating that L-selectin–mediated interactions are also dependent on an intact actin cytoskeleton.43 Similar to the data presented in this report for PSGL-1, truncation of the L-selectin cytoplasmic tail also eliminated adhesion to high endothelial venules and rolling in vivo.43,44 L-selectin also interacts with the actin cytoskeleton, but these interactions appear to be mediated by α-actinin, not ERM proteins.11Thus, both L-selectin and PSGL-1 require interactions between their cytoplasmic domains and an intact actin cytoskeleton, but not de novo actin polymerization, for leukocyte rolling. Our findings therefore suggest a new paradigm for leukocyte migration: that the function of leukocyte “rolling receptors” requires interactions with the pre-existing actin cortical network, and therefore that ligand recognition is necessary but not sufficient for cell adhesion.
PSGL-1 is localized primarily to the tips of microvilli on resting leukocytes, which is thought to be important for interactions with selectins.34 Because ERM proteins are concentrated in microvilli and available evidence suggests that ERM proteins may be necessary for microvilli formation in leukocytes,45,46 it is reasonable to hypothesize that interactions with moesin are essential for both subcellular localization and PSGL-1 adhesive functions. However, PSGL-1 displayed a random pattern of distribution on K562 cells, being found on the microvilli, ruffles, and planar body of this cell line7 (Figure 8A), suggesting that interaction with moesin is not sufficient for preferential localization to microvilli. An identical pattern of surface expression was present on transfectants expressing PSGL-1Δcyto (Figure 8B), and a similar random pattern of distribution was also found with BJAB cells transfected with either PSGL-1 or PSGL-1Δcyto (data not shown). Thus, the significant decreases in rolling by PSGL-1Δcyto transfectants cannot be attributed to improper cellular localization. Whether localization of PSGL-1 to microvilli is essential for leukocyte rolling has yet to be definitively determined.
Our findings confirm and extend a previous report that the ERM protein moesin appears to serve as a linker protein between PSGL-1 and the actin cytoskeleton. Moesin and PSGL-1 colocalize to the uropods of activated neutrophils,26 moesin in HL60 lysates selectively bound to GST fusion proteins containing the PSGL-1 cytoplasmic tail (Figure 6A),26 and moesin was coimmunoprecipitated in transfectants expressing full-length PSGL-1 but not PSGL-1Δcyto transfectants (Figure 6B). ERM proteins exist in both active and inactive forms within cells, with inactivation resulting from an intramolecular association of the N- and C-terminal halves of the protein.27,28,47 The generation and maintenance of the active cross-linking form of moesin, which is essential for interactions with F-actin, requires phosphorylation of Thr558.32,33,48 Phosphorylation at this site is inhibited by treatment with staurosporine, which converts moesin into its inactive, unphosphorylated form and prevents it from interacting with F-actin.33 We observed a dose-dependent decrease in rolling on P-selectin after exposure to staurosporine, which supports a role for moesin in PSGL-1–mediated adhesion (Figure 7). Staurosporine also inhibits phosphorylation of other cellular proteins including ezrin and radixin,32 33 so it is possible that staurosporine treatment might be disrupting these interactions and therefore inhibits PSGL-1–mediated adhesion. However, we were unable to detect interactions between the PSGL-1 cytoplasmic tail and ezrin by either GST binding assays or immunoprecipitation. In addition, neutrophils express no detectable ezrin by Western blotting (data not shown) and freshly isolated neutrophils roll extremely well on P-selectin, suggesting that ezrin is not essential for PSGL-1–mediated rolling on P-selectin. These data further support a role for interactions between PSGL-1, moesin, and F-actin in rolling on P-selectin, but do not rule out a role for other as yet unidentified cytoplasmic proteins in leukocyte rolling.
This is the first report describing an essential role for the highly conserved PSGL-1 cytoplasmic domain in rolling on P-selectin. Truncation of the PSGL-1 cytoplasmic domain abrogated rolling of cells on P-selectin and sharply reduced interactions with the actin cytoskeleton. This loss of rolling was recapitulated in cells expressing native PSGL-1 by treatment of cells with reagents that disrupt either the pre-existing actin cytoskeleton or interactions between moesin and F-actin. These data collectively indicate that attachment of PSGL-1 to the actin cytoskeleton is essential for cell adhesion and suggest that moesin participates in this interaction. It is likely that some level of interaction between PSGL-1 and the actin cytoskeleton is constitutive, and that these associations can be modulated by various factors such as chemokines or cytokines.
The authors gratefully acknowledge Dr Robert D. Nelson and Michael Herron (Department of Dermatology, University of Minnesota, Minneapolis) for their excellent technical support in generating the scanning electron photomicrographs of PSGL-1 and PSGL-1Δcyto distribution on K562/FucT-VII transfectants.
Supported by the American Cancer Society, Illinois Chapter, grant 99-47 (to K.R.S.), the American Heart Association grant 003003N (to K.R.S.), and grant RPG-96-097-04-CSM from the National American Cancer Society (to G.S.K.). G.S.K. is an Established Investigator of the American Heart Association.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Karen R. Snapp, Northwestern University Medical School, Department of Microbiology/Immunology, Tarry 6-728, 303 E Superior Ave, Chicago, IL 60611; e-mail: krs133@northwestern.edu.