Caspase-8 (Fas-associating protein with death domain–like interleukin-1β– converting enzyme [FLICE]/MACH/Mch5) belongs to a family of cysteine proteases presumed to be the apex of the apoptotic signaling pathways. We recently reported the presence of a novel isoform of caspase-8, named caspase-8L, generated by the alternative splicing of human caspase-8 gene, from human peripheral blood lymphocytes by reverse transcriptase–polymerase chain reaction. We herein report a functional analysis of caspase-8L in the Fas-mediated apoptotic pathway. Caspase-8L is missing the catalytic site of caspase-8 but retains 2 N-terminal repeats of the death-effector domain. The caspase-8L messenger RNA was detected in various tissues but not in any cell lines examined. In human peripheral blood lymphocytes, caspase-8L was strongly suggested to be expressed at the protein level. In MCF-7 cells, caspase-8L transfection itself did not affect cell viability but instead inhibited the apotosis induced by the cotransfection of caspase-8 in a dominant negative manner. Moreover, Fas-mediated apoptosis was inhibited in caspase-8L–transfected Jurkat cells, which were associated with a reduction in the caspase-8 catalytic activity. In vitro binding assays demonstrated that caspase-8L bound to FADD (Fas-associating protein with death domain) and caspase-8a and blocked the binding of caspase-8 to FADD. In in vivo binding assays, transfected caspase-8L bound to endogeneous FADD. Thus, caspase-8L acts as an inhibitor of caspase-8 by interfering with the binding of caspase-8 to FADD and is involved in the regulation of Fas-mediated apoptosis.
Introduction
Apoptosis (programmed cell death) is a strictly regulated cell suicide mechanism that is morphologically and biochemically distinct from necrosis and plays a crucial role in the development, homeostasis, and defense of multicellular organisms.1-5
The morphologic changes observed in apoptosis, including mitochondrial damage, nuclear membrane breakdown, DNA fragmentation, chromatin condensation, and the formation of apoptotic bodies, are conducted by a specialized cellular mechanism consisting of 3 major components: the caspases, the Bcl-2 family proteins, and the Apaf-1/Caenorhabditis elegans cell death protein-4 (CED-4) protein.6 7
Among these components, caspases, which belong to a family of cysteine proteases, are the core of this mechanism and participate in the apoptosis cascade that is triggered in response to proapoptotic signals by cleaving a set of proteins.6 They are synthesized as inactive proenzymes that have to be activated by proteolytic cleavage after specific aspartate residues, and once the caspases are activated, they cleave their substrates, thus resulting in the disintegration of the cells.6 Among them, caspase-8 is the key enzyme, and it is presumed to be the apex of the Fas-mediated apoptosis pathway. Caspase-8 is activated in association with the Fas death-inducing signaling complex (DISC).8 The binding of Fas ligand to Fas receptor induces the trimerization of Fas, and the cytoplasmic region of Fas, containing a death domain (DD), recruits a DD-containing adaptor molecule, FADD (Fas-associating protein with death domain).9-11 FADD also contains a DD at its C-terminus and binds to Fas via interactions between the DDs.9-11 The N-terminal region of FADD, termed the death-effector domain (DED), is responsible for downstream signal transduction and binds to caspase-8.12,13 Caspase-8 carries 2 DEDs in its N-terminal region, through which it binds to FADD.12,13 The Fas oligomerization induced by the binding of Fas ligand results in the formation of DISC and the oligomerization of caspase-8. Subsequently, caspase-8 is fully activated by self-cleavage and thereby cleaves many cellular substrates.14-17
There are various distinct inhibitory molecules involved in the regulation of the activation of caspases triggered by proapoptotic signals from death receptors.7 One group of these inhibitory molecules belongs to a family of viral proteins, FADD-like interleukin-1β–converting enzyme (ICE) inhibitory proteins (v-FLIPs), containing 2 DEDs.18 These proteins inhibit the recruitment of procaspases to the DISC by competing with the procaspases when binding to the DED of FADD. Cellular Fas-associating protein with death domain–like interleukin-1β–converting enzyme (FLICE)–inhibitory protein (c-FLIP, also called Casper/I-FLICE/FLAME-1/CASH/ CLARP/MRIT/Usurpin) is a mammalian homolog of v-FLIPs and acts as an endogeneous inhibitor of the apoptosis cascade.18-26 Another group of these inhibitory molecules are the inhibitors of apoptosis (IAP) family proteins, such as NAIP, c-IAP1/HIAP-2, c-IAP2/HIAP-1, XIAP/hILP, survivin, and BRUCE.27-32 IAPs inhibit some members of the caspase family, either directly or indirectly.27
In addition to these endogenous regulatory molecules, various isoforms of different caspases, such as ICE-ε (caspase-1 isoform), caspase-2S, CASP-2L-Pro (caspase-2 isoforms), caspase-9S, and caspase-9b (caspase-9 isoforms), have been suggested to act as dominant negative, endogeneous inhibitors of apoptosis.33-37 For caspase-8, 8 different isoforms (designated as caspase-8/a-h) have been described.12,13 38 Caspase-8a and -8b are the complete forms, which are known to mediate apoptosis; however, the physiologic role of other isoforms has yet to be clarified.
We recently reported a novel isoform of caspase-8, named caspase-8L, generated by the alternative splicing of human caspase-8 gene, from human peripheral blood lymphocytes (PBLs) by reverse transcriptase–polymerase chain reaction (RT-PCR).39 In the present study, we show evidence for the antiapoptotic activity of caspase-8L.
Materials and methods
Cell culture and media
MCF-7 cells, a human breast cancer–derived cell line, were donated by the Cell Resource Center for Biomedical Research Institute of Development, Aging and Cancer, Tohoku University (Sendai, Japan), and cultured in Dulbecco modified Eagle medium (Life Technologies, Gaithersburg, MD) supplemented with 10% fetal calf serum (FCS) and 10 μg/mL tobramycin. COS-7 cells were cultured in the same medium. Jurkat cells, a human T-cell leukemia cell line, were maintained in an RPMI 1640 medium containing 10% FCS, 5 μM 2-mercaptoethanol, 100 IU/mL penicillin, and 400 μg/mL streptomycin. All cultures were maintained in a humidified environment of 5% CO2 in air.
Antibodies
The following antibodies were purchased from the indicated companies: rabbit anti–human caspase-8 polyclonal antibody directed against the N-terminus of caspase-8 (BD Pharmingen, San Diego, CA), mouse anti–human caspase-8 monoclonal antibody directed against the C-terminus of caspase-8 (Medical & Biological Laboratories [MBL], Nagoya, Japan), mouse anti–human FADD monoclonal antibody (MBL), horseradish peroxidase (HRP)–conjugated goat anti–rabbit IgG antibody (Amersham, Arlington Heights, IL), HRP-conjugated goat anti–mouse IgG antibody (Cappel, Aurora, OH), and antihuman Fas monoclonal antibody (clone CH-11) (MBL).
RNA extraction and reverse transcriptase reaction
Total RNA was isolated using the ISOGEN reagent (Nippongene, Tokyo, Japan) following the manufacturer's instructions. The RT reaction was conducted using First-Strand cDNA Synthesis Kit (Amersham Pharmacia Biotech, Piscataway, NJ) according to the manufacturer's instructions.
Molecular cloning of caspase-8L cDNA
Human caspase-8L complementary DNA (cDNA) and caspase-8a cDNA were cloned by RT-PCR from healthy human PBLs. PBLs were prepared by Lymphocyte Separation Medium (ICN Biomedicals, Aurora, OH) from the heparinized blood of healthy individuals. RT-PCR was conducted using 2 primers, 5′-GGAGTTAGGCAGGTTAGGGG-3′ (5′-primer) and 5′-GCGACAGAGCGAGATTCTG-3′ (3′-primer).39 After 5 minutes incubation at 94°C, PCR was carried out for 30 seconds at 94°C, 30 seconds at 60°C, and 90 seconds at 72°C for 35 cycles. The resulting PCR products (1723 base pairs [bp]: caspase-8L; 1587 bp: caspase-8a) were cloned into pCR2.1 vector by Original TA cloning kit (Invitrogen, Carlsbad, CA) and sequenced using the Big Dye Terminator Cycle Sequencing Kit (Applied Biosystems, Tokyo, Japan) and the ABI PRISM 310 genetic analyzer (Perkin-Elmer, Norwalk, CT).
Expression vectors
To generate caspase-8L or -8a expression vectors, RT-PCR was performed as described previously.40 RT-PCR of caspase-8L was conducted using 2 primers, 5′-CGGGGTACCATGGACTTCAGCAGAAATC-3′ (5′-primer) and 5′-CGCGGATCCTCCTGTCCATCAGTGCCATA-3′ (3′-primer). RT-PCR of caspase-8a was performed using 2 primers, 5′-CGGGGTACCATGGACTTCAGCAGAAATC-3′ (5′-primer) and 5′-TCAATCAGAAGGGAAGACAA-3′ (3′-primer). Each PCR reaction was carried out for 30 seconds at 94°C, 30 seconds at 60°C, and 90 seconds at 72°C for 35 cycles. Before the cycle, a denaturation step for 5 minutes at 94°C was included. The resulting products were subcloned into pcDNA3 mammalian expression vector (Invitrogen). To obtain N-terminal polyhistidine-tagged (His-tagged) caspase-8L or -8a expression plasmids, pCR2.1 vectors encoding caspase-8L or -8a were digested with BstXI, and the resulting fragments were subcloned into a pcDNA3.1/HisA mammalian expression vector (Invitrogen). Full-length FADD cDNA containing cosmid pAxCAhFADD41 (obtained from RIKEN Gene Bank, the Institute of Physical and Chemical Research, Tsukuba, Japan) was digested withKpnI and BamHI, and FADD cDNA fragment was subcloned into pcDNA3 mammalian expression vector (Invitrogen).
In vitro translation
In vitro translation was performed using the TNT-Coupled Reticulocyte Lysate Systems (Promega, Madison, WI) in the presence or the absence of Transcend biotinylated transfer RNA (tRNA) (Promega) according to the manufacturer's instruction. In vitro–translated proteins were analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gel and transferred to the nitrocellulose membrane (BIO-RAD Laboratories, Hercules, CA). The membrane was blocked with Tris-buffered saline (TBS) containing 0.5% Tween 20 (TBS-T), probed with HRP-conjugated streptavidin, and visualized by Transcend Chemiluminescent Non-Radioactive Translation Detection Systems (Promega).
RT-PCR analysis of various caspase-8 isoforms' mRNA expression
To determine the expression of caspase-8L, -8a, -8b, and other isoforms of caspase-8 messenger RNA (mRNA) in different tissues or in human tumor cell lines, cDNAs of selected human normal tissues (CLONTECH, Palo Alto, CA), the synthesized cDNAs prepared from human PBLs, or human tumor cell lines were amplified by means of the PCR as described previously.40 PCR was conducted using 2 primers, 5′-TCTGTGCCCAAATCAACAAG-3′ (5′-primer) and 5′-GCCACCAGCTAAAAACATTCC-3′ (3′-primer). After 5 minutes' incubation at 94°C, PCR was carried out for 30 seconds at 94°C, 30 seconds at 60°C, and 30 seconds at 72°C for 35 cycles. The resulting products were subjected to 1.2% agarose gel electrophoresis and visualized by ethidium bromide staining. As an internal control for RT-PCR, glyceraldehyde-3-phosphate dehydrogenase cDNA was amplified by PCR using 2 primers, 5′-TGAAGGTCGGAGTCAACGGATTTGGT-3′ (5′-primer) and 5′-CATGTGGGCCATGAGGTCCACCAC-3′ (3′-primer) at the same experimental conditions and then were used for normalization.
Expression of caspase-8L in COS-7 cells
COS-7 cells were transiently transfected with caspase-8L expression vector using LipofectAMINE reagent (Life Technologies) according to the manufacturer's instructions. Forty-eight hours after transfection, a Western blotting analysis was performed to detect caspase-8L protein.
Western blot analysis
The cells (2.5 × 106 cells) were lysed in 50 μL SDS-PAGE sample buffer (0.625 M Tris-HCl [pH 8.8], 2% SDS, 10% β-mercaptoethanol, 10% glycerol, and 0.01% bromophenol blue), and lysates were boiled for 5 minutes and then analyzed by SDS-PAGE followed by transfer to nitrocellulose membrane. The membranes were incubated for 1 hour in phosphate-buffered saline containing 5% nonfat milk. After incubation, the membranes were blotted using rabbit anti–caspase-8 polyclonal antibody (BD Pharmingen) or using mouse anti–caspase-8 monoclonal antibody (MBL) overnight. After washing with TBS-T, the membranes were incubated with peroxidase-conjugated secondary antibody for 2 hours and visualized with ECL Western Blotting Detection Reagents (Amersham Pharmacia Biotech).
Ribonuclease protection assay
Using RT-PCR, a probe for ribonuclease (RNase) protection was constructed with a sense primer (5′-GGATTTAAACATATTTCCCTGTGG-3′) and an antisense primer (5′-TCCATGGGAGAGGATACAGC-3′) to clone the 288-bp probe. The resulting PCR product was cloned into the pCR2.1 vector using a TA cloning kit (Invitrogen), and the vector was sequenced to rule out any point mutations. Plasmid DNA was linearized withBamHI, and in vitro transcription was performed using Riboprobe in vitro transcription system (Promega) in the presence of 50 M [α-32P]CTP (cytidine triphosphate) according to the manufacturer's protocol. The hybridization of probe and sample RNA and the RNase digestion of hybridized probe and sample RNA were performed using the RPAII Ribonuclease Protection Assay Kit (Ambio, Austin, TX) according to the manufacturer's instructions. Briefly, after gel purification, the 4 × 105 cpm probe was hybridized to sample total RNA (20 μg) overnight at 42°C. RNase digestion of hybridized probe and sample RNA was performed using RNase A (0.25 U/μL) and RNase T1 (10 U/μL) solution at 37°C for 30 minutes. Next, the digestion mixtures were treated with RNase Inactivation/Precipitation Mixture to inactivate RNase and precipitate RNA. The precipitated products were loaded on denaturing polyacrylamide gel. After electrophoresis, the gel was transferred to filter paper, and autoradiographic exposure to x-ray film was performed overnight.
Cytotoxicity assays
MCF-7 cells (3 × 105 cells in 3 cm dishes) were transiently transfected with the cDNAs of the indicated proteins together with the β-galactosidase expression vector (pcDNA3.1/His/LacZ, Invitrogen) using LipofectAMINE reagent (Life Technologies). In cotransfection assays, 0.5 μg caspase-8a expression vector plus various amounts of empty vector or caspase-8L expression vector were transiently transfected with 0.75 μg β-galactosidase expression vector into MCF-7 cells. The cells were rinsed 5 hours after transfection and were further incubated for 18 hours without any additional treatment. The extent of cell death was assessed by determining the percentage of apoptotic cells in morphology in β-galactosidase–positive cells, as described previously.42
Stable transfection and evaluation of the transfected cells
A total of 20 μg linearized empty vector (pcDNA3.1/HisA, Invitrogen) (mock) or 20 μg linearized vector expressing the His-tagged caspase-8L cDNA was transfected into Jurkat cells by electroporation using Gene Pulser apparatus (BIO-RAD Laboratories) at 250 V, 960 microfarads. Cells were immediately plated to prewarmed medium and cultured at 37°C. Two days after transfection, transfected cells were selected in the presence of 2 mg/mL G418 (Sigma, St Louis, MO). The clones were established by limiting dilution, and the expression of the transfected caspase-8L was assessed by RT-PCR. In these clones, 3 clones were used for apoptosis assay by flow cytometry because of its high expression level of caspase-8L.
Cell death evaluation by flow cytometry
Fas-induced cell death of empty vector–transfected (mock) and caspase-8L–transfected Jurkat cells were evaluated by membrane permeability cell death analysis. Cells (2.0 × 105) were left either untreated or were treated with anti-Fas monoclonal antibody (0.5 μg/mL) for 12 hours. After stimulation, the cells were stained with 40 μg/mL propidium iodide in phosphate-buffered saline containing 2% FCS and 0.05% NaN3 for 5 minutes on ice. Then the percentage of propidium iodide–positive (dead) and –negative (alive) cells was determined with FACScan (Becton Dickinson, San Jose, CA) flow cytometer using CellQuest software (Becton Dickinson).
Caspase activity assays
Fas-induced caspase-8 catalytic activity in empty vector–transfected (mock) and caspase-8L–transfected Jurkat cells was measured using a Caspase-8 Colorimetric Protease Assay Kit (MBL) according to the manufacturer's instructions. In brief, 1 × 106 cells were left either untreated or were treated with anti-Fas monoclonal antibody (0.5 μg/mL) for 4 hours and were harvested by centrifugation. The cells were lysed by lysis buffer and recentrifuged at 10 000g for 1 minute. Then, 50 μL of the cytosolic extracts (1 mg of protein contained) was diluted in 50 μL of reaction buffer and was added with fluorogenic substrates (IETD-pNA; Ile-Glu-Thr-Asp–P-nitroanilide) and incubated at 37°C for 12 hours. Release of pNA was measured by spectrofluorometry at 400 nm.
In vitro binding assays
The cDNA of the indicated proteins was in vitro translated, and the proteins were incubated with 50 μL Ni-NTA Magnetic Agarose Beads (QIAGEN, Valencia, CA) overnight at 4°C in protein binding buffer (50 mM NaH2PO4 [pH 8.0], 300 mM NaCl, 0.2 mM phenylmethylsulfonyl fluoride, and 20 mM imidazole) to selectively collect His-tagged proteins. After incubation, the Ni-NTA beads were collected using a magnetic separator and then were washed 3 times with wash buffer (50 mM NaH2PO4 [pH 8.0], 300 mM NaCl, 0.005% Tween 20, 0.2 mM phenylmethylsulfonyl fluoride, and 20 mM imidazole). Next, Ni-NTA beads bearing His-tagged proteins were resuspended in interaction buffer (50 mM NaH2PO4 [pH 8.0], 300 mM NaCl, 0.005% Tween 20, 0.2 mM phenylmethylsulfonyl fluoride, and 20 mM imidazole) and incubated with in vitro–translated FADD or caspase-8L for 2 hours at room temperature. Ni-NTA beads were collected on a magnetic separator. After washing, the bound proteins were eluted by adding 50 μL SDS-PAGE buffer, analyzed by 10% SDS-PAGE, and detected with anti-FADD monoclonal antibody (MBL) or with anti–caspase-8 polyclonal antibody (BD Pharmingen) and HRP-conjugated secondary antibody.
In vivo interaction assay of caspase-8L with FADD in MCF-7 cell lines
MCF-7 cells (2 × 107) were transiently transfected with 4 μg empty vector (pcDNA3.1/HisA, Invitrogen) or His-tagged caspase-8L expression vector using LipofectAMINE (Life Technologies) according to the manufacturer's instructions. Eighteen hours after transfection, cells were lysed, resuspended in lysis buffer (50 mM NaH2PO4 [pH 8.0], 300 mM NaCl, 10 mM imidazole, and 0.05% Tween 20), and sonicated on ice to lyse cells. After sonication, lysates were cleared by centrifugation at 10 000g for 30 minutes at 4°C , and the supernatants were collected. Next, those supernatants were incubated with 100 μL Ni-NTA Magnetic Agarose Beads overnight at 4°C. After incubation, Ni-NTA beads were collected on a magnetic separator. After washing, the bound proteins were eluted by adding 50 μL SDS-PAGE buffer, analyzed by 10% SDS-PAGE, and detected with anti-FADD monoclonal antibody (MBL) or with anti-HisG antibody and HRP-conjugated secondary antibody.
Results
Molecular cloning of caspase-8L
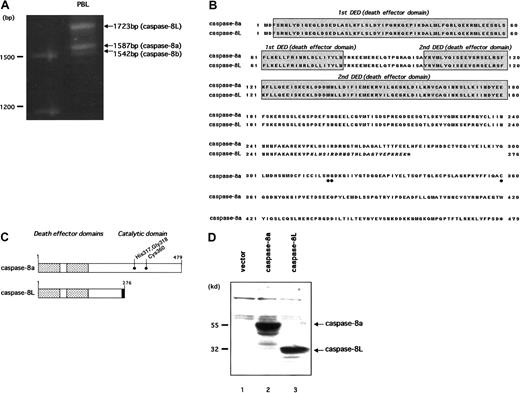
We previously reported a novel isoform of caspase-8, named caspase-8L (caspase-8 long form [GenBank accession number AF 380342]), in human PBLs.39 To clone caspase-8L, we performed RT-PCR on mRNA from human PBL with 2 primers encompassing the initiation and stop codons of caspase-8. In addition to caspase-8a (1587 bp) and caspase-8b (1542 bp), we identified a long amplification product (1723 bp) representing caspase-8L (Figure1A). Similar results were obtained from an RT-PCR analysis in purified human peripheral T cells and B cells (data not shown). As a result, we cloned caspase-8L and entirely sequenced it. Sequence data concurred with the size of the amplified product and with the published human caspase-8a cDNA sequence, except for the 136 bp insertion of the 5′ end sequence of intron 8 as previously described.39 When compared with the consensus sequences at the 5′ splice site,43 no difference was observed among the caspase-8L 5′ splice site, normal caspase-8a 5′ splice site, and the consensus sequences. This 136 bp insertion resulted in a frame shift of the transcript. Thus, this caspase-8L cDNA encoded a putative caspase-8L protein lacking the catalytic domain but retaining 2 N-terminal repeats of the DED (Figure 1B-C). The molecular mass of the predicted protein was estimated to be 32 kd, and the in vitro–translated product migrated as a 32 kd protein on SDS gels (Figure 1D).
Molecular cloning and deduced amino acid sequences of caspase-8L.
(A) Identification of caspase-8L, -8a, and -8b in human PBLs by RT-PCR analysis. The total RNA from the PBLs of a healthy human individual were reverse transcribed and then amplified by PCR with primers encompassing the entire coding region of caspase-8a and -8b. The resulting RT-PCR products were resolved on 1.2% agarose gel and visualized by ethidium bromide staining. In addition to 2 expected bands (1587 bp and 1542 bp) representing caspase-8a and -8b, a long amplification product, representing caspase-8L, was identified (1723 bp). (B) Deduced amino acid sequence of caspase-8L compared with caspase-8a (GenBank accession number X98172). The DEDs are shaded. Amino acids that were implicated for the catalytic activity of caspase-8a are marked by ● below the alignment. Caspase-8L, a splice variant of caspase-8a, is truncated at amino acid residue 276, including C-terminal 8 amino acids different from caspase-8a (italics). The C-termini of these proteins are denoted by asterisks. (C) Molecular structures of caspase-8a and -8L are shown schematically. The DEDs are denoted by the checked areas. The catalytic cysteine residue and amino acids that were implicated in catalytic activity are indicated by ●. Amino acid residues in the unique C-terminal sequences of caspase-8L are heavily shaded. (D) Western blots of in vitro–translated caspase-8a and -8L. Full-length cDNA fragments encoding caspase-8a and -8L were subcloned into pcDNA3 and in vitro translated as described. The translated products (caspase-8a, 2 μL; caspase-8L, 4μL) were fractionated on 10% SDS-PAGE, transferred to nitrocellulose membrane, and visualized as described. In lane 1, an empty vector (pcDNA3) was in vitro translated and analyzed as a negative control.
Molecular cloning and deduced amino acid sequences of caspase-8L.
(A) Identification of caspase-8L, -8a, and -8b in human PBLs by RT-PCR analysis. The total RNA from the PBLs of a healthy human individual were reverse transcribed and then amplified by PCR with primers encompassing the entire coding region of caspase-8a and -8b. The resulting RT-PCR products were resolved on 1.2% agarose gel and visualized by ethidium bromide staining. In addition to 2 expected bands (1587 bp and 1542 bp) representing caspase-8a and -8b, a long amplification product, representing caspase-8L, was identified (1723 bp). (B) Deduced amino acid sequence of caspase-8L compared with caspase-8a (GenBank accession number X98172). The DEDs are shaded. Amino acids that were implicated for the catalytic activity of caspase-8a are marked by ● below the alignment. Caspase-8L, a splice variant of caspase-8a, is truncated at amino acid residue 276, including C-terminal 8 amino acids different from caspase-8a (italics). The C-termini of these proteins are denoted by asterisks. (C) Molecular structures of caspase-8a and -8L are shown schematically. The DEDs are denoted by the checked areas. The catalytic cysteine residue and amino acids that were implicated in catalytic activity are indicated by ●. Amino acid residues in the unique C-terminal sequences of caspase-8L are heavily shaded. (D) Western blots of in vitro–translated caspase-8a and -8L. Full-length cDNA fragments encoding caspase-8a and -8L were subcloned into pcDNA3 and in vitro translated as described. The translated products (caspase-8a, 2 μL; caspase-8L, 4μL) were fractionated on 10% SDS-PAGE, transferred to nitrocellulose membrane, and visualized as described. In lane 1, an empty vector (pcDNA3) was in vitro translated and analyzed as a negative control.
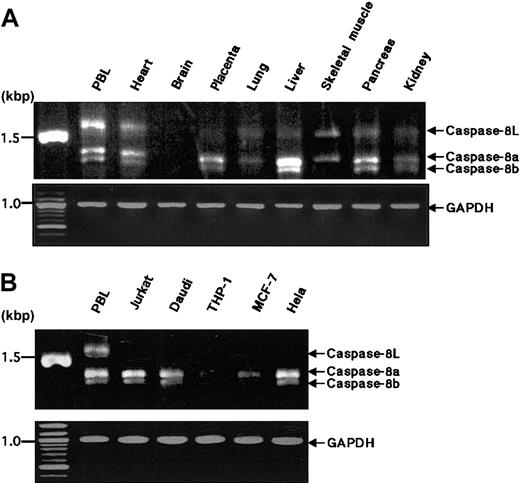
Expression of caspase-8L mRNA in normal human tissues and various cell lines
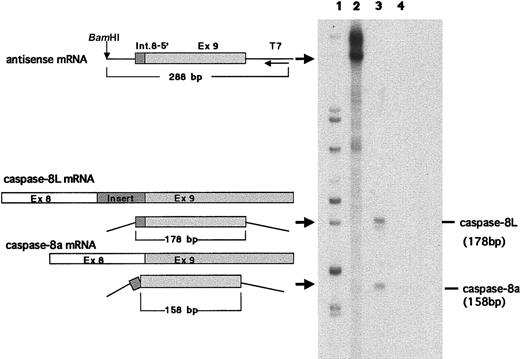
We examined the expression of caspase-8L mRNA in a series of normal human tissues by RT-PCR. Caspase-8a and -8b mRNAs were detected in most normal tissues but not in the brain (Figure2A), and these expression patterns of caspase-8a and -8b were compatible with the results demonstrated by a Northern blotting analysis in previous reports.11 12 The tissue distribution of caspase-8L mRNA was similar to that of caspase-8a and -8b. The ratio of caspase-8L to caspase-8a or -8b varied in these tissues. In PBLs, the caspase-8L expression was most prominent (Figure 2A). An RT-PCR analysis in various cell lines did not demonstrate the expression of caspase-8L mRNA in Jurkat (human T-cell leukemia cells), Daudi (human lymphoblastoid cells), MCF-7 (human breast cancer cells), or Hela (human cervical cancer cells) despite the expression of caspase-8a or -8b mRNA (Figure 2B). To evaluate the expression level of caspase-8L mRNA more precisely, we performed an RNase protection assay on mRNA from human PBLs. The probe was obtained by amplifying a smaller fragment of caspase-8L cDNA in RT-PCR using primers discussed in “Materials and methods.” This probe was constructed to include the 3′ end 20 bp of the insertion and the 5′ end 158 bp of exon 9, and therefore this probe protects fragments of 178 bp (caspase-8L) and 158 bp (caspase-8a) (Figure3, left). Two fragments were equally evident after hybridization with the labeled probe in human PBLs (Figure 3, lane 3). The longer fragment represented caspase-8L mRNA, and the smaller fragment represented caspase-8a mRNA, respectively. As a negative control, tRNA was hybridized with a probe and digested with RNase, but no fragments were observed (Figure 3, lane 4). These data indicate that in human PBLs caspase-8L mRNA is expressed almost at the same level as caspase-8a.
RT-PCR analysis of the expression of caspase-8a, -8b, and -8L mRNA in normal human tissues and tumor cell lines.
(A) Complementary DNAs of selected human normal tissues (obtained from CLONTECH) were amplified by PCR with primers that detect caspase-8a, -8b, and -8L. (B) Total RNAs from human tumor cell lines were reverse transcribed and then amplified by PCR with the same primers used in panel A. The resulting PCR products were analyzed on 1.2% agarose gel and stained by ethidium bromide. Caspase-8a, -8b, and -8L fragments are indicated by arrows. As an internal control for RT-PCR, glyceraldehyde-3-phosphate dehydrogenase cDNA was amplified by PCR under the same experimental conditions and was used for normalization.
RT-PCR analysis of the expression of caspase-8a, -8b, and -8L mRNA in normal human tissues and tumor cell lines.
(A) Complementary DNAs of selected human normal tissues (obtained from CLONTECH) were amplified by PCR with primers that detect caspase-8a, -8b, and -8L. (B) Total RNAs from human tumor cell lines were reverse transcribed and then amplified by PCR with the same primers used in panel A. The resulting PCR products were analyzed on 1.2% agarose gel and stained by ethidium bromide. Caspase-8a, -8b, and -8L fragments are indicated by arrows. As an internal control for RT-PCR, glyceraldehyde-3-phosphate dehydrogenase cDNA was amplified by PCR under the same experimental conditions and was used for normalization.
RNase protection assay in human PBLs.
An RNase protection assay was performed as described. Antisense mRNA for probe (288 bp), part of caspase-8L mRNA, caspase-8a mRNA, and protected bands (178 bp and 158 bp) are schematically shown on the left. The probe includes a portion of 136 bp insertion and a portion of exon 9. Lane 1, size marker; lane 2, an undigested probe; lane 3, a probe digested after hybridization with 20 μg RNA of PBLs from a healthy human individual; lane 4, probe digested after hybridization with 20 μg tRNA (negative control). The base pair length demonstrated on the right was derived from sequencing reactions.
RNase protection assay in human PBLs.
An RNase protection assay was performed as described. Antisense mRNA for probe (288 bp), part of caspase-8L mRNA, caspase-8a mRNA, and protected bands (178 bp and 158 bp) are schematically shown on the left. The probe includes a portion of 136 bp insertion and a portion of exon 9. Lane 1, size marker; lane 2, an undigested probe; lane 3, a probe digested after hybridization with 20 μg RNA of PBLs from a healthy human individual; lane 4, probe digested after hybridization with 20 μg tRNA (negative control). The base pair length demonstrated on the right was derived from sequencing reactions.
Caspase-8L protein expression in human PBLs
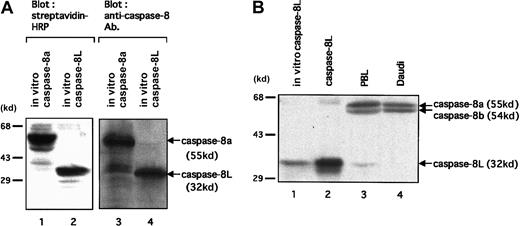
To investigate the expression of caspase-8L protein in human PBLs, at first we tested an anti–human caspase-8 polyclonal antibody directed against the N-terminus of caspase-8 for its capacity to detect caspase-8L. As shown in Figure 4A, this antibody could detect in vitro–translated caspase-8L as well as in vitro–translated caspase-8a (lanes 3 and 4). Thus, we performed immunoblot assay in human PBLs. In PBLs, we detected 2 larger bands (55 kd and 54 kd), representing caspase-8a and -8b, and a 32 kd band (Figure 4B, lane 3). This endogeneous 32 kd protein comigrated with the in vitro–translated caspase-8L (Figure 4B, lane 1) and with recombinant caspase-8L (lane 2). Additionally, this 32 kd band was not detected in Daudi cell lysate (lane 4). The Daudi cell line was used as a negative control for caspase-8L expression, because RT-PCR analysis has shown that caspase-8L mRNA was not detected in Daudi cell line (Figure 2B). These data indicated that this 32 kd band most probably represents caspase-8L. The possibility that this 32 kd band represents other isoforms of caspase-8 that mimic caspase-8L in molecular weight must be denied because this antibody can also recognize such isoforms of caspase-8 (caspase-8f: 32.4 kd; caspase-8g: 30.8 kd, respectively). To exclude this possibility, we conducted an RT-PCR analysis on human PBLs, and we detected only 3 transcripts, representing caspase-8a, -8b, and -8L (Figure 2A). Moreover, we detected no significant transcript even after a long exposure or increased PCR cycle (data not shown), except for those 3 transcripts corresponding to caspase-8a, -8b, and -8L. These results strongly suggest that this endogeneous 32 kd protein represents caspase-8L.
Expression analysis of caspase-8L protein in human PBLs.
(A) To test an anti–human caspase-8 polyclonal antibody directed against the N-terminus of caspase-8 (BD Pharmingen) for its capacity to detect caspase-8L, in vitro–translated caspase-8a (lanes 1 and 3) and -8L (lanes 2 and 4) were analyzed by SDS-PAGE. The reaction mixtures (2 μL for caspase-8a, 4 μL for caspase-8L) were separated on 10% SDS-PAGE, transferred to nitrocellulose membranes, and detected by HRP-conjugated streptavidin (left column) or by anti–human caspase-8 antibody (right column). They are revealed by Transcend Chemiluminescent Non-Radioactive Translation Detection System (Promega) (left column) or by HRP-conjugated secondary antibody and the ECL reagents (Amersham Pharmacia Biotech). (B) A Western blot analysis of the expression of caspase-8a, -8b, and -8L on resting human PBLs. In vitro–translated caspase-8L (lane 1) and cellular extracts from COS-7 cells transfected with a construct encoding caspase-8L (lane 2), human PBLs (lane 3), and Daudi cells (lane 4) were fractionated by 10% SDS-PAGE and transferred to a nitrocellulose membrane. Next, the membrane was probed with anti–human caspase-8 polyclonal antibody (BD Pharmingen) and visualized with secondary antibody as described above. The positions of molecular mass markers (in kilodaltons) are shown on the left in all panels.
Expression analysis of caspase-8L protein in human PBLs.
(A) To test an anti–human caspase-8 polyclonal antibody directed against the N-terminus of caspase-8 (BD Pharmingen) for its capacity to detect caspase-8L, in vitro–translated caspase-8a (lanes 1 and 3) and -8L (lanes 2 and 4) were analyzed by SDS-PAGE. The reaction mixtures (2 μL for caspase-8a, 4 μL for caspase-8L) were separated on 10% SDS-PAGE, transferred to nitrocellulose membranes, and detected by HRP-conjugated streptavidin (left column) or by anti–human caspase-8 antibody (right column). They are revealed by Transcend Chemiluminescent Non-Radioactive Translation Detection System (Promega) (left column) or by HRP-conjugated secondary antibody and the ECL reagents (Amersham Pharmacia Biotech). (B) A Western blot analysis of the expression of caspase-8a, -8b, and -8L on resting human PBLs. In vitro–translated caspase-8L (lane 1) and cellular extracts from COS-7 cells transfected with a construct encoding caspase-8L (lane 2), human PBLs (lane 3), and Daudi cells (lane 4) were fractionated by 10% SDS-PAGE and transferred to a nitrocellulose membrane. Next, the membrane was probed with anti–human caspase-8 polyclonal antibody (BD Pharmingen) and visualized with secondary antibody as described above. The positions of molecular mass markers (in kilodaltons) are shown on the left in all panels.
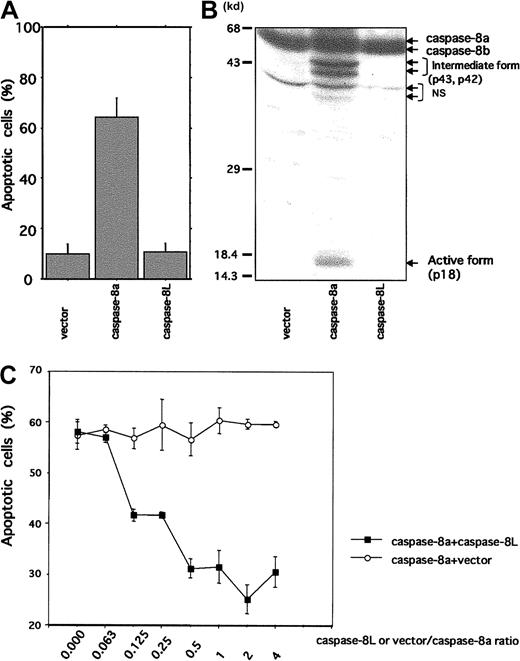
Caspase-8L is not toxic to MCF-7 cells and protects MCF-7 cells from caspase-8–induced apoptosis
Because caspase-8L, which carries N-terminal 2 repeats of DED but lacks catalytic domain, structurally resembles c-FLIP (also called Casper/I-FLICE/FLAME-1/CASH/CLARP/MRIT/Usurpin), we expected that caspase-8L might act as an inhibitor of caspase-8. We therefore performed a transient transfection study using an MCF-7 cell line. As shown in Figure 5A, the MCF-7 cells transfected with caspase-8a exhibited cell death (64.18% ± 7.7%). This was accompanied by the production of intermediate forms (p43 and p42) and an active form (p18) of caspase-8a (Figure 5B). In contrast, no death was observed in the cells transfected with either an empty vector or with caspase-8L (10.03% ± 3.97% and 10.65% ± 3.5%, respectively). In vector- or caspase-8L–transfected cells, active form of caspase-8a was not detected (Figure 5B). These data suggest that caspase-8L itself does not exhibit any cytotoxic activity. We next performed a coexpression study of caspase-8a and -8L by titrating the amount of caspase-8L that effectively inhibits caspase-8a–mediated apoptosis (Figure 5C). The coexpression of caspase-8a and an empty vector at various ratios induced similar levels of apoptosis at approximately 60% (Figure 5C, ○), while coexpression of caspase-8L dose-dependently protected cells from apoptosis induced by caspase-8a. Interestingly, caspase-8L significantly inhibited caspase-8a–mediated apoptosis by approximately 30% even at a ratio of 0.125 (caspase-8L/caspase-8a) (Figure 5C, ▪). These results indicated that caspase-8L did not exhibit any cytotoxic activity like caspase-8a but might act as a dominant negative inhibitor of caspase-8a.
Cell death mediation by caspase-8a and -8L and inhibitory role of caspase-8L in MCF-7 cell lines.
(A) MCF-7 cells were transiently transfected with 1.5 μg β-galactosidase expression vector (pcDNA3.1/His/LacZ, Invitrogen) plus 1.0 μg empty vector (pcDNA3.1/HisA, Invitrogen), caspase-8a, and caspase-8L expression vector as described in “Material and methods.” Twenty-three hours after transfection, the extent of cell death was quantified by determining the portion of β-galactosidase–expressing cells exhibiting apoptotic morphology. They are expressed as the mean percentage of the blue cells exhibiting signs of apoptosis as a fraction of the total number of blue cells counted (about 200 cells per sample). (B) MCF-7 cells were transiently transfected with empty vector, caspase-8a, and caspase-8L expression vector using LipofectAMINE (Life Technologies) as described in “Materials and methods.” Twenty-three hours after transfection, cell lysates were fractionated by SDS-PAGE and blotted with anti–human caspase-8 antibody (MBL), which could detect intermediate form (p43 and p42) and active form (p18) caspase-8. NS indicates nonspecific bands. (C) A total of 0.5 μg caspase-8a expression vector plus various amounts of empty vector or caspase-8L expression vector were transiently transfected with 0.75 μg β-galactosidase expression vector into MCF-7 cells. The ratios of vector or caspase-8L to caspase-8a are indicated on the bottom of the graph. Twenty-three hours after transfection, apoptotic cells were determined as described in “Materials and methods.” In each experiment, the data are from 3 independent experiments. The bar indicates the SE.
Cell death mediation by caspase-8a and -8L and inhibitory role of caspase-8L in MCF-7 cell lines.
(A) MCF-7 cells were transiently transfected with 1.5 μg β-galactosidase expression vector (pcDNA3.1/His/LacZ, Invitrogen) plus 1.0 μg empty vector (pcDNA3.1/HisA, Invitrogen), caspase-8a, and caspase-8L expression vector as described in “Material and methods.” Twenty-three hours after transfection, the extent of cell death was quantified by determining the portion of β-galactosidase–expressing cells exhibiting apoptotic morphology. They are expressed as the mean percentage of the blue cells exhibiting signs of apoptosis as a fraction of the total number of blue cells counted (about 200 cells per sample). (B) MCF-7 cells were transiently transfected with empty vector, caspase-8a, and caspase-8L expression vector using LipofectAMINE (Life Technologies) as described in “Materials and methods.” Twenty-three hours after transfection, cell lysates were fractionated by SDS-PAGE and blotted with anti–human caspase-8 antibody (MBL), which could detect intermediate form (p43 and p42) and active form (p18) caspase-8. NS indicates nonspecific bands. (C) A total of 0.5 μg caspase-8a expression vector plus various amounts of empty vector or caspase-8L expression vector were transiently transfected with 0.75 μg β-galactosidase expression vector into MCF-7 cells. The ratios of vector or caspase-8L to caspase-8a are indicated on the bottom of the graph. Twenty-three hours after transfection, apoptotic cells were determined as described in “Materials and methods.” In each experiment, the data are from 3 independent experiments. The bar indicates the SE.
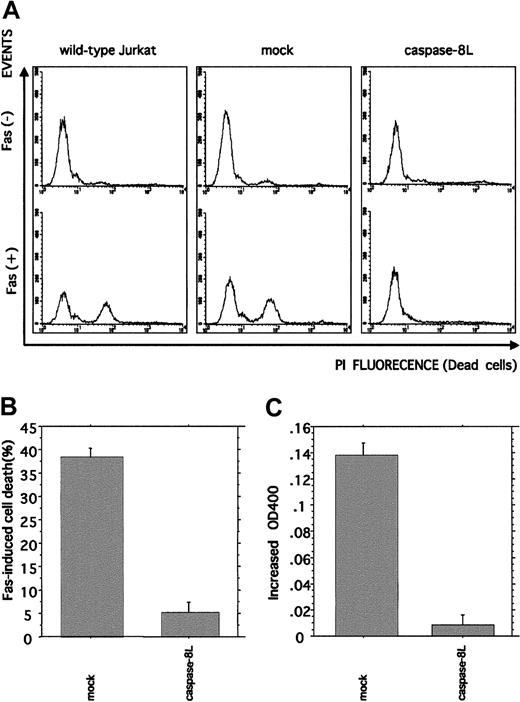
Caspase-8L expression protects Jurkat cells from Fas-mediated apoptosis and interferes with Fas-induced caspase-8 protease activity
To further investigate the caspase-8L function in the Fas-mediated apoptosis pathway, Jurkat cells were stably transfected with caspase-8L. The transfection of empty vector (mock) or caspase-8L expression vector did not influence the expression level of endogeneous caspase-8a assessed by Western blot analysis (data not shown). We induced cytotoxic activity in mock-transfected or caspase-8L–transfected cells by treating them with anti-Fas monoclonal antibody (Figure 6A-B). In mock-transfected Jurkat cells, the administration of anti-Fas antibody induced cell death effectively (38.33% ± 1.86%), which was compatible with wild-type Jurkat cells. In contrast, caspase-8L–transfected Jurkat cells were less sensitive to Fas stimulation (5.17% ± 0.27%). These data suggest that caspase-8L has a protective effect on the Fas-induced apoptosis pathway by inhibiting Fas-induced caspase-8 catalytic activity. We next determined whether or not the caspase-8 catalytic activity is inhibited in caspase-8L–transfected cells (Figure 6C). Caspase-8 catalytic activity was measured by detecting pNA release as described. In mock-transfected cells, Fas stimulation induced the catalytic activity of caspase-8 (0.138 ± 0.01). In contrast, pNA release was hardly detected in caspase-8L–transfected Jurkat cells (0.01 ± 0.01). Taken together, these results indicate that caspase-8L acts as an antiapoptotic molecule by blocking caspase-8 activation.
Flow cytometric analysis of Fas-induced cell death and colorimetric analysis of Fas-induced caspase-8 catalytic activity in caspase-8L–transfected Jurkat cells.
(A,B) Empty vector– or caspase-8L–transfected Jurkat cells and wild-type Jurkat cells were untreated or treated with 0.5 μg/mL mouse anti–human Fas monoclonal antibody (clone CH11, MBL) for 12 hours. After Fas stimulation, the cells were stained with 40 μg/mL propidium iodide, and the percentage of propidium iodide–positive (dead) and –negative (live) cells was determined by FACScan. Fluorescence-activated cell sorter profiles of empty vector– or caspase-8L–transfected Jurkat cells and wild-type Jurkat cells without or with Fas stimulation are shown in panel A. The presented data are representative of experiments using 3 independent clones of each transformant. The percentage of cell death in vector- or caspase-8L–transfected cells is shown in panel B. The data are from experiments using 3 independent clones of each transformant and represent mean ± SD. (C) Colorimetric analysis of caspase-8 catalytic activity. Either empty vector– or caspase-8L–transfected Jurkat cells were left untreated or treated with 0.5 μg/mL mouse anti–human Fas monoclonal antibody for 4 hours, and the caspase-8 catalytic activity was examined by measuring pNA release as described. All data are from experiments using 3 independent clones of each transformant and represent the mean ± SD.
Flow cytometric analysis of Fas-induced cell death and colorimetric analysis of Fas-induced caspase-8 catalytic activity in caspase-8L–transfected Jurkat cells.
(A,B) Empty vector– or caspase-8L–transfected Jurkat cells and wild-type Jurkat cells were untreated or treated with 0.5 μg/mL mouse anti–human Fas monoclonal antibody (clone CH11, MBL) for 12 hours. After Fas stimulation, the cells were stained with 40 μg/mL propidium iodide, and the percentage of propidium iodide–positive (dead) and –negative (live) cells was determined by FACScan. Fluorescence-activated cell sorter profiles of empty vector– or caspase-8L–transfected Jurkat cells and wild-type Jurkat cells without or with Fas stimulation are shown in panel A. The presented data are representative of experiments using 3 independent clones of each transformant. The percentage of cell death in vector- or caspase-8L–transfected cells is shown in panel B. The data are from experiments using 3 independent clones of each transformant and represent mean ± SD. (C) Colorimetric analysis of caspase-8 catalytic activity. Either empty vector– or caspase-8L–transfected Jurkat cells were left untreated or treated with 0.5 μg/mL mouse anti–human Fas monoclonal antibody for 4 hours, and the caspase-8 catalytic activity was examined by measuring pNA release as described. All data are from experiments using 3 independent clones of each transformant and represent the mean ± SD.
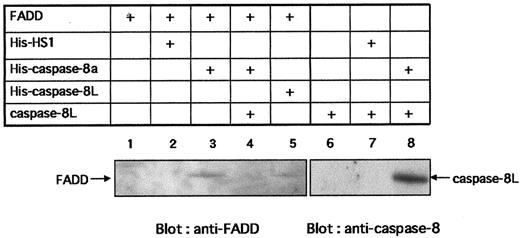
Caspase-8L binds to FADD and caspase-8 and interferes with the binding of caspase-8 to FADD
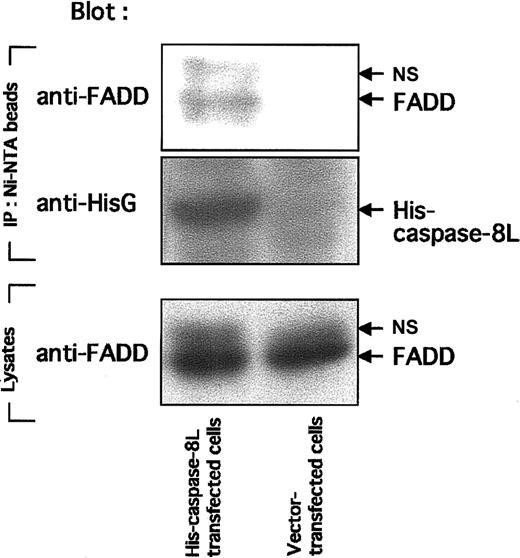
To activate caspase-8, FADD must interact with Fas through its DD and also interact with caspase-8 by its DED. To study the possibility that caspase-8L competes with caspase-8 for binding to FADD, preventing caspase-8 activation, we performed in vitro binding assays. Figure7 shows that in vitro–translated FADD bound to His–caspase-8a (lane 3) and to His–caspase-8L (lane 5), while FADD bound to neither Ni-NTA beads alone nor His-HS1 (lanes 1 and 2). HS1 is a 75 kd intracellular adaptor molecule,44 which is used as a negative control to exclude the possibility of nonspecific binding of FADD to His-tagged proteins. These results demonstrate the specific binding of FADD to caspase-8 or caspase-8L. Interestingly, in the presence of caspase-8L, the interaction of caspase-8a and FADD was abrogated (Figure 7, lane 4). Additionally, in vitro–translated caspase-8L bound to His–caspase-8a directly (Figure 7, lane 8), while caspase-8L bound to neither Ni-NTA beads alone nor His-HS1 (lanes 6 and 7). Taken together, in vitro–translated caspase-8L bound not only FADD but also caspase-8a. Finally, we examined the interaction of caspase-8L and endogeneous FADD in vivo (Figure 8). Endogeneous FADD was detected in the eluate from His-tagged caspase-8L–transfected MCF-7 cells but not from empty vector–transfected cells (Figure 8, upper column), indicating that caspase-8L associates with FADD in vivo. These results and our functional study demonstrated that caspase-8L acts as an endogeneous inhibitor of apoptosis by competing with the caspase-8a/FADD interaction by binding to FADD or to caspase-8a. It is thus strongly suggested that caspase-8L participates in the regulation of apoptosis in vivo.
In vitro interaction of caspase-8L with FADD and caspase-8a and the inhibition of the binding of caspase-8a to FADD by caspase-8L.
HS1, caspase-8a, and caspase-8L fused at its N-terminus with the polyhistidine epitope (His) were generated by in vitro translation (denoted His-HS1, His–caspase-8a, and His–caspase-8L, respectively). FADD and caspase-8L (not tagged) were also prepared by in vitro translation (denoted FADD, caspase-8L, respectively). His-tagged proteins were incubated with Ni-NTA Magnetic Agarose Beads overnight. Ni-NTA beads bearing His-tagged proteins were washed and resuspended in protein reaction buffer. Next, 50 μL in vitro–translated FADD or caspase-8L was added to each reaction mixture. After 2 hours' incubation, the beads were collected on a magnetic separator. After washing, the bound proteins were eluted by adding 50 μL SDS-PAGE buffer, analyzed by SDS-PAGE, and detected with anti-FADD monoclonal antibody (MBL) (lanes 1-5) or with anti–caspase-8 polyclonal antibody (lanes 6-8). For the blocking experiment (lane 4), His–caspase-8 bearing Ni-NTA beads were incubated with in vitro–translated FADD in the presence of 50 μL translated caspase-8L. In vitro–translated FADD (lane 1) or caspase-8L (lane 6) were incubated with Ni-NTA beads to exclude nonspecific binding of FADD or caspase-8L to Ni-NTA beads. The bands corresponding to FADD or caspase-8L are indicated by arrows.
In vitro interaction of caspase-8L with FADD and caspase-8a and the inhibition of the binding of caspase-8a to FADD by caspase-8L.
HS1, caspase-8a, and caspase-8L fused at its N-terminus with the polyhistidine epitope (His) were generated by in vitro translation (denoted His-HS1, His–caspase-8a, and His–caspase-8L, respectively). FADD and caspase-8L (not tagged) were also prepared by in vitro translation (denoted FADD, caspase-8L, respectively). His-tagged proteins were incubated with Ni-NTA Magnetic Agarose Beads overnight. Ni-NTA beads bearing His-tagged proteins were washed and resuspended in protein reaction buffer. Next, 50 μL in vitro–translated FADD or caspase-8L was added to each reaction mixture. After 2 hours' incubation, the beads were collected on a magnetic separator. After washing, the bound proteins were eluted by adding 50 μL SDS-PAGE buffer, analyzed by SDS-PAGE, and detected with anti-FADD monoclonal antibody (MBL) (lanes 1-5) or with anti–caspase-8 polyclonal antibody (lanes 6-8). For the blocking experiment (lane 4), His–caspase-8 bearing Ni-NTA beads were incubated with in vitro–translated FADD in the presence of 50 μL translated caspase-8L. In vitro–translated FADD (lane 1) or caspase-8L (lane 6) were incubated with Ni-NTA beads to exclude nonspecific binding of FADD or caspase-8L to Ni-NTA beads. The bands corresponding to FADD or caspase-8L are indicated by arrows.
In vivo interaction of caspase-8L with FADD in MCF-7 cell lines.
MCF-7 cells (2 × 107 cells) were transiently transfected with 4 μg empty vector (pcDNA3.1/HisA, Invitrogen) and His-tagged caspase-8L expression vector using LipofectAMINE (Life Technologies) as described in “Materials and methods.” Eighteen hours after transfection, cells were lysed and cleared by centrifugation. The supernatants were incubated with Ni-NTA Magnetic Agarose Beads, and Ni-NTA beads were collected using a magnetic separator. After washing, the proteins bound to His-tagged protein were eluted with SDS-PAGE buffer and fractionated by SDS-PAGE. In the upper column, eluates were blotted with anti-FADD antibody. In the middle column, eluates were blotted with anti-HisG antibody to confirm the binding of His-tagged caspase-8L to the Ni-NTA beads. In the bottom column, cell lysates of transfected MCF-7 cells were analyzed by SDS-PAGE and blotted with anti-FADD antibody. NS indicates nonspecific band.
In vivo interaction of caspase-8L with FADD in MCF-7 cell lines.
MCF-7 cells (2 × 107 cells) were transiently transfected with 4 μg empty vector (pcDNA3.1/HisA, Invitrogen) and His-tagged caspase-8L expression vector using LipofectAMINE (Life Technologies) as described in “Materials and methods.” Eighteen hours after transfection, cells were lysed and cleared by centrifugation. The supernatants were incubated with Ni-NTA Magnetic Agarose Beads, and Ni-NTA beads were collected using a magnetic separator. After washing, the proteins bound to His-tagged protein were eluted with SDS-PAGE buffer and fractionated by SDS-PAGE. In the upper column, eluates were blotted with anti-FADD antibody. In the middle column, eluates were blotted with anti-HisG antibody to confirm the binding of His-tagged caspase-8L to the Ni-NTA beads. In the bottom column, cell lysates of transfected MCF-7 cells were analyzed by SDS-PAGE and blotted with anti-FADD antibody. NS indicates nonspecific band.
Discussion
In this study, we cloned and characterized a novel isoform of caspase-8, named caspase-8L, generated by alternative splicing of the human caspase-8 gene. A functional study demonstrated that caspase-8L is an endogeneous inhibitor of the caspase cascade.
Caspase-8L carries N-terminal 2 repeats of DED of the full-length caspase-8 but lacks a C-terminal half catalytic domain (Figure 1C). Our transient transfection assays in MCF-7 cells demonstrated that caspase-8L protected cells from caspase-8–mediated apoptosis in a dominant negative fashion (Figure 5C). In addition, Jurkat cells that stably expressed caspase-8L showed resistance to Fas-mediated cell death and decreased catalytic activity of caspase-8 induced by Fas stimulation (Figure 6). In in vitro binding assays, we demonstrated that caspase-8L directly bound to FADD and caspase-8a and also interferes with the binding of caspase-8a to FADD (Figure 7). Additionally, we showed direct interaction of caspase-8L and FADD in in vivo transfection assay (Figure 8). These results strongly suggest that caspase-8L is a novel antiapoptotic molecule that modulates the activation of caspase-8, the most important and apical enzyme in the caspase cascade. Several isoforms of caspase-8 other than caspase-8L have been previously described.11,12,38 These caspase-8 isoforms have been suggested to function as modulators of the activation of caspase-8 in Fas-induced apoptosis.12Caspase-8c (MACHα3) has been demonstrated to protect against Fas-induced apoptosis, whereas caspase-8d (MACHβ1) was suggested to enhance the cytotoxic activity of the active caspase-8 isoforms (caspase-8a and -8b).12 However, Scaffidi et al demonstrated that only 2 active forms of caspase-8 (caspase-8a and -8b) were predominantly expressed in the cell lines of different origin in their immunoblot assay using a panel of monoclonal antibodies.45 In fact, we were unable to detect other isoforms of caspase-8 (caspase-8∼8h) in various tissues and cell lines by RT-PCR analysis (data not shown). Instead, caspase-8L was expressed in almost all the tissues studied except in brain. This tissue distribution was completely identical to that of caspase-8 (Figure 2A), thus suggesting the importance of caspase-8L in caspase-8 regulation. Moreover, caspase-8L expression is strongly suggested at the protein level. Although the protein expression of caspase-8L seems to be low compared with that of caspase-8a and -8b (Figure 4B), caspase-8L significantly inhibited caspase-8a–mediated apoptosis even at the one-eighth amount of caspase-8a in transient transfection assay (Figure 5C). This dominant negative effect of caspase-8L suggests that caspase-8L primarily participates in the regulation of caspase-8–mediated apoptosis even at a relatively low expression level as in the case of PBLs.
Several structurally and functionally similar molecules have been reported, such as viral proteins, v-FLIPs, and their mammalian homolog, c-FLIPs. In the case of c-FLIPs, there are 2 alternatively spliced forms, c-FLIP–long (c-FLIPL) and c-FLIP–short (c-FLIPS). In addition to the N-terminal 2 repeats of DED, FLIPL possesses a C-terminal domain resembling caspase-8 missing protease activity. The c-FLIPS possesses only 2 DEDs and resembles caspase-8L. Both isoforms bind to FADD or to caspase-8 through DED-DED interaction, which competitively blocked the recruitment of caspase-8 to the DISC.7,19,20,22,23 25 This strict regulation of caspase-8 activation by multiple molecules is conceivable considering the molecular ordering of caspase-8 in caspase cascade.
In fact, DED-containing proteins, such as FADD, c-FLIP, caspase-8d (MACHβ1), and artificially constructed caspase-8 prodomains, induce apoptosis when highly overexpressed.10-12,46 However, it remains controversial as to whether or not these DED-containing proteins have a proapoptotic effect under various conditions. This seemingly opposite effect by DED-containing proteins might be explained by apoptotic activity being a concentration-dependent phenomenon.46 Namely, at lower expression levels, DED-containing proteins block Fas-induced apoptosis by interfering with the recruit of caspase-8 to DISC, whereas when highly overexpressed they can induce apoptosis by the formation of structure called death-effector filaments (DEFs). DEF is an insoluble filamentous perinuclear structure that is generated by the assembly of overexpressed DED-containing proteins and recruits procaspase zymogens for apoptosis. However, the precise concentration of DED-containing proteins, which enables them to form DEF, is still unclear, and further investigation on this issue is needed.
The dysregulation of apoptosis has been implicated for the pathogenesis of disease such as autoimmunity (eg, systemic lupus erythematosus [SLE]) and cancer.5,47 SLE is an autoimmune disease characterized by the production of autoantibodies by the breakdown of self-tolerance and subsequent immune complex deposition and tissue injury in several organ systems.48-50 In patients with SLE, apoptosis of PBLs has been shown to increase in comparison to that of healthy controls.51 In addition, degenerated proteins during apoptosis have been shown to contribute to the supply of autoantigens.52-55 Taken together, the increased apoptosis of PBL in SLE patients has thus been suggested to be associated with the development of SLE by the increased supply of autoantigens and the production of autoantibodies. We previously reported that the expression of caspase-8L mRNA decreased in the PBLs of patients with SLE,39 thus raising the possibility that the increased sensitivity to apoptosis in PBLs from SLE patients is, at least in part, due to the decreased expression of caspase-8L. In addition, we also demonstrated the decreased expression of caspase-8L mRNA in several tumor cell lines (Figure 3B). In normal physiology, the apoptotic machinery must be strictly controlled at several levels to avoid dysregulated cell death, and this control is exerted by several antiapoptotic proteins. In combination with these findings, it is thus speculated that the decreased expression of antiapoptotic caspase-8L in certain tumor cell lines may correlate with an abnormal response of tumor cells to various proapoptotic and antiapoptotic stimuli. There is supporting evidence demonstrating an involvement of caspases and other apoptosis regulators beyond cell death, such as cell-cycle progression.56 Clearly, much remains to be investigated regarding the contribution of the imbalanced expression of caspase-8L to the pathogenesis of these diseases.
In conclusion, caspase-8L is a novel splice variant of caspase-8, expressed in various normal tissues, including PBLs, but not in several cancer cell lines. The expression of caspase-8L protein is strongly suggested in PBLs. In transient transfection assays, caspase-8L could thus contribute to the prevention of apoptosis by an overexpression of caspase-8 in a dominant negative manner. Jurkat cells that stably expressed caspase-8L are resistant to Fas-stimulation, which was associated with a significantly decreased amount of caspase-8 catalytic activity. In vitro binding assays showed that caspase-8L bound to FADD and caspase-8a and prevented the binding of caspase-8 to FADD. The association of caspase-8L and FADD was confirmed in an in vivo system using MCF-7 cells transfected with caspase-8L. These results indicate that caspase-8L is an endogeneous competitive inhibitor of caspase-8—namely, an initiator of cascade. Regulation by their own alternative splicing products might be common in proteins belonging to the caspase cascade due to the fact that a similar inhibitory mechanism has been described in caspase-1, -2, and -9 as well.
We thank Dr Hirohumi Hamada for providing cosmid pAxCAhFADD.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Takahiko Horiuchi, Medicine and Biosystemic Science, Kyushu University Graduate School of Medical Sciences, 3-1-1 Maidashi, Higashi-ku, Fukuoka 812-8582, Japan; e-mail:horiuchi@intmed1.med.kyushu-u.ac.jp.