Recognition of the essential role of dendritic cells (DCs) as professional antigen-presenting cells has prompted investigators to search for methods to use DCs as natural adjuvants in immunotherapy. A number of antigenic oligopeptides, recognized by CD8+cytotoxic T lymphocytes (CTLs) specific for cancer cells, have been applied in clinical trials using DCs. Such a monovalent vaccine with a single epitope for a particular type of HLA class 1 molecule would be effective. However, a polyvalent vaccine might be more potent. We designed a novel protein delivery system consisting of hydrophobized polysaccharides complexed with target proteins. The truncated HER2 protein encompassing 147 N-terminal amino acids, including the 9-mer HER2p63-71 peptide (HER2p63), TYLPTNASL, the human homologue of an antigenic murine tumor rejection peptide, was prepared. We report here that HLA-A2402+ DCs could incorporate hydrophobized polysaccharide–truncated HER2 protein complexes and process the protein to present major histocompatibility complex class 1-binding HER2p63 peptide. The complexes enter DCs by phagocytosis, and then the truncated protein is processed through a pathway similar to that for endogenous proteins. DCs sensitized by these complexes primed and boosted HER2p63-specific CD8+T cells in the context of HLA-A2402. Vaccination with DCs incorporating these complexes completely suppressed lung metastases in a HER2-expressing murine tumor model. We also generated 3 CD4+ clones reactive with different HER2- derived 25-mer peptides from lymph node cells in mice treated with CHP/HER2-147. Thus, hydrophobized polysaccharide–protein complexes are promising candidates for the construction of polyvalent vaccines.
Introduction
Recent studies have demonstrated that dendritic cells (DCs) act as professional antigen-presenting cells (APCs) in host immune responses and are particularly more important than other APCs in priming naive T-cell populations for the cognate antigen peptide.1-4 The use of DCs for cancer immunotherapy as natural adjuvants for defined tumor antigen peptides has been explored. In fact, vaccination of tumor-bearing mice with target antigen peptide-pulsed DCs is effective in suppressing tumor growth.5-9 Based on these encouraging results in experimental tumor models, early-phase clinical trials with peptide-pulsed DCs are under way for patients with melanoma and other types of cancer.10-14
T cells recognize specific peptides bound to self-major histocompatibility complex (MHC) molecules, a fundamental feature of antigen recognition by T cells. This unique characteristic of antigen recognition by T cells indicates that a particular oligopeptide can be antigenic, depending on HLA type. Despite the identification of an increasing number of antigenic peptides that bind to various HLA types, the use of these oligopeptides for cancer vaccine is restricted by the expression of proteins with target peptide sequences in tumor cells and the HLA type of patients.
In comparison with oligopeptides used as monovalent vaccine with a single epitope, polyvalent vaccines with multiple epitopes are more desirable. Protein molecules or their cDNAs may be used to load DCs with multiple potential epitopes binding to various types of HLA. However, exogenous soluble-protein antigens incorporated by APCs are in general inefficient in sensitizing CD8+ T lymphocytes because the proteins are hardly processed by the MHC class 1 pathway. Instead they are internalized into endosomes and are then taken into the MHC class 2 pathway.15-17 To overcome this issue, we designed a novel simple protein delivery system consisting of hydrophobized polysaccharide, pullulan (CHP) or mannan (CHM), complexed with soluble protein molecules.18-20 As reported previously in murine tumor systems, we prepared the truncated HER2 protein containing 147 amino acid residues from N-terminus (HER2-147), including the 9-mer HER2p63-71 peptide (HER2p63), TYLPTNASL, the human homologue of a murine tumor rejection antigen peptide, TYLPANASL, and its complexes with CHP (CHP/HER2-147) or CHM (CHM/HER2-147).21,22 When BALB/c mice were immunized with CHP/HER2-147 or CHM/HER2-147 complexes, HER2-specific CD8+cytotoxic T lymphocytes (CTLs) recognizing HER2p63 peptide with restriction of Kd were generated.21 Murine and human MHC class 1 molecules, H-2Kd and HLA-A2402, have some similarity in their anchor motifs for peptide binding,23,24 and the HER2p63 amino acid sequence is compatible with binding to HLA-A2402. In fact, HER2-specific human CTLs restricted with HLA-A2402 were induced by the use of HER2p63 peptide.25
With the aim of designing more advanced strategies for cancer vaccines, we investigated in the present study whether human DCs prepared from persons with HLA-A2402+ can incorporate and process CHP/HER2-147 complexes and then present HER2-specific peptide on HLA-A2402 molecules. The therapeutic effects of DCs incorporating CHP/HER2-147 complexes were studied using in vivo murine models. We also established 3 different HER2-specific CD4+ T-cell clones from mice treated with the complexes. Our results suggest the potential usefulness of CHP/HER2-147 complexes as a polyvalent vaccine.
Materials and methods
Cell lines
SKOV3, which expresses HER2 but not HLA-A2402 or HLA-A0201, is a human ovarian cancer cell line supplied by the American Type Culture Collection (Rockville, MD). The cDNAs of HLA-A2402 and -A0201 inserted into the pcDNA3.1 vector were transfected into SKOV3. T2-A24 is a B-blast line T2-transfected with HLA-A2402 cDNA.26 CMS5mHE is a transfectant of human HER2 cDNA to CMS5m, a HER2-negative sarcoma line of BALB/c origin.
Antibodies and immunostaining
Human.
Anti–HLA-A02 (MA2.1), anti–HLA-A24 (A11.1M), anti–HLA-A, -B, -C (W6/32), and anti–HLA-DR (HDR1) monoclonal antibodies (mAbs) were gifts from Dr K. Itoh (Kurume University, Japan). Anti–HLA-A30, -A31 (0273HA) mAb was purchased from One Lambda (Los Angeles, CA). Anti-CD1a, anti-CD14, anti-CD80, and anti-CD86 mAbs were from Becton Dickinson (San Jose, CA). Anti-CD83 mAb was purchased from Immunotech (Marseilles, France). Anti-CD3 was from Ortho Biotech (Raritan, NJ). Mouse IgG1, IgG2 (Becton Dickinson), or IgG2b (Coulter, Hialeah, FL) served as an isotype control. Antihuman interferon-γ (IFN-γ) mAbs, 1-D1K, and biotinylated antihuman IFN-γ mAb, 7-B6-1, were purchased from Mabtech (Stockholm, Sweden).
Murine.
Anti-L3T4/CD4 (GK1.5), anti-Lyt2.2/CD8 (19/178), anti-B220/CD45R (RA3-3A/6.2), anti–H-2Kd (20-8-4), and anti–I-Ad (MKD6) mAbs were purified from supernatant of hybridomas. Anti–ICAM-1/CD54, anti–LFA-1, anti–Mac-1/CD11b, anti-B7.1/CD80, and anti-B7.2/CD86 were purchased from PharMingen (San Diego, CA). Anti-DEC205 (NLDC-145) mAb was a gift from Dr B. Kraal (Leiden University, The Netherlands).
For membrane staining, the cells were stained with fluorescence-conjugated mAbs for 30 minutes on ice. In some experiments, the cells were stained with mAbs, washed 3 times with Ca++-, Mg2+-free phosphate-buffer saline (PBS), and incubated with fluorescence-conjugated rat antimouse mAbs for 30 minutes. The phenotype of cell samples was analyzed using a FACScan flow cytometer (Becton Dickinson). Data analysis was performed with CellQuest software (Becton Dickinson).
Reagents
Human HER2-derived peptides, HER2p63 (amino acids 63-71, TYLPTNASL) and HER2p780 (amino acids 780-788, PYVSRLLGI), were synthesized by Sawady Technology (Tokyo, Japan).22 The truncated HER2 protein encompassing 147 N-terminal amino acids, HER2-147, was prepared as described previously.21Complexes of CHP and either of recombinant HER2-147 or carbonic anhydrase 2 (CAB), CHP/HER2-147 and CHP/CAB, were synthesized as described previously.18-20 Ten 25-mer peptides of HER2, starting from the N-terminus and overlapping by 10 residues, were also synthesized by Sawady Technology.
Lactacystin, a specific proteasome β subunit inhibitor, was purchased from Kyowa Medix (Tokyo, Japan).27-29 Cytochalasin D, amiloride, chloroquine, brefeldin A, and cycloheximide were purchased from Sigma Chemical (St Louis, MO). Cytochalasin D disrupts actin microfilaments and blocks phagocytosis. Amiloride, the Na+/H+ channel inhibitor, inhibits macropinocytosis.30 Chloroquine inhibits protein hydrolysis by cathepsins.31,32 Brefeldin A blocks the exocytosis of protein from the endoplasmic reticulum and Golgi complex and, thus, prevents newly assembled peptide–MHC class 1 complexes from reaching the cell surface.33,34 Cycloheximide blocks protein biosynthesis and, hence, inhibits newly synthesized MHC molecules within the endoplasmic reticulum of the APC.35Purified recombinant human and murine granulocyte macrophage–colony-stimulating factor (GM-CSF) were generous gifts from Kirin Brewery (Tokyo, Japan). Purified recombinant human interleukin-4 (IL-4) was kindly provided by Ono Pharmaceutical (Osaka, Japan). Purified recombinant human tumor necrosis factor-α (TNF-α) was a gift from Dainippon Pharmaceutical (Suita, Japan). Purified recombinant IL-2 was provided by Takeda Pharmaceutical (Osaka, Japan). Concentrations of cytokines used in this study were as follows: human GM-CSF, 10 ng/mL; murine GM-CSF, 10 ng/mL; IL-4, 10 ng/mL; TNF-α, 20 ng/mL; and IL-2, 20 IU/mL.
Preparation of human monocyte–derived DCs
Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized whole blood of consenting healthy donors by density gradient centrifugation with Ficoll-Hypaque (Pharmacia, Uppsala, Sweden). DCs were prepared from CD14+ cells in PBMCs, as described previously.36 Briefly, CD14+ cells were separated from PBMCs using the MACS CD14 Microbeads (Miltenyi Biotec, Auburn, CA). CD14+ cells were cultured at a density of 5 × 105 cells/mL in 6-well plates (Nunc, Roskilde, Denmark) in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS; Interoen, New York, NY) in the presence of GM-CSF and IL-4. After a 7-day culture period, the cells were harvested and used as immature DCs. Approximately 75% to 85% of the cells had typical immature DC morphology and displayed typical cell surface markers of immature DCs—CD1a+, CD14−, CD80+, CD86+, HLA-A, -B, -C+, HLA-DR+, and CD83low. We also cultured CD14+ cells in the presence of GM-CSF and IL-4 for 5 days, followed by TNF-α for 2 additional days. The cells obtained expressed high levels of CD80, CD86, HLA-A, -B, -C, HLA-DR, and CD83, a finding consistent with the features of mature DCs.
Sensitization of human monocyte–derived DCs by CHP/HER2-147 complexes or peptides
DCs (1 × 106) were incubated in 10% FCS RPMI 1640 with 20 μg/mL CHP/HER2-147, CHP/CAB, HER2-147 protein, or CAB protein for 3 hours at 37°C in a humidified atmosphere flushed with 5% CO2, followed by washing 3 times with 10% FCS RPMI 1640 and reculturing in 10% FCS RPMI 1640 with GM-CSF and IL-4 for 18 hours until CTL assay.37 DCs, PBMCs, or T2-A24 cells (1 × 106) were pulsed with 10 μM peptides for 1 hour at room temperature and an additional hour at 37°C and were used as stimulator and target cells.
Treatment of sensitized DCs with inhibitors
DCs were cultured with 30 μg/mL cytochalasin D, 0.2 mM amiloride, 100 mM chloroquine, 100 μM lactacystin, 5 μg/mL brefeldin A, or 10 μg/mL cycloheximide for 1 hour at 37°C in a serum-free AIM-V medium (Gibco-BRL, Grand Island, NY) and were incubated with or without CHP/HER2-147 in the presence of the inhibitors for 3 hours. Then they were washed 3 times with PBS and cultured in 10% FCS RPMI 1640 supplemented with GM-CSF and IL-4 for 18 hours. DCs cultured without CHP/HER2-147 were pulsed with HER2p63 for 1 hour at room temperature and an additional hour at 37°C. They were then harvested and used for antigen presentation.
Generation of human killer cells and CD8+ T-cell clones
PBMCs (1 × 106 cells/well) were cultured with autologous HER2p63 peptide- or CHP/HER2-147–pulsed and irradiated (46 Gy) DCs (5 × 105 cells/well) in 24-well plates (Nunc) in RPMI 1640 medium supplemented with 10% heat-inactivated human AB serum. After a 7-day culture period, the cells were restimulated with HER2p63 peptide- or CHP/HER2-147–pulsed, irradiated DCs and irradiated (33 Gy) PBMCs (1 × 106 cells/well). IL-2 was added on days 1 and 4 after restimulation. Subsequent restimulation was performed weekly with HER2p63 peptide-pulsed, irradiated PBMCs and irradiated (80 Gy) allogeneic Epstein-Barr virus–transformed B lymphoblastoid cell lines (LCLs; 1 × 106 cells/well). Responder populations were tested for their cytotoxic activity against HER2-expressing cells at the end of the second restimulation culture period. Before the fourth stimulation, CD8+ T cells were separated using the MACS CD8 Microbeads (Miltenyi Biotec).
CTL clones were established as described previously, with some modifications.9,25 38 Cloning was performed by limiting dilution at 0.3 to 0.5 cells/well of CD8+ killer T cells in 96-well round-bottom plates (Nunc) in the presence of irradiated autologous PBMCs (5 × 104 cells/well), irradiated LCLs (1 × 104 cells/well), IL-2 (20 IU/mL), and anti-CD3 mAb (30 ng/mL). CD8+ killer T-cell clones that specifically lysed HER2-expressing cells were expanded in the presence of irradiated PBMCs, irradiated LCLs, and anti-CD3 mAbs.
Chromium Cr51 release cytotoxicity assay
Cytotoxicity assays were performed, as described previously.39 Target cells labeled with 100 μCi chromium (3.7 × 106 Bq) Cr51(1 × 104 cells) were incubated with serial numbers of effector cells in 96-well V-bottom plates (Nunc) at 37°C. After 5 hours, 100 μL supernatant was collected, and the mean percentage of specific lysis of triplicate wells was calculated as follows: % specific lysis = [(cpm experimental release − cpm spontaneous release)/(cpm detergent release − cpm spontaneous release)] × 100.
Antigen presentation assay
We fixed DCs onto the flat bottoms of 96 wells by incubation in 1% paraformaldehyde PBS for 10 minutes at room temperature and quenched the well with 0.1 M glycine PBS for 10 minutes. The wells were washed thoroughly with medium. CTLs (5 × 104 cells/well) were cultured with DCs (105 cells/well) fixed in 96-well round-bottom plates in 200 μL 10% FCS RPMI 1640 for 18 hours. IFN-γ production by CTL was measured in duplicate using an ELISA kit (Endogen, Woburn, MA).
Enzyme-linked immunospot assay
Ninety-six–well nitrocellulose enzyme-linked immunospot (ELISPOT) plates (MAHA S4510; Millipore, Bedford, MA) were coated with 75 μL antihuman IFN-γ mAb (1-D1K) at a concentration of 2 μg/mL in PBS overnight at 4°C. The plates were washed 6 times with PBS and treated with 10% human AB serum RPMI 1640 for 2 hours to block nonspecific binding. PBMCs were stimulated with autologous DCs at 1-week intervals for 3 weeks. After stimulation, CD8+ T cells were separated from cultured PBMCs, using MACS CD8 Microbeads (Miltenyi Biotec), and were used as the effector cells. In the next step, 5 × 104 effector cells and 1 × 105peptide-pulsed T2/A24 cells were placed in each well of the ELISPOT plate. After incubation for 18 hours at 37°C with 5% CO2, the plates were extensively washed 6 times with PBS containing 0.05% Tween 20 (PBS-Tween). They were then incubated with 75 μL biotinylated antihuman IFN-γ mAb (7-B6-1) at a concentration of 0.2 μg/mL in PBS for 2.5 hours at 37°C, washed 6 times with PBS-Tween, and reacted with 1.0 μg/mL streptavidin–alkaline phosphatase conjugate (Mabtech) in 75 μL PBS for 90 minutes at room temperature. Wells were washed 6 times with PBS-Tween and then stained with an alkaline phosphatase conjugate substrate kit (BioRad, Hercules, CA). The reaction was stopped by rinsing the plates with distilled water. After drying the plates, the spots were counted using the Axioplan 2 imaging system (Carl Zeiss Vision, Hallbergmoos, Germany).
In vivo tumor model
Seven-week-old female BALB/c mice were obtained from Japan SLC (Hamamatsu, Japan). Bone marrow–derived DCs were prepared, as described previously.14 40 Briefly, single-cell suspensions were obtained from pooled femurs and tibiae, and the marrow cells were enriched for progenitors by negative selection using a cocktail of mAbs including anti-CD4, anti-CD8, anti-B220, and anti-Ia antibodies and rabbit complement (Cedarlane, Ontario, Canada). The enriched cells were cultured at a density of 1 × 106cells/mL in 6-well plates in RPMI 1640 medium supplemented with 10% FCS in the presence of murine GM-CSF. Cultures were incubated for 7 days at 37°C in a humidified atmosphere flushed with 5% CO2. On days 3 and 5 of culture, floating cells were removed and half the culture media were replaced with fresh medium containing murine GM-CSF. Nonadherent and loosely adherent cells were collected and replated at a density of 106 cells/mL in a 100-mm Petri dish (Nunc). After a 10-day culture period, the cells were harvested. Approximately 80% to 90% of the cells had DC morphology. These cells expressed high levels of Kd, Ia, CD86, CD54, and LFA-1 and moderate levels of CD80, CD11b, and DEC-205, which is typical for murine mature DCs. Seven-week-old female BALB/c mice were inoculated with 1 × 106 human HER2-expressing tumor cells, CMS5mHE cells, in a total volume of 0.1 mL through the tail vein. Four days later, subcutaneous vaccination with 4 × 105 of DCs alone, DCs pulsed with HER2p63, or DCs incorporating CHP/HER2-147 was performed twice at a 1-week interval. One week after the second vaccination, the mice were killed. Lungs were rinsed with PBS and fixed in 10% neutral-buffered formalin. Tumor nodules in the lungs were counted using a dissecting microscope. Lungs were prepared for histopathologic examination using standard methods.
Generation of murine CD4+T-cell lines and clones and their proliferation assay
Seven-week-old female BALB/c mice were used. CHP/HER2-147 at 10 μg was subcutaneously administered through footpads. After 10 days, single-cell suspensions were prepared from popliteal lymph nodes of the mice. First, the lymph node cells (1 × 106cells/well) were cultured with 20 μg/mL CHP/HER2-147 alone in 24-well plates in 10% FCS RPMI 1640 for 1 week. Cells were restimulated in 10% FCS RPMI 1640 with syngeneic CHP/HER2-147–pulsed spleen cells (5 × 106 cells/well) that had been treated with 50 μg/mL mitomycin C (Sigma Chemical) for 30 minutes at 37°C. For approximately 3 months, these cells were stimulated every week with the mitomycin C–treated spleen cells that had been pulsed with CHP/HER2-147. Responding cells were screened for the expression of CD4 by flow cytometry. Cloning of the proliferating population was performed by limiting dilution in 96-well round-bottom plates containing CHP/HER2-147–pulsed and mitomycin-treated spleen cells. Three CD4+ clones, A6, A8, and C8, were generated.
In the next step, 5 × 106 mitomycin C–treated spleen cells were reacted in 10% FCS RPMI 1640 with 20 μg/mL CHP/HER2-147 or CHP/CAB for 3 hours at 37°C in a humidified atmosphere flushed with 5% CO2, washed 3 times with 10% FCS RPMI 1640, recultured in 10% FCS RPMI for 18 hours, and used as stimulator cells in the proliferation assay. Furthermore, 5 × 106mitomycin C–treated spleen cells were also pulsed with 10 μM 25-mer peptides for 1 hour at room temperature and an additional hour at 37°C and were used as stimulator cells. The stimulator cells (5 × 105) were cultured in 96-well tissue culture plates (Nunc) in 100 μL RPMI 1640 supplemented with 10% FCS with 1 × 105 CD4+ clones as responding cells. Plates were incubated for 5 days at 37°C with 5% CO2. To assess the proliferation of CD4+ clones, tritiated thymidine (3H-TdR; specific activity, 25 Ci/mM [0.925 MBq]) uptake was carried out by using a modification of the method described previously.37 On day 5, the cells were pulsed with .037 MBq/well of 3H-TdR for the last 18 hours of incubation. Cells were harvested onto nitrocellulose filters using an automatic cell harvester, and radioactivity was measured in a scintillation counter. The results were expressed as the mean count per minute of duplicate experiments.
Statistics
Student t test was used to determine statistical significance.
Results
Monocyte-derived DCs can incorporate CHP/HER2-147 and present HER2p63 peptide with HLA-A2402
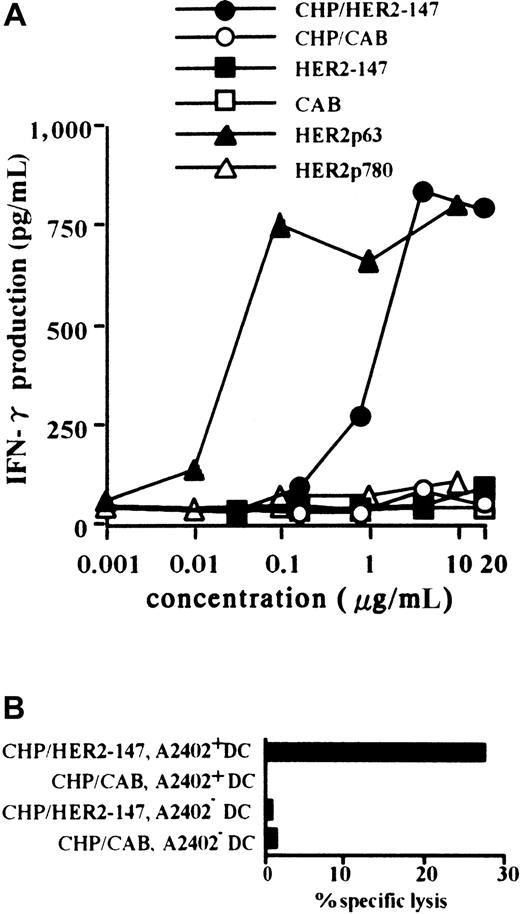
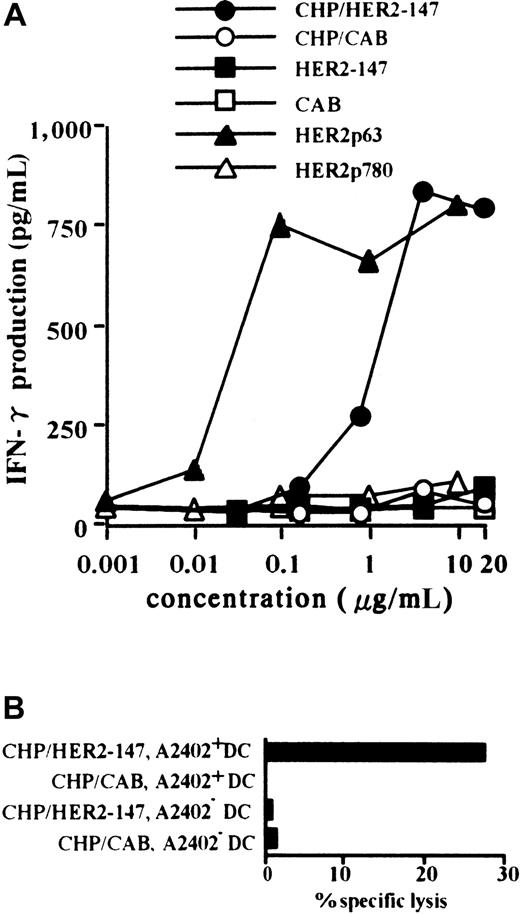
Primary CTLs were established by PBMCs from a person with HLA-A2402+ using autologous DCs pulsed with HER2p63. A T-cell clone, Y.I.1a, which recognizes HER2p63 peptide in the context of HLA-A2402, was generated, as described in “Materials and methods.” Monocyte-derived DCs were prepared from CD14+cells in PBMCs obtained from persons with HLA-A2402+ and HLA-A2402−. DCs were incubated with CHP/HER2-147 or CHP/CAB and were used as target cells of HER2p63 peptide-specific Y.I.1a cells. HLA-A2402+ DCs were treated with 0.001 to 20 μg/mL CHP/HER2-147, CHP/CAB, HER2-147 protein, CAB protein, HER2p63, or HER2p780 peptides, and their stimulatory activity on the CTL clone, Y.I.1a, was examined. DCs treated with CHP/HER2-147 or HER2p63 stimulated the CTL clone, Y.I.1a, in a dose-dependent manner, as determined by the production of IFN-γ (Figure1A). The stimulatory effects of CHP/HER2-147 and HER2p63 reached a plateau at the concentrations of 2 and 0.1 μg/mL, respectively. This finding is in agreement with the fact that the relative concentration of amino acids in CHP/HER2-147 is approximately 0.06 (9 of 147) because CHP/HER-147 and HER2p63 contain 147 and 9 amino acids, respectively. DCs treated with CHP/CAB, HER2-147 protein, CAB protein, or HER2p780 peptides did not increase the production of IFN-γ by Y.I.1a. As shown in Figure 1B, HLA-A2402+ DCs treated with CHP/HER2-147, but not those treated with CHP/CAB, were lysed by the CTL clone at an effector-target ratio of 2.5. HLA-A2402− DCs treated with CHP/HER2-147 or CHP/CAB were not susceptible to Y.I.1a cells. Similar results were obtained at effector-target ratios of 5 and 10 (data not shown). These findings suggest that DCs incorporating CHP/HER2-147 present HER2p63 peptide on HLA-A2402 molecules. Treatment of DCs with CHP/HER2-147 or CHP/CAB did not modify the cell surface markers, including CD80, CD86, HLA-A, B, C, HLA-DR, and CD83 (data not shown), implying that DCs incorporating CHP/HER2-147 or CHP/CAB retained the phenotype of immature DCs.
DCs incorporating CHP/HER2-147 effectively present HER2p63 peptide on HLA-A2402 molecules.
(A) HLA-A2402+ DC (1 × 106) were cultured with 0.001 to 20 μg/mL CHP/HER2-147, CHP/CAB, HER2-147 protein, HER2p63, or HER2p780 at room temperature for 1 hour and were recultured for an additional hour at 37°C. DCs (1 × 105cells/well) were incubated with 5 × 104 cells/well Y.I.1a for 18 hours, and IFN-γ production was measured in duplicate using an ELISA kit. Data represent the average of duplicate measurements. (B) HLA-A2402+ (1 × 106) and HLA-A2402− DCs (1 × 106) were cultured with 20 μg/mL protein CHP/HER2-147 or CHP/CAB for 3 hours at 37°C, washed, recultured in 10% FCS RPMI 1640 alone for an additional 18 hours, and incubated with the CTL clone, Y.I.1a, for 5 hours at an effector-target ratio of 2.5. Cytotoxic activity was measured using 51Cr release assay.
DCs incorporating CHP/HER2-147 effectively present HER2p63 peptide on HLA-A2402 molecules.
(A) HLA-A2402+ DC (1 × 106) were cultured with 0.001 to 20 μg/mL CHP/HER2-147, CHP/CAB, HER2-147 protein, HER2p63, or HER2p780 at room temperature for 1 hour and were recultured for an additional hour at 37°C. DCs (1 × 105cells/well) were incubated with 5 × 104 cells/well Y.I.1a for 18 hours, and IFN-γ production was measured in duplicate using an ELISA kit. Data represent the average of duplicate measurements. (B) HLA-A2402+ (1 × 106) and HLA-A2402− DCs (1 × 106) were cultured with 20 μg/mL protein CHP/HER2-147 or CHP/CAB for 3 hours at 37°C, washed, recultured in 10% FCS RPMI 1640 alone for an additional 18 hours, and incubated with the CTL clone, Y.I.1a, for 5 hours at an effector-target ratio of 2.5. Cytotoxic activity was measured using 51Cr release assay.
Induction of a HER2p63 peptide-specific, HLA-A2402–restricted CTL clone by HLA-A2402+ DCs incorporating CHP/HER2-147
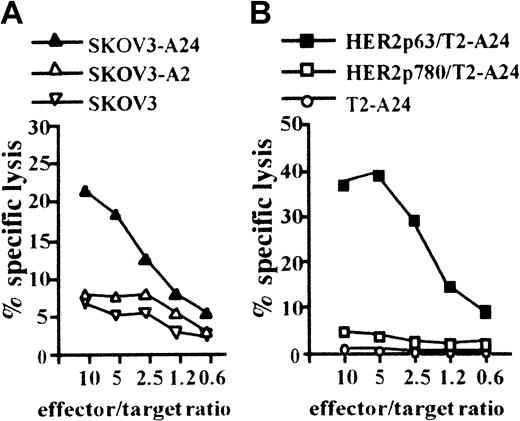
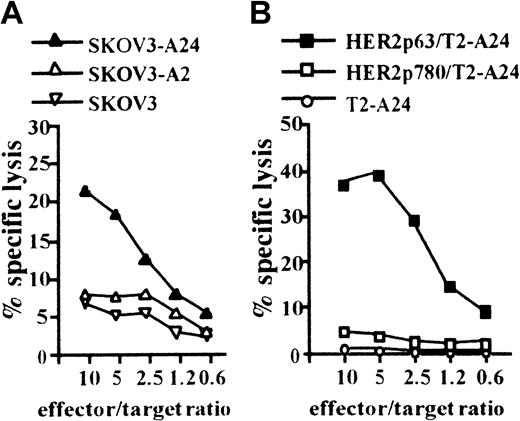
We next attempted to induce a CTL clone specific for HER2 using HLA-A2402+ DCs incorporating CHP/HER2-147. PBMCs obtained from a person with HLA-A2402+ were stimulated with autologous DCs treated with CHP/HER2-147. A CTL clone, PHYI8/63, was generated, and its specificity for HER2 and restriction to HLA-A2402 were examined. The CTL clone, PHYI8/63, lysed the human HER2-expressing ovarian cancer cell line, SKOV3, transfected with HLA-A2402 cDNA (SKOV3-A24) but not SKOV3 transfected with HLA-A0201 cDNA (SKOV3-A2) or HLA-A2402–negative parental SKOV3 (Figure2A). We also confirmed using flow cytometry that the expression level of HLA-A2402 on SKOV3-A24 was almost identical to that of HLA-A0201 on SKOV3-A2 (data not shown). In addition, PHYI8/63 cells also lysed HER2− T2-A24 pulsed with HER2p63 but not T2-A24 pulsed with HER2p780 or without peptides (Figure 2B).
CTL clone, PHYI8/63, generated by DCs treated with CHP/HER2-147, is specific for HER2p63.
(A) To assay the cytotoxicity of PHYI8/63 cells, 1 × 104HER2-expressing ovarian cancer cells, SKOV3, transfected with HLA-A2402 cDNA (SKOV3-A24), SKOV3 transfected with HLA-A0201 cDNA (SKOV3-A2), and HLA-A2402− HLA-A0201− parental SKOV3 were incubated with PHYI8/63 cells at serial effector-target ratios for 5 hours. Cytotoxic activity was measured using 51Cr release assay. (B) HLA-A2402+HER2− T2-A24 cells (1 × 104) were pulsed with 10 μM HER2p63 (HER2p63/T2-A24) or HER2p780 (HER2p780/T2-A24) for 1 hour at room temperature and for an additional hour at 37°C. PHYI8/63 cells were incubated with HER2p63/T2-A24, HER2p780/T2-A24, or T2-A24 at serial effector-target ratios for 5 hours. Cytotoxic activity was measured using 51Cr release assay.
CTL clone, PHYI8/63, generated by DCs treated with CHP/HER2-147, is specific for HER2p63.
(A) To assay the cytotoxicity of PHYI8/63 cells, 1 × 104HER2-expressing ovarian cancer cells, SKOV3, transfected with HLA-A2402 cDNA (SKOV3-A24), SKOV3 transfected with HLA-A0201 cDNA (SKOV3-A2), and HLA-A2402− HLA-A0201− parental SKOV3 were incubated with PHYI8/63 cells at serial effector-target ratios for 5 hours. Cytotoxic activity was measured using 51Cr release assay. (B) HLA-A2402+HER2− T2-A24 cells (1 × 104) were pulsed with 10 μM HER2p63 (HER2p63/T2-A24) or HER2p780 (HER2p780/T2-A24) for 1 hour at room temperature and for an additional hour at 37°C. PHYI8/63 cells were incubated with HER2p63/T2-A24, HER2p780/T2-A24, or T2-A24 at serial effector-target ratios for 5 hours. Cytotoxic activity was measured using 51Cr release assay.
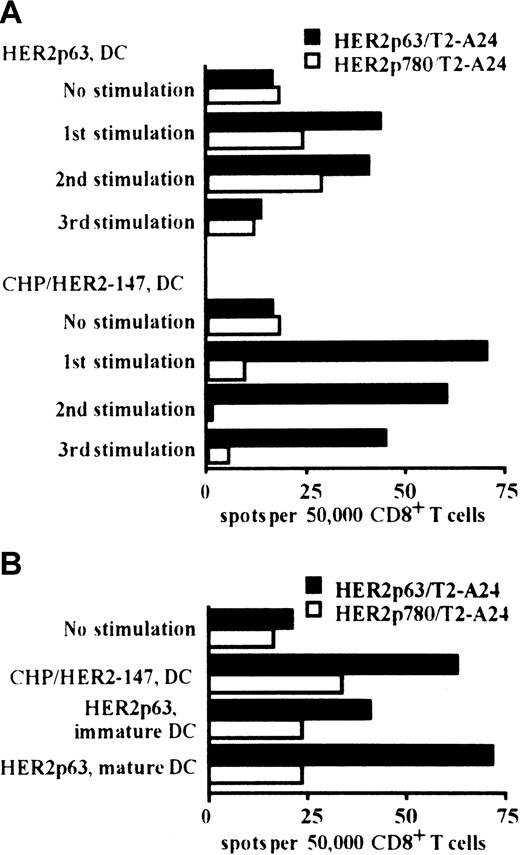
Comparative assessment of HER2p63-specific T-cell induction by DCs incorporating CHP/HER2-147 or DCs pulsed with HER2p63
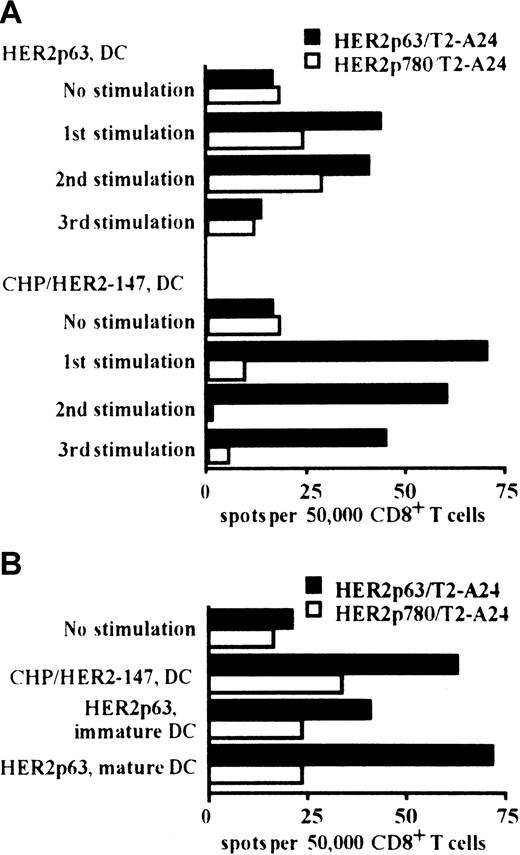
We compared the induction of HER2p63-specific CD8+ T cells by DCs incorporating CHP/HER2-147 and pulsed with HER2p63. HLA-A2402+ PBMCs were stimulated at 1-week intervals for 3 weeks with autologous DCs incorporating CHP/HER2-147 or pulsed with HER2p63. As shown in Figure 3A, DCs incorporating CHP/HER2-147 or pulsed with HER2p63 induced HER2p63-specific IFN-γ spot-forming CD8+ cells. In the experiments following 1, 2, or 3 stimulations, the potential of DCs incorporating CHP/HER2-147 to induce HER2p63-specific IFN-γ spot-forming CD8+ cells was apparently high, compared with that of DCs pulsed with HER2p63. In contrast, the number of nonspecific IFN-γ spot-forming CD8+ cells induced by DCs incorporating CHP/HER2-147 was lower than that induced by DCs pulsed with HER2p63 when control HER2p780-pulsed T2-A24 cells were used as target cells. These findings indicate that CHP/HER2-147–treated DCs induce HER2p63-specific T cells more efficiently than HER2p63-pulsed DCs.
DCs incorporating CHP/HER2-147 induce HER2p63-specific CD8+ T cells.
(A) HLA-A2402+ PBMCs were stimulated with autologous DCs either incorporating CHP/HER2-147 or pulsed with HER2p63 for 3 weeks at 1-week intervals. One, 2, and 3 weeks later, 5 × 104CD8+ T cells and either of 1 × 105 HER2p63- or HER2p780-pulsed T2-A24 cells were placed in each well of the ELISPOT plate. After 18 hours, IFN-γ spot-forming cells were assessed, as described in “Materials and methods.” Each bar represents the average of duplicates. (B) HLA-A2402+ PBMCs were stimulated with autologous immature DCs incorporating CHP/HER2-147 or pulsed with HER2p63 or autologous mature DCs pulsed with HER2p63 for 2 weeks at 1-week intervals. Fifty thousand CD8+ T cells and either 1 × 105 HER2p63- or HER2p780-pulsed T2-A24 cells were placed in each well of the ELISPOT plate. After 18 hours, IFN-γ spot-forming cells were assessed, as described in “Materials and methods.” Each bar represents the average of duplicate measurements.
DCs incorporating CHP/HER2-147 induce HER2p63-specific CD8+ T cells.
(A) HLA-A2402+ PBMCs were stimulated with autologous DCs either incorporating CHP/HER2-147 or pulsed with HER2p63 for 3 weeks at 1-week intervals. One, 2, and 3 weeks later, 5 × 104CD8+ T cells and either of 1 × 105 HER2p63- or HER2p780-pulsed T2-A24 cells were placed in each well of the ELISPOT plate. After 18 hours, IFN-γ spot-forming cells were assessed, as described in “Materials and methods.” Each bar represents the average of duplicates. (B) HLA-A2402+ PBMCs were stimulated with autologous immature DCs incorporating CHP/HER2-147 or pulsed with HER2p63 or autologous mature DCs pulsed with HER2p63 for 2 weeks at 1-week intervals. Fifty thousand CD8+ T cells and either 1 × 105 HER2p63- or HER2p780-pulsed T2-A24 cells were placed in each well of the ELISPOT plate. After 18 hours, IFN-γ spot-forming cells were assessed, as described in “Materials and methods.” Each bar represents the average of duplicate measurements.
The capacity to induce HER2p63-specific IFN-γ spot-forming CD8+ cells was also compared among DCs incorporating CHP/HER2-147, HER2p63-pulsed immature DCs, and HER2p63-pulsed mature DCs (Figure 3B). DCs incorporating CHP/HER2-147 and HER2p63-pulsed mature DCs exhibited efficient induction of HER2p63-specific IFN-γ spot-forming CD8+ cells, compared with HER2p63-pulsed immature DCs. The potential of DCs incorporating CHP/HER2-147 was similar to that of HER2p63-pulsed mature DCs.
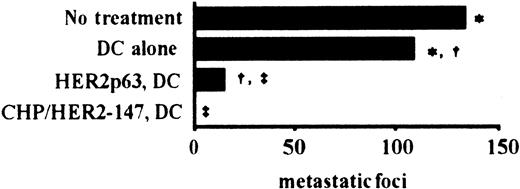
Effect of vaccination using DCs incorporating CHP/HER2-147 and HER2p63-pulsed DCs in an in vivo murine tumor model
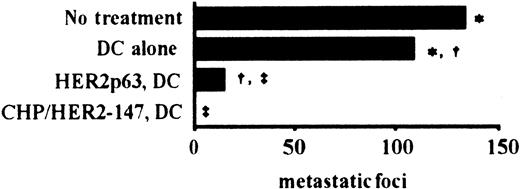
To examine the effects of vaccination using DCs incorporating CHP/HER2-147 and HER2p63-pulsed DCs on tumor growth in vivo, we used a murine model with human HER2-expressing tumor cells, CMS5mHE cells. As described in “Materials and methods,” the phenotype of prepared DCs was that of mature DCs. BALB/c mice were intravenously injected with syngeneic CMS5mHE cells and were examined for lung metastases. Mice bearing CMS5mHE cells were treated twice by weekly subcutaneous injection with DCs alone, DCs incorporating CHP/HER2-147, or HER2p63-pulsed DCs. DCs alone reduced lung metastases, though the extent was approximately 20% (Figure 4). Treatment of mice with HER2p63-pulsed DCs resulted in more than 85% reduction in the number of metastatic foci in the lungs, compared with mice treated with DCs alone. Interestingly, treatment with DCs incorporating CHP/HER2-147 resulted in complete absence of lung metastases, which was confirmed by histologic examination.
DCs incorporating CHP/HER2-147 are more potent than HER2p63-pulsed DCs in an in vivo tumor model.
Five mice in each group were injected intravenously with 1 × 106 syngeneic human HER2-expressing tumor cells, CMS5mHE cells. The mice were immunized with 4 × 105 DCs incorporating CHP/HER2-147, HER2p63-pulsed DCs, or DCs alone 4 and 11 days after tumor challenge. The number of pulmonary metastatic foci was counted 18 days after the administration of CMS5mHE cells. Each bar represents the mean value of 5 mice. Numbers of metastatic foci in each group are as follows: no treatment, 133 ± 13; DCs alone, 108 ± 18; HER2p63-pulsed DCs, 15 ± 6; DCs incorporating CHP/HER2-147, 0. *Difference is significant at P < .05. †‡Differences are significant at P < .01.
DCs incorporating CHP/HER2-147 are more potent than HER2p63-pulsed DCs in an in vivo tumor model.
Five mice in each group were injected intravenously with 1 × 106 syngeneic human HER2-expressing tumor cells, CMS5mHE cells. The mice were immunized with 4 × 105 DCs incorporating CHP/HER2-147, HER2p63-pulsed DCs, or DCs alone 4 and 11 days after tumor challenge. The number of pulmonary metastatic foci was counted 18 days after the administration of CMS5mHE cells. Each bar represents the mean value of 5 mice. Numbers of metastatic foci in each group are as follows: no treatment, 133 ± 13; DCs alone, 108 ± 18; HER2p63-pulsed DCs, 15 ± 6; DCs incorporating CHP/HER2-147, 0. *Difference is significant at P < .05. †‡Differences are significant at P < .01.
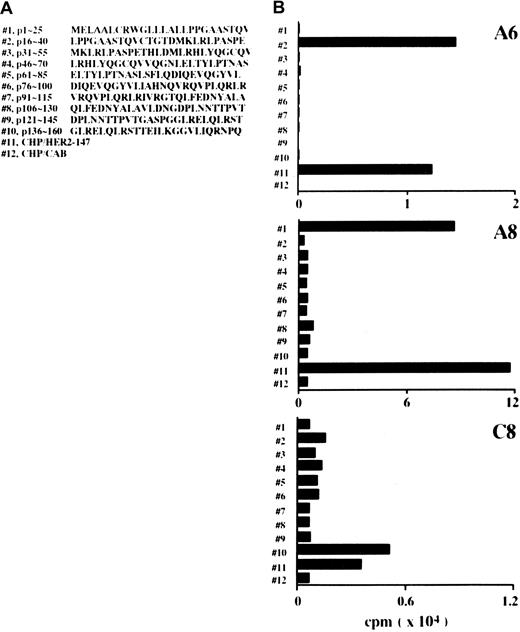
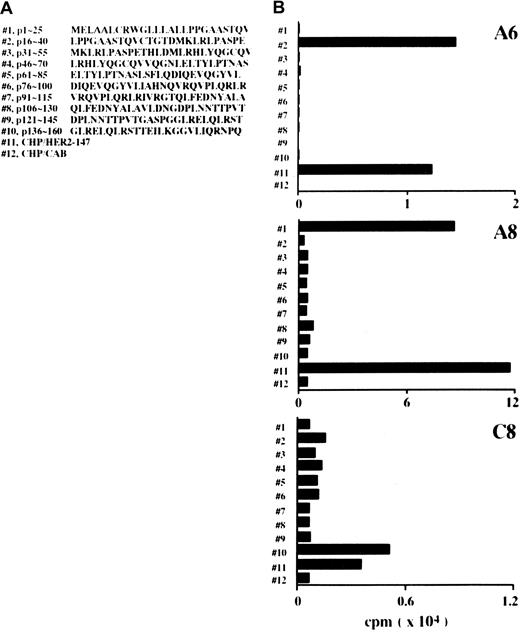
CD4+ clones generated with CHP/HER2-147 recognize the 25-mer peptides of truncated HER2 protein, HER2-147
CD4+ lines were generated in response to CHP/HER2-147 from lymph node cells of mice treated with CHP/HER2-147. Cloning of the lines gave rise to 3 CD4+ clones: A6, A8, and C8. The responses of these 3 CD4+ clones to 10 different 25-mer peptides of HER2, which were continuous from the N-terminus and overlapped by 10 residues, were tested. The sequences of the 25-mer peptides are shown in Figure 5A. When A6 cells were incubated with syngeneic spleen cells pulsed with the 10 different 25-mer peptides, A6 cells were reactive against spleen cells pulsed with the peptide p16-40 but not with those of the other peptides (Figure 5B). A8 and C8 cells showed reactivity to spleen cells pulsed with peptides p1-25 and p136-160, respectively, though the specific reactivity of C8 cells was low compared with that of A6 and A8 cells. Moreover, A6, A8, and C8 cells proliferated in response to spleen cells pulsed with CHP/HER2-147, but not with those pulsed with CHP/CAB.
CD4+ clones recognize HER2-derived peptides.
(A) Position and amino acid sequences of 25-mer peptides derived from HER2 protein. (B) Mitomycin C–treated spleen cells (5 × 106) were reacted with 10 μM 25-mer peptides for 1 hour at room temperature and an additional hour at 37°C and were used as stimulator cells. Mitomycin C–treated spleen cells (5 × 106) were also incubated with 20 μg/mL CHP/HER2-147 or CHP/CAB for 3 hours at 37°C, washed, recultured for 18 hours, and used as stimulator cells. Furthermore, 1 × 105 cells/well CD4+ T cell clones A6, A8, and C8 were cultured with 5 × 105stimulator cells/well for 5 days. Proliferation of responding cells was measured using 3H-TdR uptake assay. Each bar represents the average of duplicate measurements.
CD4+ clones recognize HER2-derived peptides.
(A) Position and amino acid sequences of 25-mer peptides derived from HER2 protein. (B) Mitomycin C–treated spleen cells (5 × 106) were reacted with 10 μM 25-mer peptides for 1 hour at room temperature and an additional hour at 37°C and were used as stimulator cells. Mitomycin C–treated spleen cells (5 × 106) were also incubated with 20 μg/mL CHP/HER2-147 or CHP/CAB for 3 hours at 37°C, washed, recultured for 18 hours, and used as stimulator cells. Furthermore, 1 × 105 cells/well CD4+ T cell clones A6, A8, and C8 were cultured with 5 × 105stimulator cells/well for 5 days. Proliferation of responding cells was measured using 3H-TdR uptake assay. Each bar represents the average of duplicate measurements.
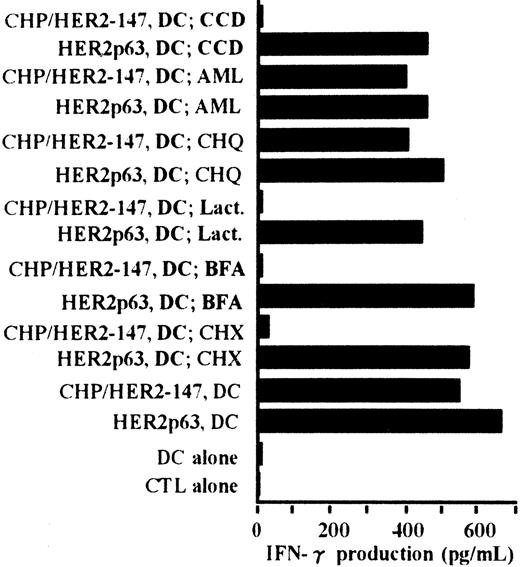
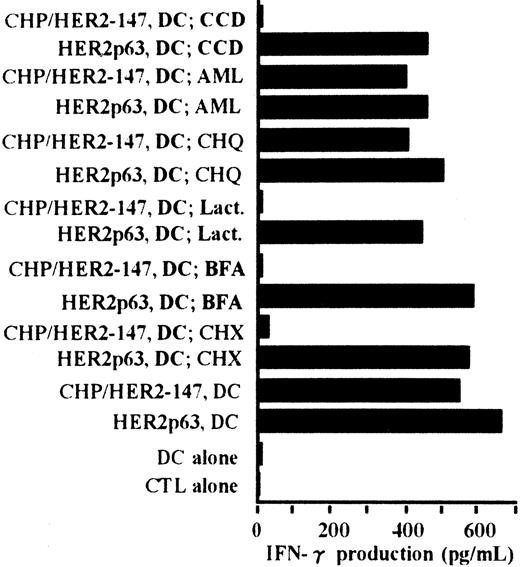
Processing pathway of CHP/HER2-147 in DCs for presentation of HER2p63 bound to HLA-A2402
The processing pathway of CHP/HER2-147 in DCs was examined using various inhibitors. Cytochalasin D was used as an inhibitor of phagocytosis, amiloride as an inhibitor of macropinocytosis, chloroquine as an inhibitor for protein hydrolysis, lactacystin as an inhibitor of proteasome, brefeldin A as an inhibitor of exocytosis, and cycloheximide as an inhibitor of protein synthesis. As shown in Figure6, the stimulatory activity of DCs incorporating CHP/HER2-147 on the CTL clone, Y.I.1a, was inhibited when DCs were treated with cytochalasin D, lactacystin, brefeldin A, or cycloheximide. The stimulatory activity of DCs incorporating CHP/HER2-147 was, however, persistent when treated with amiloride or chloroquine. Neither of these inhibitors suppressed the stimulation of Y.I.1a cells by DCs pulsed with HER2p63. These results indicate that DCs incorporating CHP/HER2-147 process HER2-147 and present MHC class 1–binding HER2p63 through the conventional processing pathway for endogenous protein molecules.
Peptides derived from CHP/HER2-147 are presented on HLA-A2402 molecules through the conventional MHC class I pathway.
DCs (1 × 106) were cultured with cytochalasin D (CCD, 30 μg/mL), amiloride (AML, 0.2 mM), chloroquine (CHQ, 100 μM), lactacystin (Lact, 100 μM), brefeldin A (BFA, 5 μg/mL), or cycloheximide (CHX, 10 μg/mL) for 1 hour in the serum-free AIM-V medium, followed by incubation with or without 20 μg/mL protein CHP/HER2-147 for 3 hours. DCs were then cultured in the absence of the inhibitors, CHP/HER2-147, or HER2p63 for 18 hours in 10% FCS RPMI 1640 supplemented with GM-CSF and IL-4. DCs not cultured with CHP/HER2-147 were incubated with 10 μM HER2p63 for 1 hour at room temperature and for an additional hour at 37°C. DCs (1 × 106) that were not treated with the inhibitors were also cocultured with CHP/HER2-147 or HER2p63, as described in Figure 2B. For IFN-γ production assay, 1 × 105 cells/well of these DCs were incubated with 5 × 104 cells/well of the CTL clone Y.I.1a for 18 hours. IFN-γ production was measured in duplicate using an ELISA kit. Data are the average of duplicate measurements.
Peptides derived from CHP/HER2-147 are presented on HLA-A2402 molecules through the conventional MHC class I pathway.
DCs (1 × 106) were cultured with cytochalasin D (CCD, 30 μg/mL), amiloride (AML, 0.2 mM), chloroquine (CHQ, 100 μM), lactacystin (Lact, 100 μM), brefeldin A (BFA, 5 μg/mL), or cycloheximide (CHX, 10 μg/mL) for 1 hour in the serum-free AIM-V medium, followed by incubation with or without 20 μg/mL protein CHP/HER2-147 for 3 hours. DCs were then cultured in the absence of the inhibitors, CHP/HER2-147, or HER2p63 for 18 hours in 10% FCS RPMI 1640 supplemented with GM-CSF and IL-4. DCs not cultured with CHP/HER2-147 were incubated with 10 μM HER2p63 for 1 hour at room temperature and for an additional hour at 37°C. DCs (1 × 106) that were not treated with the inhibitors were also cocultured with CHP/HER2-147 or HER2p63, as described in Figure 2B. For IFN-γ production assay, 1 × 105 cells/well of these DCs were incubated with 5 × 104 cells/well of the CTL clone Y.I.1a for 18 hours. IFN-γ production was measured in duplicate using an ELISA kit. Data are the average of duplicate measurements.
Discussion
To develop an effective immuno-cell therapy, different approaches for loading DCs with antigenic peptides have been sought. The use of proteins containing multiple antigenic peptides is one such approach. It is, however, well known that professional APCs—DCs—can hardly present MHC class 1–binding peptides when they incorporate exogenous soluble proteins containing the antigenic peptides by phagocytosis.15-17 After processing of exogenous proteins, DCs may effectively stimulate CD4+ helper T cells with MHC class 2–binding peptides but not CD8+ T cells, which recognize MHC class 1–binding peptides. Transfection of cDNA encoding antigenic proteins is an alternative approach.41,42Although much effort has been made to transduce cDNA effectively into DCs,43-47 further technical improvement is needed to perform efficient and consistent transduction of cDNA.
Our antigen molecule delivery system using the hydrophobized polysaccharide–protein complex has several advantages. DCs incorporating hydrophobized polysaccharide–protein complex can process and present MHC class 1–binding peptides, as shown clearly by the fact that DCs incorporating CHP/HER2-147 complexes can specifically stimulate a HER2p63-specific CTL clone and also can generate a CTL clone with similar specificity. Experiments with inhibitors for a variety of molecules that are required for processing and presenting antigen molecules indicated that CHP/HER2-147 complex is incorporated into DCs by phagocytosis because the pretreatment of DCs by cytochalasin D completely abrogated antigen presentation. Although we still do not know how CHP/HER2-147 or HER2-147 protein alone enters the cytosol through the phagosomes, HER2-147 protein was subsequently processed like endogenously synthesized proteins for MHC class 1–binding peptides because pretreatment of DC with lactacystin, brefeldin A, or cycloheximide totally abolished their capability for peptide presentation. Other strategies for delivering peptides derived from exogenous proteins to MHC class 1 molecules have been reported.48-55 These include the use of particle-associated antigens,48-50 the use of antigen-IgG complexes internalized through Fcγ receptors,51 the use of antigens coupled to heat shock proteins,52,53 the use of sterically stabilized liposomes as a vehicle of proteins,54 and the use of outer membrane protein A fromKlebsiella pneumoniae coupled to antigens bound to and endocytosed by DCs.55 Clinical outcome and efficacy of these novel agents remain to be investigated.
In the ELISPOT assay for IFN-γ–producing cells, which allows a direct count of the number of peptide-specific T cells, there was an obvious increase in IFN-γ–producing cells reactive with HER2p63 peptide in PBMCs primed by CHP/HER2-147–treated DCs. Most of these IFN-γ–producing cells are, in fact, reactive with HER2p63 peptide because the number of IFN-γ–producing cells was much lower when those cells were exposed to control HER2p780 peptide-pulsed target cells. This is in sharp contrast to the cells sensitized by HER2p63 peptide-pulsed DCs, which included a significantly higher number of IFN-γ–producing cells even when they were exposed to control HER2p780 peptide-pulsed target cells. We consistently experienced this sort of nonspecific activation of lymphocytes when they were exposed to peptide-pulsed DCs. Using an in vivo tumor model, we were able to show that CHP/HER2-147–treated DCs resulted in the complete absence of lung metastases of HER2-expressing tumor cells, whereas a small number of metastatic foci were persistently seen in mice treated with HER2p63-pulsed DCs. Several possible mechanisms could explain the superiority of CHP/HER2-147–treated DCs to HER2p63 peptide-pulsed DC for specific priming of T cells. One attractive and likely interpretation is the involvement of helper T cells specific for HER2-derived peptides. As reported previously, immunization of animals with CHP/HER2-147 led to the production of antibodies of IgG class specific for HER2-147 protein with much higher titers than antibodies produced in animals immunized with HER2-147 protein alone.21 In our study, we generated murine CD4+ T-cell clones that specifically recognized several distinct antigenic peptides in the truncated HER2-147 protein from mice treated with CHP/HER2-147. These findings indicate that murine DCs incorporating CHP/HER2-147 can present MHC class 2–binding peptides to stimulate CD4+ helper T cells. Although we still have no similar observation in the human system, CHP/HER2-147–treated DCs, but not HER2p63 peptide-pulsed DCs, may activate HER2-specific helper T cells required for efficient activation of HER2p63-specific CD8+ T cells.
Taken together, hydrophobized polysaccharide–protein complexes appear to be able to load DCs with antigenic peptide epitopes that can bind to MHC class 1 and class 2 molecules as well, implying that these complexes could be used for immuno-cell therapy as polyvalent vaccine. Hydrophobized polysaccharides of a simple structure are expected to be nonhazardous; in fact, no adverse effects have been observed in animals treated with CHP alone or with CHP/HER2-147.21 An early-phase clinical trial of immuno-cell therapy with CHP/HER2-147–treated DCs will be launched in our hospital in the near future in patients with HER2-expressing advanced cancer refractory to conventional therapy.
Supported in part by a Grant-in Aid for Scientific Research on Priority Areas from the Ministry of Education, Culture, Science, Sports, and Technology and from the Ministry of Health, Labour, and Welfare, Japan.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Hiroshi Shiku, The Second Department of Internal Medicine, Mie University School of Medicine, 2-174 Edobashi, Tsu, Mie 514-8507, Japan; e-mail:shiku@clin.medic.mie-u.ac.jp