Abstract
Fractalkine is the only member of the CX3C chemokine family. Polymorphism of the fractalkine receptor gene may influence the prognosis of human immunodeficiency virus (HIV) infection, but the nature of the cells expressing fractalkine or its receptor in HIV-infected patients remains unknown. We show that, in contrast to HIV-uninfected individuals, a large number of cells expressed fractalkine in T-cell zones of lymph nodes from HIV-infected patients. CD83+ mature and CD123+ plasmacytoid dendritic cells as well as plasma cells are involved in this increased expression of fractalkine. Increased numbers of plasmacytoid dendritic cells and plasma cells were present in T-cell zones of HIV-infected patients. CD83+ dendritic cells were present in similar number in HIV-infected patients and controls, but an increased fraction of these cells produced fractalkine in HIV-infected patients. Many plasma cells in the gut-associated lymphoid tissue from HIV-infected patients also produced fractalkine, whereas few cells produced fractalkine in the gut of controls. The fraction of CD45RO+ and CD45RO− T helper (Th) cells expressing the fractalkine receptor CX3CR1 was higher in HIV-infected patients than in healthy individuals, and these cells were abnormally sensitive to fractalkine stimulation. This increased response correlated with HIV viremia, and it returned to normal levels in patients successfully treated with antiretroviral drugs. The increased expression of the fractalkine/fractalkine receptor complex associated with HIV infection may affect adhesion and migration of Th lymphocytes and their interaction with dendritic cells. Thus, it may influence the equilibrium between depletion and renewal of the Th lymphocyte compartment.
Introduction
Chemokines are cytokines orchestrating the traffic of leukocytes throughout the body. Unlike other chemokines, fractalkine (FKN) is produced as a membrane-bound form presented at the cell surface by a mucinlike stalk. FKN can be released as a soluble form (sFKN) following proteolytic cleavage.1 In healthy individuals, FKN is constitutively expressed by neurons and in several nonlymphoid tissues, mainly by endothelial cells.2-5 No FKN expression is detected in Peyer patches. In reactive lymph nodes, high endothelial venule (HEV) cells, dendritic cells (DCs), follicular DCs, and a few germinal center lymphocytes express FKN.4FKN expression is increased in the brain of patients with human immunodeficiency virus (HIV)–related encephalitis.6
In healthy individuals, the FKN receptor (CX3CR1) is expressed by monocytes, microglial cells, natural killer cells, and subpopulations of T lymphocytes.4,7,8 Leucocytes expressing CX3CR1 rapidly and firmly adhere to cells expressing the membrane-bound form of FKN. This adhesion is unaffected by pertussis toxin and does not require selectins or integrins,9,10 in contrast to adhesion mediated by other chemokines.1,11,12 Therefore, modulation of the expression of FKN and of its receptor presumably directly modifies leukocyte adherence and traffic. The sFKN is a chemoattractant for leukocytes expressing CX3CR1, but it antagonizes their adherence to cells expressing the membrane-bound form of FKN.9
Polymorphism of the CX3CR1 gene may be associated with the prognosis of HIV infection. In 3 Caucasian HIV cohorts in France, patients homozygous for an allele of the receptor gene containing 2 mutations (I249M280) progressed more rapidly from infection to acquired immunodeficiency syndrome than patients with 2 wild-type alleles or those who were heterozygous.13 In 3 cohorts in North America, this accelerating effect in homozygotes is not found, but heterozygosity for this allele is associated with longer median time to acquired immunodeficiency syndrome and to death.14 The function of the mutant receptor in the response to FKN has not been directly tested. Binding experiments of FKN to patients' blood mononuclear cells and HIV envelope–induced fusion assays suggest that the I249M280 allele is associated with an impaired receptor function. The function of FKN in HIV-infected patients is unknown, and indeed no study has addressed the expression of FKN and of its receptor in HIV-infected patients except in the brain.6 The mechanism by which the FKN/CX3CR1 complex may influence the course of HIV infection is thus unknown.
We report a study of the expression of FKN in tissues from HIV-infected patients, showing that FKN expression is up-regulated in lymph nodes and that this increased expression originates from DCs and plasma cells. FKN expression is also strongly up-regulated in plasma cells of the gut-associated lymphoid tissue (GALT) of HIV-infected patients. CX3CR1 expression and activity in T helper (Th) lymphocytes are increased, particularly in the naive subpopulation, and this dysregulation correlates with the rate of HIV replication.
Patients and methods
Expression of FKN in tissues
Expression of FKN in tissues was analyzed by in situ hybridization and immunohistochemistry as previously described.4,15 For immunofluorescence studies, the anti-FKN antibody was used at a 1:10 final dilution, and its binding was evidenced using a Texas red–coupled goat anti–rabbit immunoglogulin (Jackson Immunoresearch Laboratories, West Grove, PA) (final dilution 1:100). Samples from HIV-1–infected and uninfected individuals were tested in parallel. Tissues were obtained during surgery or endoscopy for diagnostic purposes. None of the patients studied were suffering opportunistic infection. Lymph node and liver biopsies were obtained for suspicion of lymphoma and contained no malignant cells. Lung biopsies were performed for pulmonary hypertension. Duodenal biopsies were performed for digestive symptoms: gastroesophageal reflux, abdominal pain, or diarrhea in 3 controls, and an isolated weight loss and a weight loss associated with diarrhea in 2 HIV-infected patients. In every case, all biopsy samples were morphologically normal. Studies were performed according to French law, with the informed consent of patients and with the approbation of the institutional review board. Mature DCs were detected using CD83 monoclonal antibody (mAb) (Clone HB15A, Immunotech, Marseille, France). Plasmacytoid DCs, B lymphocytes, T lymphocytes, and macrophages were detected using the CD123, CD79α, CD3, and CD68 mAbs, respectively (all from Dako, Glostrup, Denmark). Cells expressing FKN, CD83, CD123, and CD79α were enumerated as previously described,16independently by 2 pathologists (D.B. and A.C-L.) blind to the patient status. The T-cell zone was defined as the lymph node compartment located between the cortex and the medulla, with the exclusion of follicles. In double-labeling immunohistochemistry experiments, we detected CD83 using the supersensitive detection kit from Biogenex (San Ramon, CA) in which the link is a biotinylated anti–mouse immunoglobulin and the label an alkaline phosphatase-conjugated streptavidin, and we detected FKN using a goat anti–rabbit immunoglobulin (ICN Pharmaceuticals, Orsay, France) and an antiperoxidase complex (Dako). In double-labeling immunofluorescence studies, CD83, CD123, or CD79α was detected with a fluorescein isothiocyanate (FITC)–coupled F(ab′)2 goat anti–mouse immunoglobulin (Immunotech) (final dilution 1:100) and FKN-expressing cells with a Texas red–coupled goat anti–rabbit immunoglobulin. The specificity of the labeling was confirmed in single-labeling experiments in which we used CD83, CD79α, or CD123 (mouse mAbs) as the first antibody and the anti–rabbit immunoglobulin as the second antibody or the anti-FKN antibody (a rabbit antibody) as the first antibody and the anti–mouse immunoglobulin as the second antibody. In both cases, there was no labeling (data not shown).
Expression and function of CX3CR1
Expression and function of CX3CR1 were studied on freshly isolated peripheral blood mononuclear cells by flow cytometry and by actin polymerization experiments, respectively, as previously described.4 17 Briefly, for flow cytometry studies, freshly isolated cells were first incubated in the presence of FKN-polyhistidine–tagged recombinant protein, then in the presence of an antipolyhistidine mouse mAb, and then with an FITC-conjugated goat F(ab′)2 anti–mouse immunoglobulin. Finally, a phycoerythrin-Cy5–labeled anti-CD4 mAb and a phycoerythrin-labeled anti-CD45RO mAb were used to identify CX3CR1+ cells. For actin polymerization assays, peripheral blood mononuclear cells were first labeled with phycoerythrin-Cy5–labeled anti-CD4 mAb plus phycoerythrin-labeled CD45RO mAb. Cells then were incubated at 37°C with or without human (hu)-FKN (500 ng/mL) (R&D Systems, Minneapolis, MN). Every 15 seconds, 100 μL of the cell suspension was added to 400μL of 10−7 M FITC-labeled phalloidin (Sigma, L'Isle d'Abeau, France), 0.125 mg/mLl-α-lysophosphatidylcholine, and 4.5% formaldehyde. Fixed cells were then subjected to flow cytometry.
Patients
All HIV-infected patients tested were asymptomatic. They were recruited at 2 centers (Department of Clinical Immunology, Henri Mondor Hospital, Créteil, and Department of Internal Medicine and Clinical Immunology, Antoine Béclère Hospital, Clamart, France). Their antiretroviral (ARV) treatment (Table1) had been unchanged for at least the 4 weeks preceding the experiments. Patients treated with interleukin (IL)-2 were participating in the ANRS 079 trial and received IL-2 (Proleukin, Chiron, Emeryville, CA) (5 × 106 IU twice daily) for 5 days. They were studied during the first cycle of IL-2 administration, and they were also treated with stavudine, lamivudine, and indinavir, which were initiated 4 weeks before IL-2.
Controls for CX3CR1 studies included 10 healthy individuals and 6 patients suffering from hepatitis C virus (HCV)–related chronic viral hepatitis. The patients (Table 2) were previously untreated but presented with an active HCV infection requiring initiation of interferon-α treatment. Blood was collected just before treatment initiation. Samples from HIV-infected, HCV-infected, and uninfected individuals were tested in parallel.
Plasma concentrations of sFKN, sVCAM-1, sIL-2R, and vWF
The sFKN concentration was measured by enzyme-linked immunosorbent assay (ELISA) using a pair of antibodies, one of which was coupled to biotin, directed against hu-FKN. Antibody binding was revealed using peroxidase-labeled streptavidin and the peroxidase substrate ABTS. Recombinant hu-FKN was used to define a standard curve. The detection threshold of this method was 70 pg/mL sFKN. Plasma levels of soluble vascular cell adhesion molecule (sVCAM)-1 and soluble IL-2 receptor (sIL-2R) were measured using ELISA kits from Diaclone (Besançon, France); von Willebrand factor (vWF) was measured using an ELISA kit from Diagnostics Stago (Asnieres, France).
Results
Production of FKN in lymph nodes from HIV-infected patients
Production of FKN in lymph nodes from 4 HIV-infected patients was tested by immunohistochemistry, immunofluorescence (Figure1), and in situ hybridization (Figure2). The 3 approaches gave similar findings. As controls, reactive lymph nodes from 4 HIV-uninfected patients presenting a benign follicular hyperplasia were analyzed.
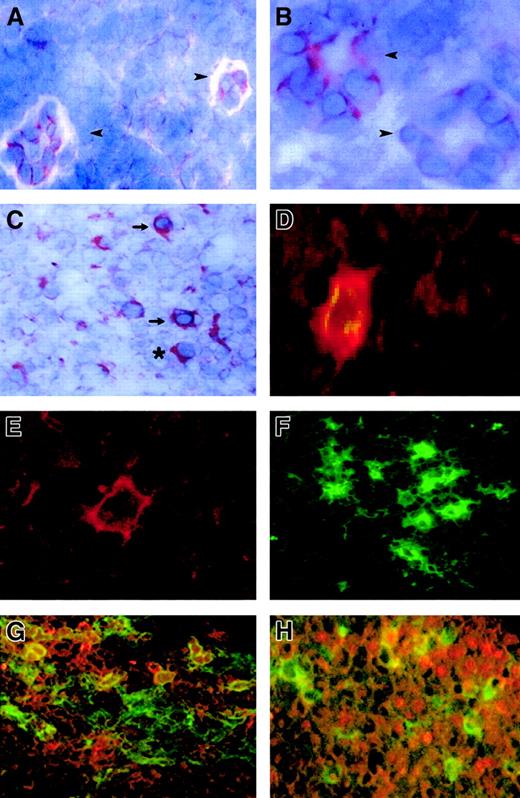
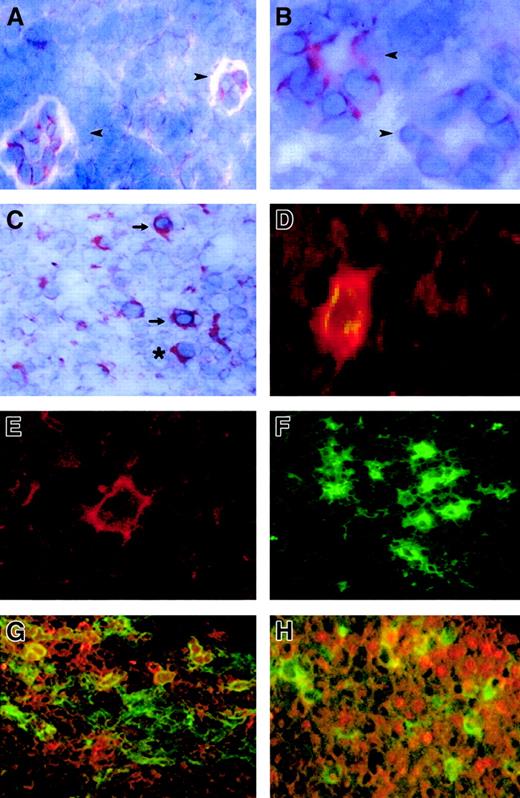
FKN expression in lymph nodes.
FKN expression was tested by immunohistochemistry (A-C) and by immunofluorescence (D-H) in lymph nodes from 4 HIV-infected patients and 4 controls. (A) Two HEV cells in a T-cell zone of an HIV-uninfected patient. (B,C) T-cell zone in an HIV-infected patient. (D,G,H) Characterization of FKN-expressing cells in an HIV-infected patient, evidenced by a double-labeling experiment combining detection of FKN (red) and CD83 (D), CD123 (G), or CD79α (H) (green). Double-labeled cells appear in yellow. Single-labeling immunofluorescence experiments with the anti-FKN or the CD83 antibody are shown in panels E and F, respectively. → indicates DCs; ➤, HEV; *, plasma cell. Original magnification × 400 in panels B, D, and E and × 200 in other panels.
FKN expression in lymph nodes.
FKN expression was tested by immunohistochemistry (A-C) and by immunofluorescence (D-H) in lymph nodes from 4 HIV-infected patients and 4 controls. (A) Two HEV cells in a T-cell zone of an HIV-uninfected patient. (B,C) T-cell zone in an HIV-infected patient. (D,G,H) Characterization of FKN-expressing cells in an HIV-infected patient, evidenced by a double-labeling experiment combining detection of FKN (red) and CD83 (D), CD123 (G), or CD79α (H) (green). Double-labeled cells appear in yellow. Single-labeling immunofluorescence experiments with the anti-FKN or the CD83 antibody are shown in panels E and F, respectively. → indicates DCs; ➤, HEV; *, plasma cell. Original magnification × 400 in panels B, D, and E and × 200 in other panels.
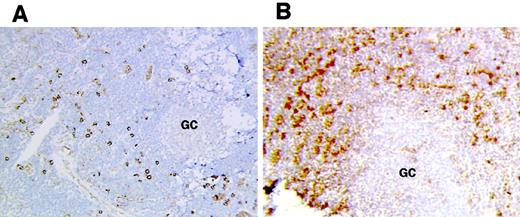
FKN gene expression in lymph nodes.
FKN gene expression was analyzed in lymph nodes from 4 HIV-infected (A,B) and 4 uninfected (not shown) patients by in situ hybridization with an antisense probe. (A) T-cell zone. (B) Border between the germinal center (GC) and the T-cell zone (T). (C) Control hybridized with a sense probe. Original magnification × 400 in panels A and B and × 200 in panel C.
FKN gene expression in lymph nodes.
FKN gene expression was analyzed in lymph nodes from 4 HIV-infected (A,B) and 4 uninfected (not shown) patients by in situ hybridization with an antisense probe. (A) T-cell zone. (B) Border between the germinal center (GC) and the T-cell zone (T). (C) Control hybridized with a sense probe. Original magnification × 400 in panels A and B and × 200 in panel C.
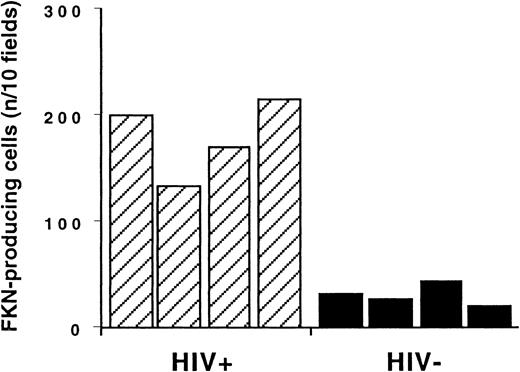
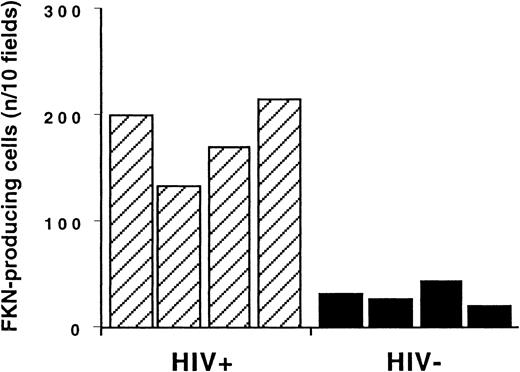
FKN was detected in all lymph nodes. FKN was expressed by cells in HEV, lymphatic sinuses, and germinal centers. FKN expression in these cells did not differ between HIV-infected patients and controls (Figure1A,B). In contrast, the density of FKN-expressing cells in T-cell zones was higher in HIV-infected patients than in controls (Figures 1C and2A). In these areas, positive cells were scattered in controls, whereas they were clustered in HIV-infected patients. Enumeration of FKN gene–expressing cells confirmed their higher density in HIV-infected patients (Figure 3).
Enumeration of FKN gene–expressing cells in T-cell zones.
In in situ hybridization experiments, FKN gene–expressing cells were enumerated in T-cell zones of lymph nodes from HIV-infected and uninfected individuals (hatched and black columns, respectively). Results are expressed as the number of positive cells detected per 10 fields at the × 400 magnification. This corresponds to an average density of FKN gene–expressing cells of 294 ± 60/cm2and 48 ± 8/cm2 in HIV-infected patients and controls, respectively.
Enumeration of FKN gene–expressing cells in T-cell zones.
In in situ hybridization experiments, FKN gene–expressing cells were enumerated in T-cell zones of lymph nodes from HIV-infected and uninfected individuals (hatched and black columns, respectively). Results are expressed as the number of positive cells detected per 10 fields at the × 400 magnification. This corresponds to an average density of FKN gene–expressing cells of 294 ± 60/cm2and 48 ± 8/cm2 in HIV-infected patients and controls, respectively.
In HIV-infected patients, 2 types of cells expressed FKN in T-cell zones: cells with the morphology of DCs and cells with the morphology of plasma cells (Figure 1C). FKN production by mature DCs was directly evidenced by double-labeling experiments combining detection of CD83 and FKN, with concordant results between immunohistochemical (not shown) and immunofluorescence (Figure 1D) techniques. Only a minority of CD83+ DCs in controls expressed FKN, whereas CD83+ DCs expressing FKN were abundant in HIV-infected patients. To determine whether FKN-expressing cells with a plasma cell morphology were plasma cells or plasmacytoid DCs, we used immunofluorescence to study FKN expression by CD79α+ B lymphocytes and by CD123+ plasmacytoid DCs. Both types of cells contained FKN (Figure 1G,H). No CD68+macrophages contained FKN, and CD3+ T cells containing FKN were rare (data not shown). No FKN-containing cells with a plasma cell morphology were observed in control lymph nodes.
Quantification of CD83+, CD123+, and plasma cells in lymph nodes
We next tested whether the increased density of FKN-expressing cells in T-cell zones of HIV-infected patients was due to an abnormal accumulation of mature DCs, plasmacytoid DCs, and plasma cells. The density of CD83+ cells was similar in HIV-infected patients and in controls (113 ± 21/cm2 and 120 ± 33/cm,2 respectively). Therefore, the increased number of CD83+ DCs expressing FKN in HIV-infected patients was not due to an abnormal accumulation of these cells but to increased induction of FKN production. In contrast, the density of CD123+ plasmacytoid DCs was much higher in T-cell zones from HIV-infected patients than from controls (235 ± 15/cm2 and 70 ± 18/cm2, respectively) (Figure 4). The density of CD79α+ plasma cells was also higher in T-cell zones from HIV-infected patients than in those from controls (290 ± 9/cm2 and 32 ± 2/cm,2respectively), as previously reported.18 Therefore, abnormal accumulation of both plasmacytoid DCs and plasma cells accounted for increased production of FKN in T-cell areas in HIV-infected patients. Presumably, deregulated production of FKN in individual plasmacytoid DCs and plasma cells also contributed to the increased FKN production in HIV lymph nodes, because cells with a plasma cell morphology did not express FKN in controls.
Accumulation of plasmacytoid DCs in HIV lymph nodes.
Plasmacytoid DCs were detected by immunohistochemistry in frozen lymph node sections from controls (A) and HIV-infected patients (B). GC indicates germinal center. Original magnification × 200.
Accumulation of plasmacytoid DCs in HIV lymph nodes.
Plasmacytoid DCs were detected by immunohistochemistry in frozen lymph node sections from controls (A) and HIV-infected patients (B). GC indicates germinal center. Original magnification × 200.
Production of FKN in nonlymphoid organs from HIV-infected patients
FKN expression in the lung and liver was tested by immunohistochemistry and by in situ hybridization. There was no difference between HIV-infected patients and controls. The main cells expressing FKN were endothelial cells. In contrast, FKN expression in the duodenum clearly differed between HIV-infected patients and controls.
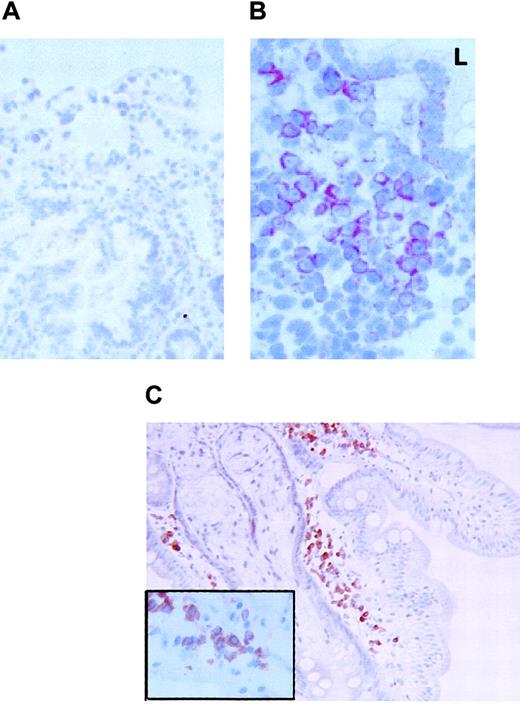
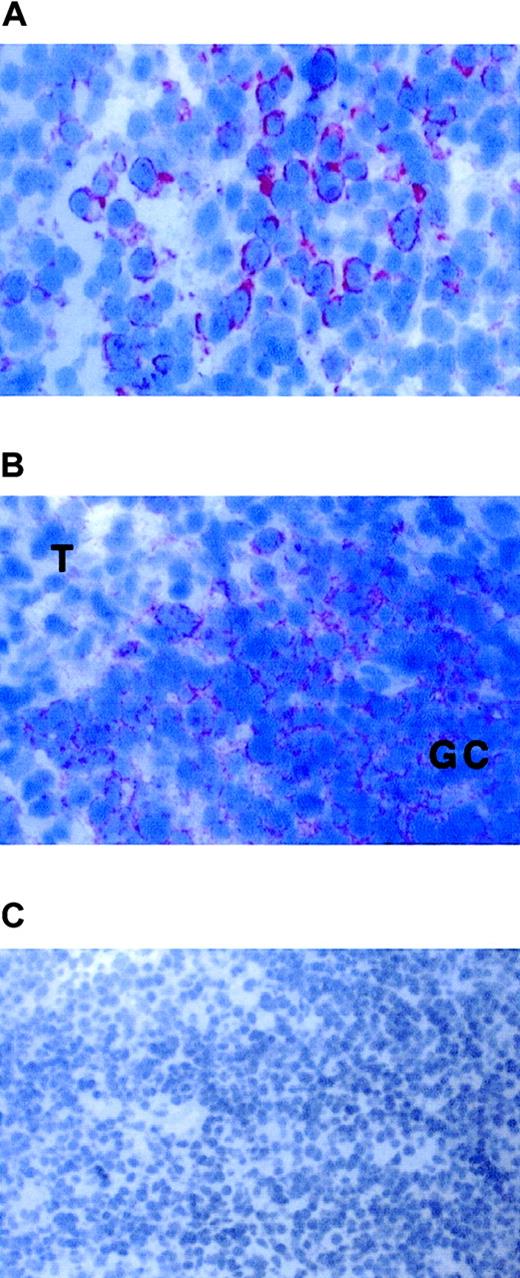
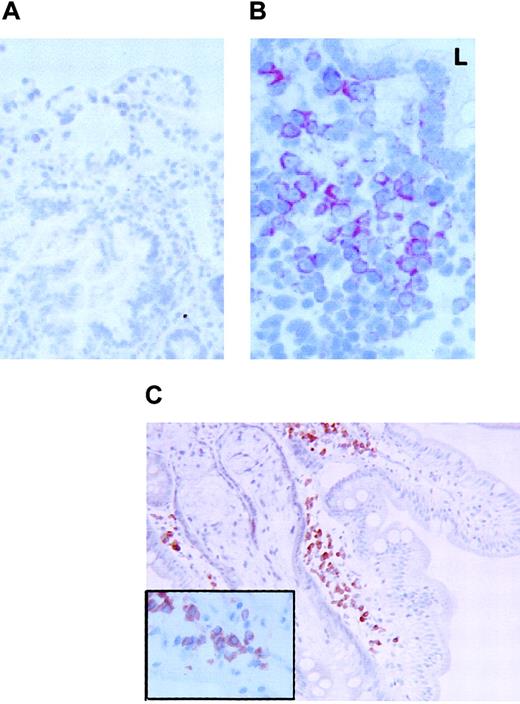
In the 3 controls tested, rare and weakly stained FKN-expressing cells were found in the superficial area of the lamina propria. In contrast, positive cells were extremely abundant and intensely stained in the 2 HIV-infected individuals tested. Stained cells were clustered in the lamina propria. Most, if not all, FKN-expressing cells in HIV-infected patients had the morphology of plasma cells (Figure5B). There were no CD123+plasmacytoid DCs, and cells with a plasma cell morphology all expressed the CD79α antigen (Figure 5C). Because CD79α+ plasma cells were also detected in controls, with no substantial difference in their density as compared with HIV-infected patients, production of FKN in the duodenum of HIV-infected patients is due to a deregulation of FKN synthesis in plasma cells. FKN was also expressed in epithelial cells from HIV-infected patients, especially at the basal pole of the cells (Figure 5B). No epithelial labeling was observed in controls (Figure 5A).
FKN expression in the duodenum.
FKN expression was tested in the duodenum of 3 HIV-uninfected (A) or 2 HIV-infected (B) patients by immunohistochemistry (data not shown) or by in situ hybridization with an antisense probe. (C) CD79α-expressing cells in the duodenum of HIV-infected patients. Original magnification × 200 in panels A and C and × 400 in panel B and in the insert of panel C. L indicates digestive lumen.
FKN expression in the duodenum.
FKN expression was tested in the duodenum of 3 HIV-uninfected (A) or 2 HIV-infected (B) patients by immunohistochemistry (data not shown) or by in situ hybridization with an antisense probe. (C) CD79α-expressing cells in the duodenum of HIV-infected patients. Original magnification × 200 in panels A and C and × 400 in panel B and in the insert of panel C. L indicates digestive lumen.
Circulating levels of sFKN in HIV-infected patients
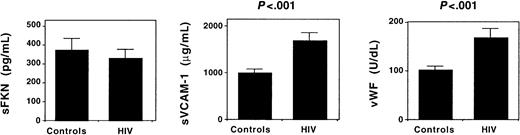
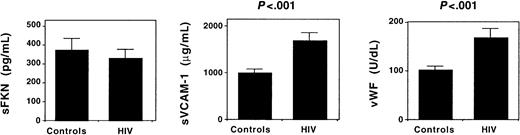
Circulating sFKN may antagonize interactions between FKN-expressing endothelial cells and CX3CR1-expressing blood leucocytes.9 To investigate whether endothelial cell activation in HIV infection up-regulates sFKN release, we measured plasma concentrations of sFKN. Identical sFKN concentrations were observed in HIV-infected patients and in healthy individuals. In contrast, plasma concentrations of sVCAM-1 and vWF, 2 markers of endothelial cell activation, were higher in HIV-infected patients (Figure 6). Therefore, endothelial cell activation in HIV-infected patients is not associated with an increased release of sFKN.
Circulating levels of sFKN and endothelial cell activation.
The plasma concentration of sFKN, sVCAM-1, and vWF was measured in 8 healthy individuals (controls) and 16 HIV-infected patients. Results shown represent the mean ± SEM plasma concentration.
Circulating levels of sFKN and endothelial cell activation.
The plasma concentration of sFKN, sVCAM-1, and vWF was measured in 8 healthy individuals (controls) and 16 HIV-infected patients. Results shown represent the mean ± SEM plasma concentration.
Increased expression and activity of CX3CR1 in Th lymphocytes from HIV-infected patients
Expression of CX3CR1 was studied by flow cytometry. In controls, only a few Th cells expressed CX3CR1. The fraction of CD4+cells expressing CX3CR1 was higher in HIV-infected patients, and this applied for both CD45RO+ and CD45RO− Th cells (Figure 7A,D).
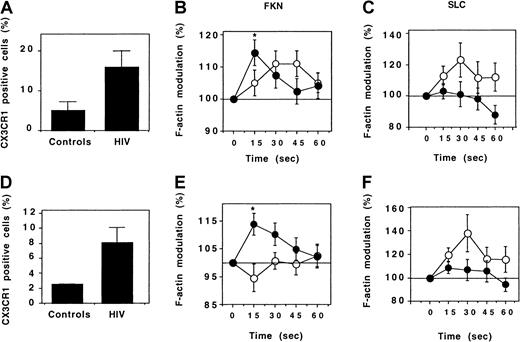
Expression and function of CX3CR1 by Th lymphocytes.
Expression of CX3CR1 by CD45RO+ (A) and CD45RO− (D) Th lymphocytes was evaluated by flow cytometry. Six healthy individuals (controls) and 14 HIV-infected patients were analyzed. Results correspond to the fraction (mean ± SEM) of CD4+ T lymphocytes expressing CX3CR1. The response of CD45RO+ (B,C) and CD45RO− (E,F) Th lymphocytes following addition of FKN (B,E) or SLC (C,F) was evaluated by determining the kinetics of actin polymerization in 10 healthy individuals (empty circles) and in HIV-infected individuals (full circles). A total of 38 and 9 HIV-infected patients were tested for response to FKN and to SLC, respectively. *P = .01 (comparison between HIV-infected patients and controls, ttest).
Expression and function of CX3CR1 by Th lymphocytes.
Expression of CX3CR1 by CD45RO+ (A) and CD45RO− (D) Th lymphocytes was evaluated by flow cytometry. Six healthy individuals (controls) and 14 HIV-infected patients were analyzed. Results correspond to the fraction (mean ± SEM) of CD4+ T lymphocytes expressing CX3CR1. The response of CD45RO+ (B,C) and CD45RO− (E,F) Th lymphocytes following addition of FKN (B,E) or SLC (C,F) was evaluated by determining the kinetics of actin polymerization in 10 healthy individuals (empty circles) and in HIV-infected individuals (full circles). A total of 38 and 9 HIV-infected patients were tested for response to FKN and to SLC, respectively. *P = .01 (comparison between HIV-infected patients and controls, ttest).
The activity of CX3CR1 was tested by evaluating the kinetics of actin polymerization following addition of FKN. In HIV-infected patients and in controls, CD45RO+ Th lymphocytes responded to FKN triggering. However, this response was faster in HIV-infected patients: It was maximal 15 seconds after FKN addition, whereas the maximal response was attained after 30 to 45 seconds in controls (Figure 7B). The difference between HIV-infected patients and controls was more evident for CD45RO− Th cells: FKN did not trigger any actin polymerization in controls. In contrast, a strong response was observed in HIV-infected patients (Figure 7E), similar in intensity and kinetics to that of CD45RO+ Th cells.
To test whether the increased CX3CR1 activity in HIV-infected patients was a specific feature of this receptor or reflected a more general hyperresponsiveness of the cytoskeleton to chemokine receptor triggering, actin polymerization induced by the CCR7-binding chemokine SLC was analyzed. SLC stimulated cytoskeleton reorganization in both CD45RO+ and CD45RO− Th lymphocytes from control individuals. In HIV-infected patients, SLC only weakly stimulated actin polymerization in CD45RO− cells, and it had no effect in CD45RO+ cells (Figure 7C,F). Therefore, the increased reorganization of the cytoskeleton following stimulation with FKN in HIV-infected patients is due to an increase of the activity of CX3CR1 and not of other chemokine receptors.
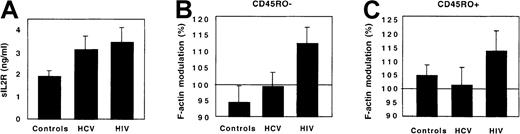
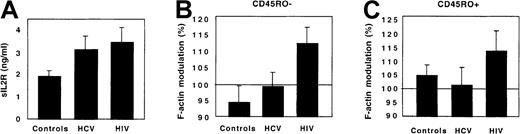
As additional controls, patients with another chronic viral infection, chronic viral hepatitis, were studied. These patients had an aggressive infection requiring initiation of interferon-α treatment. Their immune activation, reflected by a high plasma concentration of sIL-2R, was in the same range as that of HIV-infected patients. However, in contrast to HIV-infected patients, neither CD45RO+ nor CD45RO− Th cells from HCV-infected patients displayed any abnormal response to FKN (Figure 8).
CX3CR1 function in HCV-infected patients.
The plasma concentration of sIL-2R (A) and the FKN-induced actin polymerization in CD45RO− (B) and CD45RO+ (C) Th lymphocytes were compared between 10 healthy controls, 6 HCV-infected patients, and 9 HIV-infected patients. Actin polymerization was evaluated 15 seconds after FKN addition.
CX3CR1 function in HCV-infected patients.
The plasma concentration of sIL-2R (A) and the FKN-induced actin polymerization in CD45RO− (B) and CD45RO+ (C) Th lymphocytes were compared between 10 healthy controls, 6 HCV-infected patients, and 9 HIV-infected patients. Actin polymerization was evaluated 15 seconds after FKN addition.
CX3CR1 function and CD4+ cell counts
We tested whether the increased CX3CR1 activity in HIV-infected patients was a result of the CD4+ T-lymphocyte depletion. In each patient, the CX3CR1 activity was compared with the circulating CD4+ T-lymphocyte count. There was no correlation between these variables either for CD45RO+ or CD45RO−Th cells (data not shown).
CX3CR1 function and HIV replication
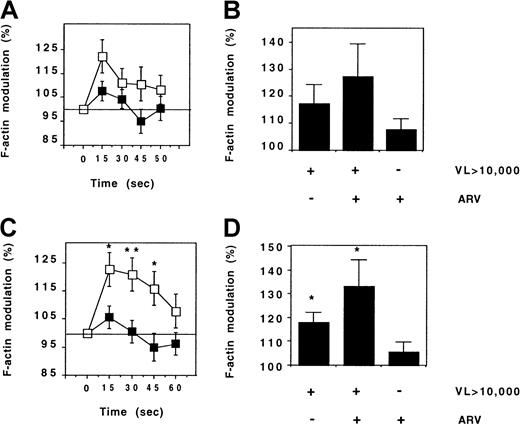
CX3CR1 activity was compared with the rate of HIV replication. FKN-induced actin polymerization was stronger in CD45RO+ Th lymphocytes from patients with a plasma HIV concentration above 10 000 copies/mL than in those from patients with a lower viral load, but the difference was not significant (Figure9A).
CX3CR1 function and HIV viremia.
FKN-induced actin polymerization was evaluated in CD45RO+(A,B) and CD45RO− (C,D) Th lymphocytes from HIV-infected patients. The kinetics of actin polymerization (A,C) was compared between 20 patients and 18 patients with HIV RNA counts below (full symbols) and above (empty symbols) 10 000 copies per milliliter, respectively. Actin polymerization 15 seconds after FKN addition (B,D) was compared between patients treated or not treated with ARV drugs. VL indicates viral load (HIV copies/mL). *P = .05; **P = .005, t test.
CX3CR1 function and HIV viremia.
FKN-induced actin polymerization was evaluated in CD45RO+(A,B) and CD45RO− (C,D) Th lymphocytes from HIV-infected patients. The kinetics of actin polymerization (A,C) was compared between 20 patients and 18 patients with HIV RNA counts below (full symbols) and above (empty symbols) 10 000 copies per milliliter, respectively. Actin polymerization 15 seconds after FKN addition (B,D) was compared between patients treated or not treated with ARV drugs. VL indicates viral load (HIV copies/mL). *P = .05; **P = .005, t test.
In CD45RO− Th lymphocytes, the intensity of actin polymerization following FKN addition was much higher in patients with a high viral load than in patients with a low viral load, and this difference persisted during the 60 seconds of the stimulation (Figure 9C). CX3CR1 activity in CD45RO− Th cells of HIV-infected patients was linearly correlated to the number of plasma HIV RNA copies (r = 0.46, P = .01). Patients with ARV treatment failure had an abnormal CX3CR1 function similar to that of patients with a spontaneously high rate of viral replication (Figure 9B,D).
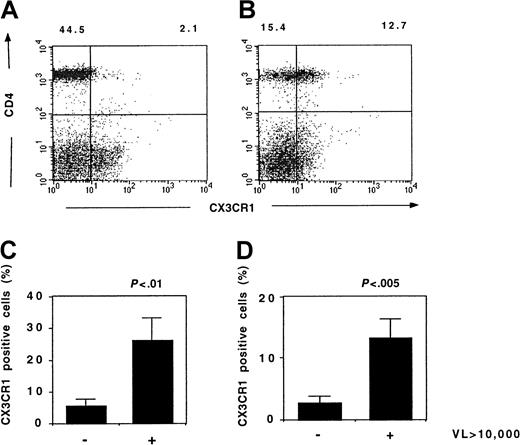
Flow cytometry studies showed that the highest CX3CR1 expression was in patients with the highest plasma HIV concentration (Figure10). The fractions of CD45RO+ and CD45RO− Th lymphocytes expressing CX3CR1 significantly correlated with the viral load (r = 0.60, P = .03 and r = 0.43,P = .01, respectively, Spearman test). Thus, the increased CX3CR1 activity in patients with uncontrolled viral replication is due to an increased expression of this receptor.
CX3CR1 expression and HIV viremia.
Expression of CX3CR1 by lymphocytes from HIV-infected patients was analyzed by flow cytometry. Typical results from a patient with a low (< 20 copies/mL) (A) or a high (36 253 copies/mL) (B) HIV viremia are shown. The fraction of CD45RO+ Th lymphocytes (C) and CD45RO− Th lymphocytes (D) expressing CX3CR1 in patients with HIV viremia below (n = 7) or above (n = 7) 10 000 copies per milliliter is shown. VL indicates viral load (HIV copies/mL); Mann-Whitney U test.
CX3CR1 expression and HIV viremia.
Expression of CX3CR1 by lymphocytes from HIV-infected patients was analyzed by flow cytometry. Typical results from a patient with a low (< 20 copies/mL) (A) or a high (36 253 copies/mL) (B) HIV viremia are shown. The fraction of CD45RO+ Th lymphocytes (C) and CD45RO− Th lymphocytes (D) expressing CX3CR1 in patients with HIV viremia below (n = 7) or above (n = 7) 10 000 copies per milliliter is shown. VL indicates viral load (HIV copies/mL); Mann-Whitney U test.
Normalization of CX3CR1 function following control of HIV replication
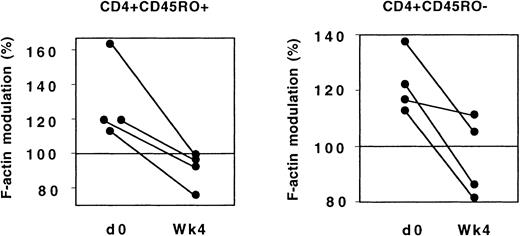
The evolution of CX3CR1 function was analyzed in 4 patients who had not previously received ARV drugs and who started treatment combining 2 nucleoside reverse transcriptase inhibitors and 1 protease inhibitor. Four weeks after treatment initiation, the response of both CD45RO+ and CD45RO− Th lymphocytes to FKN was significantly lower than before treatment (Figure11).
CX3CR1 function and ARV treatment.
In 4 patients, actin polymerization 15 seconds after addition of FKN was analyzed in CD45RO+ and CD45RO− Th lymphocytes before and 4 weeks after initiation of ARV treatment.
CX3CR1 function and ARV treatment.
In 4 patients, actin polymerization 15 seconds after addition of FKN was analyzed in CD45RO+ and CD45RO− Th lymphocytes before and 4 weeks after initiation of ARV treatment.
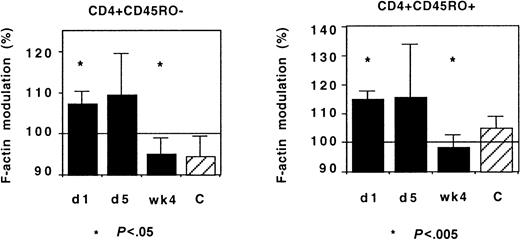
IL-2 treatment and CX3CR1 function
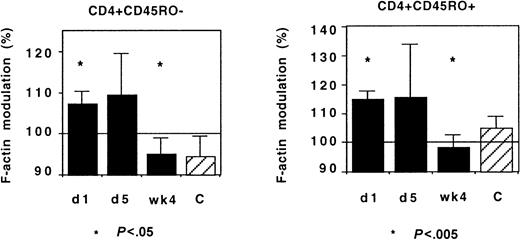
IL-2 treatment of HIV-infected patients induces an acute redistribution of both naive and effector/memory CD4+ T lymphocytes and the up-regulation of several chemokine receptors.17 We investigated whether such treatment affected CX3CR1 activity in Th lymphocytes. Although there was some interindividual diversity, IL-2 administration had no acute effect on CX3CR1 activity: FKN-induced actin polymerization was identical in cells taken before and on the last day of the IL-2 administration (Figure 12).
CX3CR1 function and IL-2 treatment.
Actin polymerization 15 seconds after addition of FKN was tested in 9 HIV-infected patients before (day 1) and on the last day (day 5) of IL-2 administration and 4 weeks later. The number of HIV RNA copies on day 1 and on week 4 was 3915 ± 3357/mL and 344 ± 279/mL, respectively. The CD4+ T lymphocyte count was 518 ± 54 and 797 ± 87 cells per microliter at inclusion and on week 4, respectively. Controls were healthy individuals.
CX3CR1 function and IL-2 treatment.
Actin polymerization 15 seconds after addition of FKN was tested in 9 HIV-infected patients before (day 1) and on the last day (day 5) of IL-2 administration and 4 weeks later. The number of HIV RNA copies on day 1 and on week 4 was 3915 ± 3357/mL and 344 ± 279/mL, respectively. The CD4+ T lymphocyte count was 518 ± 54 and 797 ± 87 cells per microliter at inclusion and on week 4, respectively. Controls were healthy individuals.
In these patients, who had started a protease inhibitor–containing regimen 4 weeks before the first cycle of IL-2 administration, the IL-2 treatment did not affect the normalization of CX3CR1 function associated with the control of HIV replication: When HIV viremia decreased below 500 copies per milliliter, a few weeks after IL-2 administration the response of CD45RO+ and CD45RO− Th lymphocytes to FKN was identical to that of controls (Figure 12). Therefore, the CD4+ T lymphocytes emerging under IL-2 treatment did not display abnormal CX3CR1 function.
Discussion
In this work, we report the production of FKN in tissues of HIV-infected patients and the expression and function of the FKN receptor CX3CR1 in Th-cell subpopulations.
The expression of FKN in lymph nodes in HIV-infected patients is very different from that of HIV-uninfected patients: The number of FKN-expressing cells is much larger in T-cell zones of HIV-infected patients. The accumulation of FKN-expressing cells was due to 3 populations of cells: CD83+ (mature) DCs, CD123+ (plasmacytoid) DCs, and plasma cells. A recent study of human tonsils showed that only a fraction of CD123+ DCs expresses CD83. CD123+CD83+ DCs display a dendritic-like morphology different from that of typical CD123+ plasmacytoid DCs.19 The production of FKN by mature DCs and by activated B lymphocytes has already been reported.2 4 This is the first report of the production of FKN by plasmacytoid DCs and by plasma cells. FKN expression is also deregulated in the GALT of HIV-infected patients. Virtually no FKN is expressed in GALT of controls, whereas large numbers of FKN-expressing cells accumulate in HIV-infected patients. In this case, only plasma cells and epithelial cells produce FKN. FKN expression during HIV infection is up-regulated only in a limited set of tissue compartments; there was no difference in FKN production between HIV-infected patients and controls in nonlymphoid organs, HEV cells, or germinal centers of lymph nodes.
Plasma cells are present in the GALT of controls but do not produce FKN and, therefore, the increased production of FKN in the GALT of HIV-infected patients is due to deregulation of its production in these cells. In lymph nodes, the increased number of plasma cells and of plasmacytoid DCs expressing FKN in HIV-infected patients was at least partly due to an abnormal accumulation of these cells. However, a deregulated FKN production in individual plasmacytoid DCs and plasma cells may also contribute to increased FKN production, because no cell with a plasma cell morphology expresses FKN in control lymph nodes, although CD123+ plasmacytoid DCs and plasma cells were definitely present in these tissues. This work is the first to show an abnormal accumulation of plasmacytoid DCs in lymph nodes from HIV-infected patients. Recent studies have shown that the number of circulating plasmacytoid DCs is decreased in HIV-infected patients.20,21 This indicates that, as for CD4+ T lymphocytes, redistribution of cells to lymphoid organs may contribute to the depletion of circulating plasmacytoid DCs in HIV-infected patients. In contrast to FKN production by plasmacytoid DCs and by plasma cells, which seems to be due to both an increased number of cells and an increased production at the single-cell level, a single mechanism explained the role of mature DCs in the increased FKN production. Indeed, the number of CD83+ DCs in T-cell zones of lymph nodes in HIV-infected patients was not different than that in controls. Only a minority of CD83+ DCs produced FKN in controls, whereas most CD83+ DCs were positive in HIV-infected patients. Thus, lymph node CD83+ DCs display specific functional abnormalities during HIV infection, resulting in an increased FKN expression. The expression of FKN by human CD83+ DCs is up-regulated upon activation and especially following triggering of CD40,2 suggesting that increased expression of FKN by CD83+ DCs from HIV-infected patients reflects an increased activation of these cells.
Dysregulation of the FKN/CX3CR1 complex associated with HIV infection also involves the receptor. This is especially clear for naive Th lymphocytes, which do not respond to FKN in healthy individuals but respond strongly in HIV-infected patients. This increased CX3CR1 activity is due to an increased number of cells expressing the receptor, as shown by flow cytometry studies. The same is not true for CCR7, another chemokine receptor tested in parallel. Increased CX3CR1 expression and activity in both naive and memory Th lymphocytes correlate with the rate of replication of HIV. This dysregulation is observed even in patients with treatment failure, ie, those with uncontrolled viral replication despite antiviral drugs.
It is unclear whether up-regulation of CX3CR1 expression in HIV infection is directly induced by viral products or results from the immune activation induced by the viral replication. Distinguishing between these 2 possible causes is difficult because the 2 are strongly linked. In an attempt to solve this issue, we studied patients with chronic and active HCV infection for reference. With a similar level of plasma sIL-2R concentration, the immune activation in these HCV-infected patients was as strong as that in HIV-infected patients. However, CX3CR1 function in HCV-infected patients was identical to that of healthy individuals, and it was clearly different from that of HIV-infected patients. This argues in favor of dysregulation of CX3CR1 expression and function being specifically caused by HIV infection independently of the level of immune activation.
Increased expression of CCR5 22 and decreased expression of CXCR5 23 have been reported on Th lymphocytes from HIV-infected patients, and lymph nodes from HIV-infected patients overproduce the chemokines RANTES, MIP-1α, and MIP-1β, all 3 being CCR5-binding chemokines.24 25 The increased expression of FKN and of its receptor and the decreased response to SLC we report underline the complexity of the deregulation of the chemokine/chemokine receptor network in HIV infection, which is expected to alter profoundly the migration of Th lymphocytes.
T lymphocytes exposed to HIV show increased adherence to vessels.26 Contact of resting CD4+ T lymphocytes with HIV enhances their migration to lymph nodes in ex vivo assays and in mice with severe combined immunodeficiency, and it stimulates their expression of CD62L, a selectin involved in the homing of lymphocytes to lymph nodes through HEV cells.26 This suggests that increased expression of CD62L plays a role in abnormal trafficking of Th lymphocytes to patients lymph nodes. CD62L-mediated binding of leucocytes is weak and transient, and stable adhesion and migration of leucocytes requires additional events involving integrins and chemokines.11,12 Possibly, CCR5-binding chemokines trigger lymphocyte migration initiated by CD62L in HIV-infected patients. However, other chemokines may also be involved in this phenomenon, because we showed that, in HIV-infected patients as in healthy individuals, CD62L+ naive T lymphocyte do not express CCR5 and only a minority of CD62L+ memory Th lymphocytes expresses this chemokine receptor.17 In contrast to CD62L, FKN mediates strong adherence of leucocytes independently of selectins, integrins, and other chemokines.9,10 As Foussat et al4 show and we show here, because FKN is expressed by endothelial cells from both lymphoid and nonlymphoid organs in vivo, increased expression of CX3CR1 by circulating Th lymphocytes probably results in their being trapped by these various tissues.
Control of HIV replication following initiation of ARV treatment rapidly normalizes the function of CX3CR1 in Th lymphocytes. This may stop FKN-mediated accumulation of Th lymphocytes in tissues, and this may account for the rapid rise of the circulating memory Th lymphocyte count in treated patients, which has been shown to reflect redistribution of these cells from tissues to blood.27 28Such redistribution has been proposed to result from the release of memory Th lymphocytes from peripheral lymphoid tissues. Our present findings, showing a wide expression of FKN in many tissues, indicate that the organs involved in Th lymphocyte sequestration during uncontrolled HIV infection and in Th-lymphocyte release during ARV treatment may be more diverse than initially thought and, in particular, include the gut.
In mice, naive Th lymphocytes only migrate to secondary lymphoid organs, in which they receive survival signals. They are excluded from nonlymphoid organs.29,30 In healthy humans, naive Th lymphocytes do not express CX3CR1,4 and FKN production in nonlymphoid organs is thus not expected to trigger recruitment of circulating naive Th cells. In HIV-infected patients, the deregulated expression of CX3CR1 by naive Th lymphocytes raises the hypothesis of an abnormal migration of these cells to nonlymphoid organs, an environment in which they may not be able to survive. The decrease of naive Th cell counts in HIV-infected patients remains uncompletely understood, because these cells do not express CCR5,17 the coreceptor used by HIV early during the infection. The expression of CX3CR1 by naive Th lymphocytes, if inducing abnormal routes of cell migration and thus impaired survival of these cells, may contribute to their early decline in patients. Were this the case, recovery of normal cell traffic following ARV treatment initiation would not result in a rapid redistribution of naive Th cells, explaining why their recovery is slow in treated patients.28 31
In addition to perturbing leukocyte traffic, deregulated expression of FKN and its receptor in HIV-infected patients may enhance interactions between Th lymphocytes and DCs in T-cell zones of lymphoid organs. The consequences of such an increased contact are difficult to predict. In mice, survival and expansion of naive Th lymphocytes is dependent on their interaction with major histocompatibility complex class II–expressing cells, which are probably DCs.32 If this also applies in HIV-infected patients, FKN-mediated contact between DCs and naive Th lymphocytes should improve survival and expansion of naive Th cells. On the other hand, DCs facilitate productive HIV infection of CD4+ T lymphocytes.33-35 The possible influence on disease outcome of the accumulation of plasmacytoid DCs expressing FKN in lymph nodes from HIV-infected patients is also unclear. Controversy persists on the role of these cells and particularly on their ability to favor Th2 immune responses.19,36,37 Such an effect could explain the increased IL-13 gene expression we evidenced in lymph nodes from HIV-infected patients.38 In vitro, plasmacytoid DCs activate CD8+ T lymphocytes in such a way that they produce high levels of IL-10 but display poor killing properties.39 If such a phenomenon occurs in vivo in lymph nodes from HIV-infected patients, it could explain the deficiency of CD8-mediated cytotoxicity observed in this disease.40Conversely, plasmacytoid DCs express high levels of interferon-α,41 a cytokine with many antiviral and immunostimulating properties.
The ability of particular alleles of the CX3CR1 gene to affect the course of HIV infection shows that the FKN/CX3CR1 complex influences the pathophysiology of HIV infection. Our results suggest that this influence involves an effect of FKN on the homing properties of Th lymphocytes and on the contact these cells establish with other immune cells, especially with DCs. Through such effects, the FKN/CX3CR1 complex may affect the equilibrium between depletion and renewal of the Th compartment in HIV-infected patients.
We acknowledge Laurence Dubey, Alain Portier, Agnès Florentin, Ghislaine Lubart, Anne-Marie Delavalle, and Jean-Dominique Magnier for technical assistance, Thomas Schall (Chemo Centryx, San Jose, CA) for the gift of polyhistidine-tagged FKN and anti-hFKN antibody, and Agnès Veyradier for vWF measurements.
Supported by the French Agency on AIDS Research. A.F. was a fellow from the Association for Research on Cancer, and D.B. was supported jointly by the National Center for Scientific Research and by the Public Assistance-Hospitals of Paris (AHR98016).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Dominique Emilie, Institut Paris-Sud sur les Cytokines, 32 rue des Carnets, 92140 Clamart, France; e-mail:emilie@ipsc.u-psud.fr.