Abstract
CD8 T cells play an important role in protection and control of HIV-1 by direct cytolysis of infected cells and by suppression of viral replication by secreted factors. However, although HIV-1–infected individuals have a high frequency of HIV-1–specific CD8 T cells, viral reservoirs persist and progressive immunodeficiency generally ensues in the absence of continuous potent antiviral drugs. Freshly isolated HIV-specific CD8 T cells are often unable to lyse HIV-1–infected cells. Maturation into competent cytotoxic T lymphocytes may be blocked during the initial encounter with antigen because of defects in antigen presentation by interdigitating dendritic cells or HIV-infected macrophages. The molecular basis for impaired function is multifactorial, due to incomplete T-cell signaling and activation (in part related to CD3ζ and CD28 down-modulation), reduced perforin expression, and inefficient trafficking of HIV-specific CD8 T cells to lymphoid sites of infection. CD8 T-cell dysfunction can partially be corrected in vitro with short-term exposure to interleukin 2, suggesting that impaired HIV-specific CD4 T helper function may play a significant causal or exacerbating role. Functional defects are qualitatively different and more severe with advanced disease, when interferon γ production also becomes compromised.
Persistence of HIV-1–infected cells in vivo despite a vigorous immune response
During primary HIV-1 infection, plasma viremia increases to peak levels of up to 109 HIV-1 copies/mL. Viremia is thereafter partially controlled by the immune response and stabilizes 3 to 6 months later. However, in situ hybridization for HIV-1 RNA and quantification of cell-associated unspliced messenger and genomic RNA and DNA indicate that HIV-replicating and latently infected cells in lymphoid tissue increase over time.1,2 The majority of cells replicating HIV-1 are CD4 T cells, both early and late in disease.3
Viral replication persists in lymphoid tissue despite a vigorous immune response. HIV-1–specific CD8 T cells, which should be able to target virally infected cells for elimination, exist at high frequencies in most HIV-1–infected patients. Previous limiting dilution estimates of the frequency of HIV-specific CD8 T cells reached around one per thousand cells.4,5 However, more sensitive techniques that use tetramer staining, which fluorescently labels T cells whose T-cell receptors (TCRs) recognize an antigenic peptide-major histocompatibility (MHC) pair, have shown that up to a few percent of circulating CD8 T cells recognize a particular HIV-1 peptide.6 The frequency of CD8 T cells producing interferon γ (IFNγ) in response to major HIV-1 proteins expressed by vaccinia virus ranges from 0.8% to 18%.7 In response to HIV-1–infected primary CD4 T cells, approximately 0.3% to 3% of HIV-seropositive donor CD8 T cells produce IFNγ.8 In those studies, the frequency of circulating HIV-specific CD8 T cells is generally above 1%. These results provide lower limits for the frequency of HIV-specific CD8 T cells because, as discussed below, not all specific cells are triggered to produce IFNγ.8
After the first week of infection when viral dissemination is reined in by the innate immune response, viral-specific CD8 T cells form the cornerstone of immune control for most viruses, including HIV-1. Viral-specific cytotoxic T lymphocytes (CTLs) suppress HIV-1 replication in vitro by direct cytotoxicity and secretion of soluble factors.9-13 Acute viremia resolves with the appearance of HIV-specific CTLs.14,15 Moreover, the likelihood of progressing to AIDS increases in patients who lackgag-specific CTLs.4,16 This finding, together with the rapid disease course in neonates whose overall T-cell immunity is immature,17 suggests that CTLs are important in controlling HIV-1. Direct evidence was provided in rhesus macaques in which elimination of CD8 T cells results in a dramatic increase in simian immunodeficiency virus (SIV) load.18 19
If there are so many HIV-1–specific CD8 T cells, why are these cells not doing a better job? In the absence of antiviral drugs, the immune response fails to halt progressive immunodeficiency in all but a small minority of long-term nonprogressor (LTNP) patients. A persistent viral reservoir might be explained by the fact that HIV-1 can establish latent cellular infection in which HIV-1 provirus is integrated into the host genome, but no viral proteins are produced to signal to roving T cells. However, the progressive nature of the disease suggests that antiviral CD8 T cells do not always efficiently destroy cells replicating HIV-1. As many as 10 billion virions are produced each day in asymptomatic, untreated individuals.20,21 The failure of CD8 T-cell function is clear when one compares the frequency of HIV-specific CD8 T cells with the numbers of HIV-1–infected cells replicating virus, generally substantially below one per thousand and frequently on the order of one per million mononuclear cells. Because the frequency of HIV-specific CD8 T cells is typically at least 10-fold higher and because in infected individuals there are usually more CD8 T cells than CD4 T cells and macrophages, the inability to control viral production is striking, especially because CTLs are “serial killers” able to lyse multiple cells.22 23
HIV-specific CD8 T cells do not lyse HIV-1–infected cells
Failure to control viral replication suggests that antiviral CD8 T cells are impaired in lysing HIV-1–infected cells and suppressing HIV-1 replication. Although early reports suggested that peripheral blood mononuclear cells (PBMCs) from 80% to 90% of HIV-1–infected donors demonstrate HIV-specific cytotoxicity above background in 6- to 16-hour assays,10,24,25 specific cytotoxicity by freshly isolated PBMCs is generally not much above background in direct 4-hour51Cr release assays.8,26-28 However, HIV-specific cytotoxicity by PBMCs is dramatically enhanced after overnight culture in an interleukin-2 (IL-2)–dependent manner.26 The rapid increase in cytotoxicity, typically to 10% to 30% specific cytotoxicity in a 4-hour assay at an effector/target (E:T) ratio of 25:1 to 50:1, cannot be explained by overnight clonal expansion of viral-specific T cells. A possible specific defect in HIV-specific cytotoxic capability was recently demonstrated by comparing HIV- and CMV-specific lysis by freshly isolated blood lymphocytes.28 HIV-specific lysis was considerably less than CMV-specific lysis, despite comparable numbers of tetramer-staining HIV-specific and CMV-specific CD8 T cells.
Viral evasion of cytotoxic T cells
Failure of cytolysis could arise either from resistance of HIV-1–infected targets to CTL recognition or lysis29,30or from a lack of effector function by the CTLs themselves.26 Many viruses have strategies to evade CTL recognition, especially by interfering with antigen presentation (reviewed in Tortorella et al31). Two HIV-1 evasion strategies have been described. Viral mutation of the epitopic sequence recognized by HIV-specific CTL can sidestep CTL recognition.29,32,33 Moreover, mutated epitopes (altered peptide ligands) can interfere with subsequent triggering by cells expressing wild-type sequence.34 Although such mutations certainly exist, it is uncertain whether they play an important role. Convincing recent evidence for a role for escape mutation comes from the rapid emergence of viral mutations of recognized tat epitopes during acute SIV infection.35
Another viral evasion mechanism is nef-induced down-modulation of MHC class I A and B molecules.36-40 Assays that measure T-cell functional responses to HIV-1–infected primary cells are invaluable for determining whether viral evasion significantly interferes with T-cell immune responses.8,40-43 In fact, CD8 clones that recognize 4 different HIV-1 proteins lyse HIV-1–infected primary CD4 T cells as well as they lyse cells infected with recombinant vaccinia virus expressing HIV-1 genes.43 Moreover, nef is a major antigen recognized by HIV-seropositive donor CD8 T cells.44 In addition, CD8 T cells can recognize target cells expressing only a few antigenic peptide-MHC molecules45 and the HLA class I C allele is unaffected by nef.46 Those results suggest that nef-mediated down-modulation of class I alleles may not block immune recognition of HIV-1–infected cells. Nef-mediated down-modulation of class I molecules, however, might be important for CD8 T cells with low-affinity TCRs or for antigens, synthesized at low levels or inefficiently processed and presented.30
T-cell anergy in HIV-1 infection
Because lysis of HIV-1–infected cells is dramatically up-regulated after brief exposure to IL-2 in vitro,26there is also a functional in vivo problem with HIV-specific CTLs. Previously activated antigen-specific T cells may be unable to proliferate or to perform the full complement of effector functions when they re-encounter antigen. Lack of proliferation or function is termed anergy.47 Although anergy can be defined as complete unresponsiveness on re-encounter with antigen, in reality cells more often become partially anergized. For example, a CD4 T cell may lose the ability to proliferate to antigen but may still express activation molecules and secrete some cytokines. Anergy is important to prevent immunopathology and autoimmunity. Anergy has been studied extensively for CD4 T cells, but less is known about regulation of CD8 T-cell function.
HIV-1–specific CD4 T-cell proliferation is undetectable within 3 months after primary infection in most HIV-1–infected individuals, with the exception of some LTNPs and early treated patients.48-54 However, HIV-specific CD4 T cells are not deleted but are present in low numbers in a partially anergized state.55 In response to HIV-1 gag p24, approximately 0.12% (range, 0%-0.66%) of CD4 T cells produce IFNγ by intracellular flow cytometry analysis. The median response is higher in nonprogressive HIV-1 disease (0.40%). However, those figures are about an order of magnitude lower than the corresponding CD8 T-cell activity, especially when the reduced numbers of CD4 T cells are considered.55 Moreover, CD4 proliferation to HIV-1 p24 can be induced in vitro by adding CD40L-trimer and IL-12, providing further support for persistence of anergized HIV-specific CD4 cells.56
CD4 T cells are important to maintain effective antiviral CD8 CTLs. They secrete IL-2, an important growth factor for CD8 cell survival. However, high concentrations of IL-2 facilitate T-cell activation–induced apoptosis. The high frequency of HIV-specific CD8 T cells may be partly due to higher concentrations of stromal cell and monocyte-derived cytokines, such as IL-7, IL-12, and IL-15, which promote T-cell survival but not apoptosis, compared with CD4 T-cell–derived IL-2.57-62 CD4 T cells also express molecules whose ligation is directly or indirectly required for CTL differentiation. For example, ligation of CD40L on CD4 T cells activates dendritic cells (DCs) to provide signals for development of CD8 CTLs.63-65 Although antiviral CD8 T cells persist in CD4 T-cell–depleted mice, their function is compromised.66,67 In this review we argue that CD8 T-cell anergy contributes significantly to the progressive immunodeficiency of untreated HIV-1 infection, as was first suggested by Miedema68 and Lewis et al.69
Potential dysfunction of antigen presentation by macrophages and dendritic cells
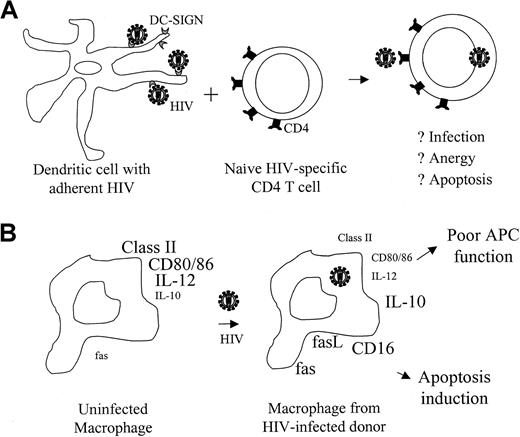
Lack of HIV-specific CD4 proliferation and CD8 cytotoxicity is usually not overcome by antiretroviral therapy. As was originally suggested by Macatonia et al70 and more recently by Shearer,71 CD4 and CD8 T-cell anergy in HIV-1 infection may be a result of changes in antigen presentation by DCs or macrophages (Figure 1). Interdigitating DCs bring antigen from peripheral tissues to the lymph nodes (LNs), where they activate naive T cells. Mature DCs strongly up-regulate MHC class II and costimulatory molecules72such as CD40, CD80, CD86, OX40L,73 and 4-1BBL,74 required for effective stimulation of a primary T-cell response. Although monocytes and macrophages are infected with HIV-1, whether DCs are infected remains controversial.75-79 HIV-1 binds to DCs through the association of gp120 with a recently described C-type lectin receptor, DC-SIGN. Tight binding of HIV to DCs facilitates CD4 T-cell infection.80,81 Because DC-SIGN is important for DC-mediated T-cell activation, HIV-1 particles or free gp120 might interfere with primary T-cell activation. However, this is just a conjecture. In fact, freshly isolated DCs from spleen or blood of HIV-1–infected donors have no significant differences in MHC class II or costimulatory molecule expression and are not defective antigen-presenting cells (APCs) after in vitro maturation.76,82-87 Although CD11c+ DCs are reduced in the blood of HIV-1–infected individuals, this reduction may be due to their migration to LNs, where they are increased.88 However, the APC function of cytokine-activated monocyte-derived DCs may have little relation to the function of DCs in vivo. Expression of critical costimulatory molecules on interdigitating DCs in situ in the paracortical regions of LNs needs to be studied.
Dendritic cells and macrophages in HIV-infected patients may hinder antigen presentation.
(A) Binding of HIV-1 gp120 to DC-SIGN on DCs enhances infection of CD4 T cells and may interfere with their activation. (B) Macrophages from HIV-infected patients have reduced expression of molecules required for effective stimulation of T cells and have increased expression of molecules that can trigger apoptosis. Macrophages with defective APC function are not necessarily infected with HIV. The size of the labels in this figure reflects the relative expression level on macrophages from healthy donors and HIV-infected donors. Part B modified from Shearer71 copyright 1998 and reprinted with permission from Elsevier Science.
Dendritic cells and macrophages in HIV-infected patients may hinder antigen presentation.
(A) Binding of HIV-1 gp120 to DC-SIGN on DCs enhances infection of CD4 T cells and may interfere with their activation. (B) Macrophages from HIV-infected patients have reduced expression of molecules required for effective stimulation of T cells and have increased expression of molecules that can trigger apoptosis. Macrophages with defective APC function are not necessarily infected with HIV. The size of the labels in this figure reflects the relative expression level on macrophages from healthy donors and HIV-infected donors. Part B modified from Shearer71 copyright 1998 and reprinted with permission from Elsevier Science.
Although no clear DC functional deficit has been demonstrated, monocytes from HIV-infected donors aberrantly express molecules important for antigen presentation to T cells. HIV-infected macrophages are important for restimulating antigen-experienced HIV-specific T cells. Monocyte/macrophages from HIV-infected donors have reduced MHC class II and costimulatory CD80 and CD86 expression.89-91Moreover, they have up-regulated fas and fasL as well as CD16, molecules that may trigger apoptosis of HIV-specific T cells instead of activating a protective immune response.92,93 In vitro HIV-1 infection of macrophages also up-regulates fasL.92,94 Moreover, HIV-1 tat down-modulates transcription of the mannose receptor, important for binding pathogens and their efficient internalization for antigen presentation.95
Monocyte-derived macrophages from HIV-infected donors are also likely to be impaired in inducing TH1 and CTL responses because they produce less IL-12 and more IL-10.91,96 This may be secondary to reduced CD4 helper function, because trimeric CD40L can help restore IL-12 production in vitro, by substituting for CD40L on activated CD4 T cells.97 CD40L is required to activate APCs to produce IL-12 and is required for generating functional CTLs.63-65 Interestingly, the combination of CD40L-trimer and IL-12 reverses CD4 and CD8 T-cell anergy in vitro.56 98
Granule-mediated killing of HIV-1–infected cells
CTLs kill cells either by the granule exocytosis pathway (by releasing cytotoxic granules that contain pore-forming perforin [PFP] and granzyme proteases) or by engagement of death domain–containing receptors, such as fas or the tumor necrosis factor receptor (reviewed in Henkart99). The granule-mediated pathway, critical for the immune defense against many viral infections, is absolutely dependent on both PFP and granzymes.100 Mice genetically deficient in PFP succumb to viral infections such as lymphocytic choriomeningitis virus.101 Children with familial hemophagocytic lymphohistiocytosis, linked to a defective perforin gene, often die of overwhelming viral infections.102 Mouse experiments have also shown that IFNγ production by viral-specific CD8 T cells is important to control some viral infections.103-105 Although HIV-1–infected CD4 T cells express twice as much fas as uninfected activated cells, HIV-1–infected primary T cells are predominantly lysed by the PFP-dependent granule-mediated pathway.43 This was shown by using HIV-specific CD8 T-cell lines and clones responding to a variety of HIV-1 proteins. Moreover, HIV-specific CD8 T cells do not express the principal death receptor ligand fasL, even after stimulation with HIV-1–infected CD4 cells.43
Dysmaturation of HIV-specific CD8 T cells
Phenotypic analysis of tetramer-stained, HIV-specific CD8 T cells indicates that circulating viral-specific CD8+ T cells have been previously activated, because they no longer express cell surface markers, such as CD62L, CCR7, CD45RA, and CD28, present on naive cells, and also express CD45RO, granzyme A (GzmA), and bcl-2, not found in naive cells.6,8,28,106,107 (Table1). This is not surprising, given the high likelihood of prior encounter with HIV-1–infected cells. However, protein expression profiles suggest that HIV-specific CD8 T cells either have not fully differentiated into mature effector CTLs, have reverted to a memory type, or have become anergized. Another possibility is that specific cells die before they fully mature; however, this is unlikely, given the high frequency of HIV-specific CD8 T cells. Although no single surface marker unambiguously identifies CTLs, differentiation into effector CTLs is associated with down-modulation of CD27 and CD28 and re-expression of CD45RA.108-110 Although HIV-1 tetramer+ cells are CD28−, they are uniformly CD27+ and CD45RA− in most donors.8,28,111 These properties are distinct to HIV-specific cells, because they do not hold for cells specific for Epstein-Barr virus (EBV) and cytomegalovirus (CMV) in the same donors.28 107
Phenotypic properties of tetramer+ CD8 T cells
| Cell marker . | HIV-1 tetramer+ cells . | EBV and CMV tetramer+ cells . |
|---|---|---|
| CD3ζ | − | − |
| CD28 | − | − |
| NKRs | ? | ? |
| CD62L | − | − |
| CD45RA | − | ± to + |
| CCR7 | − | − |
| CD27 | + | − |
| GzmA | + | + |
| Perforin | − to ± | − to ± to + |
| Bcl-2 | + | ? |
| Cell marker . | HIV-1 tetramer+ cells . | EBV and CMV tetramer+ cells . |
|---|---|---|
| CD3ζ | − | − |
| CD28 | − | − |
| NKRs | ? | ? |
| CD62L | − | − |
| CD45RA | − | ± to + |
| CCR7 | − | − |
| CD27 | + | − |
| GzmA | + | + |
| Perforin | − to ± | − to ± to + |
| Bcl-2 | + | ? |
The phenotypic properties of the majority of HIV-specific CD8 T cells are compared with those for other chronic viral infections. Data are a composite from published6,8,28,106,107,111 150 and unpublished (P.S., G. Chen, and J.L., March 2001) studies.
EBV indicates Epstein-Barr virus; CMV, cytomegalovirus; NKR, natural killer cell-inhibitory receptor; GzmA, granzyme A; ?, not known.
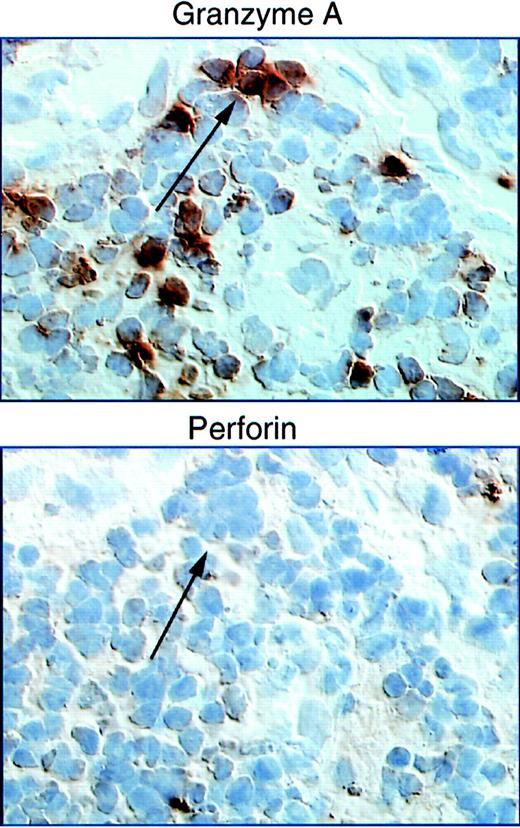
Naive CD8 T cells have limited function on their initial encounter with antigen; they are not cytolytic and do not produce effector cytokines rapidly. It takes approximately 5 days to differentiate into effector CTLs and to express cytolytic molecules.112 PFP and granzymes are up-regulated in parallel.112 Circulating CD8 T cells in HIV-1–infected subjects, compared with healthy donors, have an unusually high percentage of CD8 cells containing cytolytic granules, as measured by staining for the most abundant granule protease, GzmA.26 The overwhelming majority of HIV-1 tetramer+ CD8 T cells express GzmA.8,43However, there is a disjunction between PFP and GzmA protein expression in the LNs113 (Figure 2). Although granzyme+ CD8 T cells are increased in HIV-1–infected LNs compared with normal LNs, PFP+ cells are rare. This finding holds for both acute and chronic HIV-1 samples and is in contradistinction to acute infectious mononucleosis LNs in which PFP and GzmA are both increased.114 Lack of LN PFP-expressing CD8 T cells is not due to recent degranulation, because degranulation would deplete granzymes as well as PFP. PFP is also not expressed at high levels in circulating tetramer+HIV-specific CD8 T cells.28 Lack of PFP seems to affect HIV-specific cells preferentially, because about a third of all circulating CD8 T cells (34% ± 20%) are PFP+ in a diverse spectrum of HIV-1–infected patients.107 Moreover, in another study HIV-1 tetramer+ cells are PFP−, whereas CMV tetramer+ cells in the same patient are PFP+.28 Because PFP is required to lyse HIV-1–infected targets,43 lack of PFP may be critical for impaired cytotoxicity, especially at LN sites of viral production.
Perforin staining is reduced compared with staining for GzmA in adjacent LN sections from a donor undergoing primary HIV infection.
In contrast, comparable levels of granzymes A and perforin were found in samples from acute infectious mononucleosis. Figure taken from Andersson et al113. Copyright 1999 and reprinted with permission from Lippincott Williams & Wilkins.
Perforin staining is reduced compared with staining for GzmA in adjacent LN sections from a donor undergoing primary HIV infection.
In contrast, comparable levels of granzymes A and perforin were found in samples from acute infectious mononucleosis. Figure taken from Andersson et al113. Copyright 1999 and reprinted with permission from Lippincott Williams & Wilkins.
These results require further verification. The absence of PFP in HIV-specific CD8 T cells compared with CD8 T cells specific for another virus was reported in only one study. A word of caution also is necessary before generalizing from results for HIV-1 tetramer+ cells to conclusions about all HIV-specific CD8 T cells. HIV-1 epitopes for which tetramers are available are rarely immunodominant.115-118 CD8 T cells responding to subdominant or cryptogenic epitopes may have lower avidity interactions with target cells than T cells in the immunodominant response.119 They may revert to CD45RA−PFP− memory cells if they are not effectively activated by HIV-infected cells, especially if the viral epitope has mutated. Low avidity interactions may alter T-cell activation and differentiation, as has been shown for murine CD4 T cells.120 Therefore, lack of PFP in HIV-specific CD8 T cells needs to be confirmed by using a broader array of tetramers or other methods.8 26-28
CD8 T cells suppress HIV-1 replication by soluble factors
CD8 T cells also suppress viral production by secreting cytokines like IFNγ, inhibitory chemokines, such as macrophage inflammatory protein 1α (MIP-1α), MIP-1β, and regulated on activation, normal T-cell expressed and secreted (RANTES), and other factors, some of which, such as CD8+ cell antiviral factor (CAF), remain to be characterized.9-13 CAF is produced by CD28+ CD8 T cells, which are substantially reduced in HIV-1 infection.121 The inhibitory chemokines, and perhaps other suppressive molecules, are stored in cytotoxic granules with PFP and granzymes.122 Leukemia inhibitory factor, also produced by CD8 T cells, is a recently described potent HIV-1 suppressor factor, active at concentrations 100-fold lower than the β-chemokines.123
Reduced IFN-γ production by HIV-specific CD8 T cells in late-stage patients
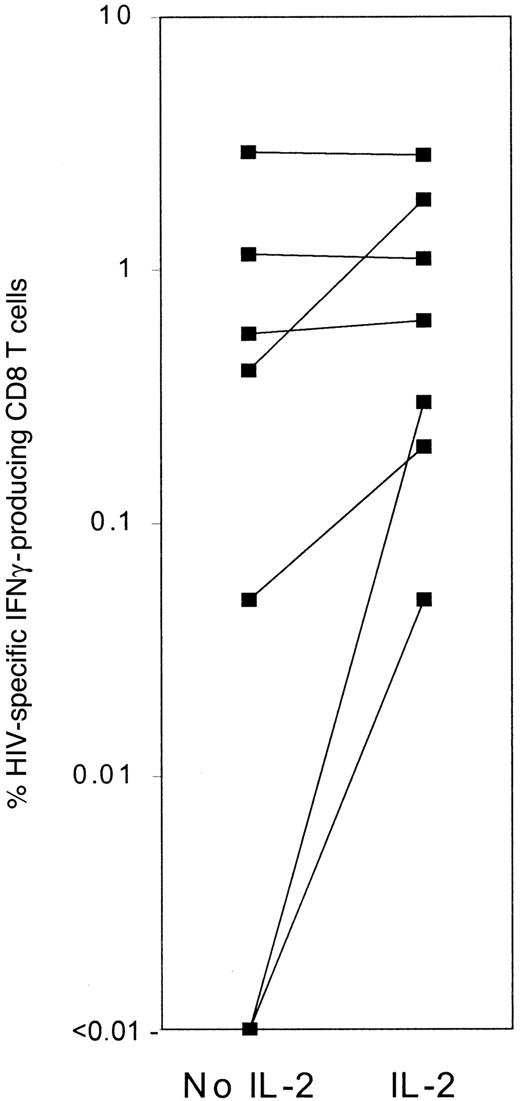
IFNγ is important in antiviral immunity in genetically targeted murine models.124-126 In more advanced patients, fewer cells produce IFNγ in response to HIV-1, and the number of IFNγ-producing cells initially increases with highly active antiretrovial therapy.127-129 On a single cell basis, IFNγ production in response to HIV-1–infected cells is impaired, especially in late-stage patient samples.7,8,130 In one study, IFNγ production was measured by intracellular staining after stimulation with HIV-1–infected CD4 blasts in the presence or absence of IL-28 (Figure 3). In subjects with undetectable plasma viremia, adding IL-2 had no effect on IFNγ production. In some subjects with measurable plasma viremia, IL-2 increased the frequency of IFNγ-producing cells 4- to 5-fold. IFNγ production was also detected after IL-2 was added to some samples that had no IFNγ-producing cells without IL-2. Therefore, in more advanced patients, IFNγ production is compromised. In another approach, the numbers of HIV-1 tetramer+ CD8 T cells were compared with the numbers of cells producing IFNγ after stimulation with the tetrameric peptide. In mice, virtually every specific memory cell produces IFNγ in response to antigenic peptide.131,132 In most HIV-infected subjects, however, fewer than 25% of tetramer+ cells produce IFNγ after stimulation with tetrameric peptide. Subjects with a high proportion of IFNγ-producing tetramer+ cells are asymptomatic and have well-maintained CD4 counts (> 650/μL). Most subjects with less than 25% IFNγ-producing tetramer+ cells have had HIV-1–related stage B or C symptoms. Culturing tetramer+cells in IL-15 and IL-2 converts them from nonresponsive to IFNγ-producing cells.8
The number of IFNγ-producing CD8 T cells in response to HIV-1–infected CD4 T cells increases substantially in some HIV-1–infected patient samples when IL-2 is added during the stimulation.
Background IFN production in the presence of IL-2 but not infected CD4 T cells was low (< 0.05%) and was subtracted to get the frequency of specific producers. Data are taken from Shankar et al.8
The number of IFNγ-producing CD8 T cells in response to HIV-1–infected CD4 T cells increases substantially in some HIV-1–infected patient samples when IL-2 is added during the stimulation.
Background IFN production in the presence of IL-2 but not infected CD4 T cells was low (< 0.05%) and was subtracted to get the frequency of specific producers. Data are taken from Shankar et al.8
Therefore, IFNγ production in response to HIV-1, like cytotoxicity, is partially impaired in vivo, especially in later-stage subjects, because adding exogenous cytokines increases it. A few studies have not found impaired IFNγ production in HIV-1 infection, but they have either focused on donors with well-controlled or early infection28 or measured production following stimulation with antigen plus auxiliary costimulatory agents to amplify IFNγ production.133 A recent study in rhesus macaques infected with SIV mac239 confirms and illuminates the human data.134 Although virtually all tetramer+ CD8 T cells produce IFNγ after vaccination or early after infection, within 6 months the proportion of specific cells that produce IFNγ drops substantially. Loss of IFNγ production by antigen-specific CD8 T cells infiltrating melanoma tumors or circulating in melanoma patients has also been described.135-137
Down-regulation of key signaling molecules CD3ζ and CD28 on HIV-specific CD8 T cells
The T-cell response can be dissected into a series of steps, any of which might be impaired in HIV-specific CD8 T cells. The TCR complex is composed of a clonotypic dimeric TCR, required for antigen recognition, in noncovalent association with CD3, a multicomponent signal transduction complex. CD3 consists of CD3δ, CD3ε, and CD3γ chains and a CD3ζ chain–containing dimer (reviewed in Weiss and Littman138). When the TCR encounters antigen on an infected cell, a signal is sent into the cell via sequential tyrosine phosphorylation of key TCR-associated signaling molecules. This signal initiates formation of an “immunologic synapse” between the T cell and its target. The TCR-CD3 complex, CD28 and associated signaling molecules, such as lck, fyn, and protein kinase Cθ, cluster in the center of the synapse, surrounded circumferentially by larger molecules like CD2 and LFA-1, which stabilize the synapse. The synapse forms within minutes of TCR engagement and lasts for more than an hour until the entire TCR complex is internalized.139 Synapse formation critically depends on the actin cytoskeleton to move molecules in and out of the forming synapse.140 CD3ζ is central to transmitting the TCR activation signal. It contains 3 immune-receptor tyrosine-activating motifs, which can be phosphorylated and serve as docking sites for SH2 domain–containing tyrosine kinases. CD3ζ also links TCR-CD3 to actin.141 142
In antigen-experienced CD8 T cells, cytokine secretion and cytolysis are triggered rapidly following TCR engagement.143Cytolytic granules, which normally contain granzymes, PFP, and some other components, are transported toward the immunologic synapse, and the granule membrane fuses to the CTL plasma membrane, releasing its contents. Failure of cytolysis by HIV-specific CD8 T cells could be due to lack of recognition of HIV-1–infected primary cells by the TCR, failure or incomplete signaling by the TCR-CD3 complex, failure of degranulation, absence of key cytolytic components such as PFP in the cytolytic granules, or resistance of HIV-1–infected cells to cytolysis. Because β-chemokines are also stored in CTL granules,122 viral suppression would probably also be impaired if granule exocytosis is blocked.
HIV-specific tetramer+ CD8 T cells uniformly have down-modulated both CD3ζ and the principal costimulatory molecule CD28.26,111,144 Down-modulation of CD3ζ was first described in lymphocytes infiltrating murine tumors and has since been found in a variety of human cancers.145-148 Not surprisingly, circulating T cells of HIV-1–infected subjects also have signaling defects.111,149 Using immunoprecipitation and Western blotting, Stefanova et al149 found that kinase activities of lck, fyn, and ZAP70 were decreased in HIV-1–infected patients at varying disease stages but not in a group of LTNPs. CD3ζ down-modulation was also found by Western blotting in T cells from both acutely infected donors and AIDS patients.111,149 However, down-modulation of CD3ζ and CD28 also occurs in CD8 T cells during other viral infections and therefore, is part of normal immune regulation.150 CD3ζ−CD28−CD8 T cells from HIV-1–infected and healthy donors have a selective defect in activating the IL-2 axis—they neither express the α-chain of the high affinity IL-2 receptor (CD25) nor produce IL-2.111 150 This finding may be especially critical in HIV-1 infection in which there is a special deficit in HIV-stimulated production of IL-2 by CD4 T cells.
Down-modulation of CD3ζ and CD28 on most HIV-specific CD8 T cells almost certainly raises the activation threshold for functions induced by TCR engagement.111,150,151 The threshold for some functions, such as cytotoxicity, may be higher than for other functions, such as IFNγ production. In fact, CD8 T cells with down-modulated CD3ζ and CD28 produce IFNγ but not IL-2 after TCR activation.111,150 Different activation thresholds also characterize CD4 T-cell cytokine production. Antigen-experienced CD4 T cells are heterogeneous in the amounts of cytokines produced and in the signals required to activate secretion.152 Cells that do not produce cytokines may be triggered in the presence of activated APCs or of antibodies to costimulatory pathways (CD28, CD49d, and CD5).153 Raising the threshold for cytotoxicity protects the host from immunopathogenic consequences of lysing uninfected cells expressing the low-affinity self-antigens on which the T cell was selected in the thymus. The requirement for participation of nearby antigen-specific helper cells may also help ensure that the target is indeed infected.
Exogenous IL-2 reconstitutes CTL function
HIV-specific cytotoxicity and IFNγ production are greatly enhanced by adding more than 100 IU/mL IL-2.8,151Cytotoxicity requires IL-2 in most patient samples; IFNγ secretion is enhanced by IL-2, especially in advanced patients. Therefore, thresholds have been raised to different levels for different functions. A high IL-2 concentration triggers the low-affinity IL-2R, but much lower concentrations (∼1 IU/mL) trigger the high-affinity IL-2R. Because activated CD3ζ–down-modulated cells do not express high-affinity IL-2R, high IL-2 concentrations are needed to improve their function. Therefore, in vivo high-dose IL-2 therapy in HIV infection may do more than increase CD4 T cell numbers; it may also improve CD8 antiviral function.154 This possibility should be examined.
Correction of CD8 T-cell function with IL-2 suggests that CD4 T help may be important for protective CD8 T-cell function, as it is in mouse lymphocytic choriomeningitis virus infection.67 In HIV-infected humans, who lack a proliferative response to CMV or HIV, antigen-specific CD8 T cells persist, as they do in CD4 T- cell–depleted mice.155 Just as antigen-specific CD8 T cells are not protective in CD4 T-cell–depleted mice, lack of effective HIV-specific CD4 help may also be responsible for lack of immune protection by HIV-specific CD8 T cells.51,151 156-158 Although this hypothesis is attractive, supporting data are mostly indirect.
Inhibitory natural killer cell receptors on CD8 T cells
Another possible contributory factor to CD8 T-cell dysfunction in vivo could be inhibitory signaling by natural killer (NK) cell-inhibitory receptors (NKRs), expressed on some CD8 T cells after activation.159 The receptors belong to 2 distinct molecular types: (1) the immunoglobulin superfamily killer inhibitory receptors (KIRs), which recognize specific HLA allotypes with common structural features in the α-1 domain, and (2) C-type lectin receptors in the CD94 family, which display broad specificity for HLA class I molecules through lectin interactions.160-162CD94/NKG2-(A-C) and NKG2D lectinlike receptors, respectively, recognize HLA-E (not down-modulated by HIV-1 nef46) and MHC class I chain–related A (MICA). Additional immunoglobulinlike receptors (ILT receptors) are expressed by activated T cells and other leukocytes, and they interact broadly with class Ia molecules. Within the receptor families, homologues, which bind the same ligands, have opposite inhibitory or stimulatory activity. However, the inhibitory receptors usually have higher affinity. CD8 T cells expressing NKRs have oligoclonal TCRs, suggesting that they correspond to antigen-specific cell expansions. Recently, NKRs on melanoma-specific CD8 T cells have been shown to inhibit both cytotoxicity and IFNγ production.137
Large numbers of CD8 T cells express NKRs in HIV-1 infection. Moreover, NKR expression is up-regulated by cytokines, such as IL-10, IL-15, and tumor growth factor β, which are increased in HIV-1 infection.159,163,164 In HIV-infected donor samples, blocking NKR engagement with specific antibodies augments in vitro HIV-specific cytotoxicity.165 Therefore, it makes sense to explore a role for NKR inhibitory signaling in blocking HIV-specific CD8 T-cell function. A first step would be to show increased expression of NKRs on HIV-1 tetramer+ CD8 T cells.
Inefficient trafficking of HIV-1–specific CD8 T cells to LNs
Another reason for incomplete HIV-1 control by CD8 T cells could be inefficient access to sites of HIV-1 infection in LNs. T-cell activation in vivo is associated with changes in surface phenotype, which reflect alterations in migration and functional capability. Initial activation of naive CD8 T cells occurs in T-cell zones of LNs where naive cells encounter DCs bearing the antigen, which fits its TCR. Naive T cells continuously recirculate from blood to LNs through specialized high endothelial venules (HEVs). Sequential engagement of L-selectin (CD62L) and LFA-1 on naive T cells to their respective ligands on HEVs sets into motion tethering, rolling, and firm adhesion to HEVs, prerequisite steps for transmigration into the surrounding LN (reviewed in Springer166). A chemokine receptor CCR7 has also emerged as an important determinant of T-cell homing to LNs.167-169 CCR7 on T cells interacts with the chemokine secondary lymphoid tissue chemokine on HEVs and regulates LN homing by delivering an activation signal for LFA-1 binding.169-171Studies in CCR7 knockout mice suggest that CCR7 is also important for the movement of T cells within LNs. If the naive cell encounters antigen, it proliferates and differentiates into effector cells in the specialized microenvironment of the LN. In keeping with the requirement of effector/memory cells to function at peripheral sites of infection, previously activated CD8 T cells up-regulate adhesins, including CD44 and β integrins, which contribute to their preferential homing to inflamed tissues, from which naive cells are normally excluded.172-175 The LN homing receptors L-selectin and CCR7 are down-modulated on effector cells but are heterogeneously expressed on memory cells, suggesting that memory cells recirculate through LNs as part of immune surveillance.110
In HIV-1 infection there is a marked reduction in CCR7+ naive and long-term memory cells.107HIV-1, EBV, and CMV tetramer+ CD8 T cells from healthy donors and HIV-1–infected donors, even with well-controlled disease, do not express the lymphoid homing molecules CCR7 and CD62L.107 Exclusion of effector cells from LNs, which normally provides efficient division of labor among CD8 T cells and may protect LNs from damage by inflammatory cytokines and cytolytic enzymes, may work to the detriment of the host during infections, such as HIV-1 or EBV, that target lymphocytes by converting LNs into immunologically privileged sites. In fact, in paired PBMC and LN aspirate samples from HIV-infected donors, there were 3- to 4-fold fewer EBV tetramer+ cells in the LN than in the circulation, despite the concentration of EBV infection in lymphoid tissues.107 In acute SIV infection in macaques, SIV-specific CD8 T cells are also preferentially excluded from LNs; there are proportionately 4-fold fewer SIV tetramer+ cells in LNs than in the circulation in the first weeks after infection (P < .007).176
Circumstantial evidence also supports selective exclusion of HIV-specific CD8 T cells from lymphoid sites of infection. The proportion of CD8 T cells in the LNs of HIV-infected patients is about half of what it is in the circulation.107,177,178Moreover, the number of copies of spliced and unspliced viral RNA per cell is much higher in LNs than in PBMCs, as is the frequency of cells carrying infectious virus, suggesting a greater failure of protective immunity in the LN.177 During primary and chronic infection, perturbations of T-cell receptor Vβ repertoire, indicative of expansions of antigen-specific CTLs, are more pronounced in blood CD8 T lymphocytes than in the LN.179 180
Exclusion of CCR7 T cells from the LNs in HIV-1 infection is probably leaky. Gene-marked HIV-specific CD8 T-cell clones infused into HIV-1–infected subjects can be identified in LNs.181 How efficiently they traffic there is unclear, because billions of cells are administered in these clinical studies. The requirement of CD62L and CCR7 expression for lymphocyte homing via HEVs might also be superseded in an inflammatory setting. Moreover, some tissue-homing lymphocytes can enter LNs through afferent lymph. Thus, additional human LN studies are required to determine how much lack of LN homing molecules interferes with the ability of HIV-specific cells to get to infection sites.
Conclusions
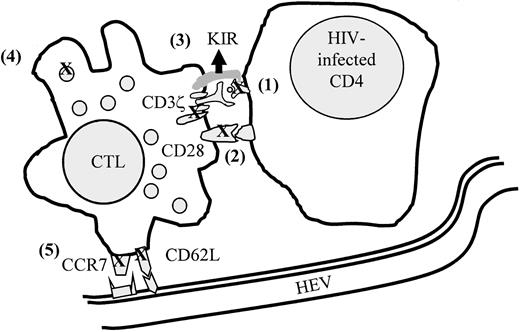
HIV-specific CD8 T cells have multiple properties that might explain their inadequate immunoprotective role in HIV-1 infection: (1) they are not cytotoxic, (2) they are impaired in trafficking to the major LN sites of viral replication, (3) they do not produce IL-2 and cannot respond to low concentrations of IL-2, and (4) they have down-modulated key molecules for T-cell signaling (Figure4). Moreover, CD8 T-cell function may be compromised even more in late-stage patients, when some of the molecular defects no longer correct in vitro with exogenous IL-2111 or when cytokine production is impaired. We now need to understand the relative contributions of the recently described novel mechanisms for CD8 T-cell dysfunction and their etiology. Closer study of the properties of HIV-specific CD8 T cells in LTNPs will help sort out what aspects of dysfunction are especially important for effective immune protection. Some of CD8 T-cell dysfunction is part of normal immune regulation of CD8 T cells to prevent these serial killers from wreaking damage by the release of cytolytic enzymes or inflammatory cytokines. For example, down-modulation of CD3ζ and CD28 and of LN homing receptors on antigen-specific CD8 T cells occurs in other infections.107 150 Although much research has focused on how CD4 T cells are regulated to prevent autoimmunity, regulation of CD8 T cells is largely terra incognito. Understanding CD8 T-cell regulation is critical for understanding immune pathogenesis and formulating strategies for immune intervention for HIV-1 and other diseases, including cancer.
Model of mechanisms that may contribute to lack of protection by antiviral CD8 T cells in HIV infection.
(1) HIV-1–infected cells are not recognized because of viral escape mutations or nef-mediated down-modulation of class I molecules, (2) signaling is impaired because of CD3ζ and CD28 down-modulation, (3) cytotoxicity is inhibited by expression of NK inhibitory receptors (KIR), (4) cytotoxicity is ineffective because of lack of PFP in cytotoxic granules, and (5) antigen-specific CTLs do not efficiently reach sites of infection because of down-modulation of homing receptors. Figure modified from Chen et al.107
Model of mechanisms that may contribute to lack of protection by antiviral CD8 T cells in HIV infection.
(1) HIV-1–infected cells are not recognized because of viral escape mutations or nef-mediated down-modulation of class I molecules, (2) signaling is impaired because of CD3ζ and CD28 down-modulation, (3) cytotoxicity is inhibited by expression of NK inhibitory receptors (KIR), (4) cytotoxicity is ineffective because of lack of PFP in cytotoxic granules, and (5) antigen-specific CTLs do not efficiently reach sites of infection because of down-modulation of homing receptors. Figure modified from Chen et al.107
Most tetramer+ HIV-specific CD8 T cells, unlike many EBV- or CMV-specific cells, do not have an effector CTL phenotype. Factors specific for HIV-1 infection that might influence CTL function are the continuous presence of high levels of infectious and noninfectious viral antigens, the paucity of functioning viral-specific CD4 helper cells, the effect of sustained inflammation on antigen-presenting function of macrophages and DCs, and the effect of particular HIV-1 gene products, such as tat or nef. A possible role of HIV proteins in blocking CD8 T-cell maturation into effector CTLs has yet to be studied. HIV-1 infection especially targets the helper CD4 T cells that are important in regulating effector CTLs. In mouse models in which CD4 T cells have been eliminated, protection against infection is lost, even though some antigen-specific CD8 T cells persist but with compromised function.66,67 These models may provide helpful parallels for HIV-1 infection, where CD8 T-cell protection may well correlate with preservation of HIV-specific CD4 T-cell function.156 182 This link needs to be studied in more depth by moving beyond counting the numbers of HIV-specific CD8 T cells to studying their function with assays that avoid more than a few hours of in vitro culture. Even after overnight culture, many CD8 functional defects correct, giving the false impression of functional competence. Studies that show no correlation between clinical status (such as LTNP) and numbers of antigen-specific CD8 T-cells need to be re-evaluated in light of new findings that many HIV-specific cells may not function in vivo.
Supported by grants AI-42519 and AI-45406 (J.L.) and AI-41536 (J.A.) from the National Institutes of Health and by grant 10850 (J.A.) from the Swedish Medical Research Council.
@ 2001 by The American Society of Hematology
References
Author notes
Judy Lieberman, The Center for Blood Research, 800 Huntington Ave, Boston, MA 02115; e-mail:lieberman@cbr.med.harvard.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal